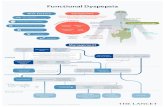
Functional Dyspepsia
-
Upload
dj-crisscross -
Category
Health & Medicine
-
view
1.368 -
download
9
description
Transcript of Functional Dyspepsia

CRISBERT I. CUALTEROS, M.D.CRISBERT I. CUALTEROS, M.D.
Department of Family and Community MedicineDepartment of Family and Community Medicine
Perpetual Succour Hospital Perpetual Succour Hospital

Gerald Holtmann, M.D., Nicholas J. Talley, M.D., Ph.D., Gerald Holtmann, M.D., Nicholas J. Talley, M.D., Ph.D., Tobias Liebregts, M.D.,Tobias Liebregts, M.D.,
Birgit Adam, M.D., and Christopher Parow, M.D.Birgit Adam, M.D., and Christopher Parow, M.D.New England Journal of Medicine New England Journal of Medicine
February 23,2006; 354: page 832-840February 23,2006; 354: page 832-840












A positive response: improvement by at least one grade





Improved symptom-severity scores on the LDQ Improved symptom-severity scores on the LDQ from baseline in all four study groups. from baseline in all four study groups.
Itopride was significantly superior to placebo Itopride was significantly superior to placebo during the testing of the global hypothesisduring the testing of the global hypothesis
Placebo and itopride given at 50 mg TID was not Placebo and itopride given at 50 mg TID was not significant (P 0.07)significant (P 0.07)
Placebo & itopride at 100 mg TID &Placebo & itopride at 100 mg TID & at 200 mg TID were both significant (P 0.05)at 200 mg TID were both significant (P 0.05)

Testing of all planned hypotheses for response Testing of all planned hypotheses for response rates to patients’ global assessment of efficacy rates to patients’ global assessment of efficacy showed significant resultshowed significant result
There was a significant association between the There was a significant association between the dose of itopride and the response ratedose of itopride and the response rate
The global hypothesis on the response rates The global hypothesis on the response rates
according to the severity of pain and fullness according to the severity of pain and fullness yielded a significant discrimination between yielded a significant discrimination between itopride & placebo itopride & placebo

Holtmann G et al. N Engl J Med 2006;354:832-840
Response Rates Based on Patients' Global Assessment of Efficacy

NDI quality-of life scores were better among NDI quality-of life scores were better among patients treated with active medication than patients treated with active medication than placebo. placebo.
The NDI quality-of-life score improved by a mean The NDI quality-of-life score improved by a mean of 13.2+/-19.4 with placebo and by 18.0+/-21.9 of 13.2+/-19.4 with placebo and by 18.0+/-21.9 with itopride (P = 0.02).with itopride (P = 0.02).
However, differences between the various doses However, differences between the various doses of itopride tested were not statistically significant.of itopride tested were not statistically significant.

Primary Outcome Variables among 523 Patients



Thank you…Thank you…