Chemistry - Lecture 5 Limiting Reactants - Power Point
Transcript of Chemistry - Lecture 5 Limiting Reactants - Power Point


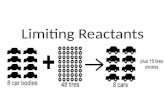
In a chemical reaction, the reaction stops as
soon as any of the reactants is totally
consumed, leaving the excess reactants as
leftovers.

CH4 + 2O2 CO2 + 2 H2OWe have
2.5 mol
We have
6.0 mol
need
3.0 mol
We have Less than needed
CH4 is limiting reactant
O2 is in excess

2 H2 + O2 2 H2O
Suppose you have a mixture of 10 mol H2 and 7
mol O2, which reacts to form water.
Since the ratio of H2 and O2 is 2:1, the number of
moles of O2 needed to react with H2 is
Moles O2 = (10 moles H2) (1 mol O2)
(2 mol H2)
= 5 mol O2

Because 7 mole O2 was available at the start of
the reaction, 7 mol O2 – 5 mol O2 = 2 mol O2 will
still be present when all the H2 is consumed.
The reactant that is consumed in a reaction is
called the limiting reactant or limiting reagent
because it determines, or limits, the amount of
product formed.
The other reactants are sometimes called
excess reactants or excess reagents.

There are no restrictions on the starting amounts
of the reactants in any reaction.
Many reactions are carried out using an excess of
one reagent.
The quantities of reactants consumed and the
quantities of products formed, however, are
restricted by the quantity of the limiting reactant.

2 H2 + O2 2 H2O
Initial quantities: 10 mol 7 mol 0 molChange (reaction): - 10 mol - 5 mol
+10 mol
Final quantities: 0 mol 2 mol 10 mol

PRACTICE EXERCISE: The most important commercial process for converting N2
from the air into nitrogen-containing compounds is based
on the reaction of N2 & H2 to form ammonia (NH3):
N2 + 3H2 2 NH3
How many moles of NH3 can be formed from 3.0 mol of N2, and
6.0 mol of H2?

Consider the following reaction:
2Na3PO4 + 3 Ba3 (PO4) 2 Ba (PO4) 2 +
6NaNO
Suppose a solution containing 3.50 g of
Na3PO4 is mixed with a solution containing
6.40 g of Ba3 (PO4) can be formed?

HOMEWORK:Page 109
3.693.70