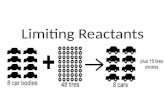
Limiting Reagent Stoichiometry!. So far In all our reactions, we assume both reactants get used up...
-
Upload
jack-collins -
Category
Documents
-
view
220 -
download
0
Transcript of Limiting Reagent Stoichiometry!. So far In all our reactions, we assume both reactants get used up...

Limiting Reagent
Stoichiometry!

So far
• In all our reactions, we assume both reactants get used up
• We assume both reactants provided are pure• We rarely see these two.– Limiting reagents– Percent Purity– Percent Yield
• So in a chemical reaction, we typically only use up one reactant and the other one is usually left over (excess)

Baking example
• You have 1 bag of flour• 1 bag of sugar• Same size• You will use up your bag of flour before you
can use up your bag of sugar.• So your bag of flour will be your limiting
reagent• Your bag of sugar is your excess

Why?
• We have excess amounts so that the one that is limiting will get used up – If it is too expensive, we want to use it up– If it is harmful to the environment, we want to
react it to a less harmful chemical• It can also be unavoidable as we usually do
have chemical that is in high quantities– Air is all around us and if the reaction involves air,
it is in excess

Key Terms
• Excess Reagent = reagent that is in excess, will not get used up in the reaction
• Limiting Reagent = reagent that will get used up fully in the reaction
• To find, we need to convert to MOLES of product.• We cannot tell just by given the mass of our
reactants.• The amount of products we form will be called
the theoretical yield.

Simulation!

Note!
• We refer to moles, not mass for limiting reagent.
• Just because something has a smaller mass doesn’t mean it is limiting!
• A limiting reagent question will always show enough information to get to moles for both reactants!

Steps to solve
• Convert mass of both reagents to moles of reagents
• Convert both moles of reagent to the moles of ONE PRODUCT!
• Whichever one is less will be the limiting reagent• Whichever one is in excess will have some
remaining, we can calculate what is leftover if we find the moles of excess used up and subtract from starting!

Example
• 2Al + 3I2 → AlI3 • Determine the limiting reagent and theoretical
yield of the product if– A) 1.20 mol Al and 2.40 mol iodine– B) 1.20g of Al and 2.40g of iodine– C) How many grams of Al are left over in part b?

Example
• 15.00g of aluminum sulfide and 10.00g of water react until the limiting reagent is used up
• Al2S3 + 6H2O → 2Al(OH)3 + 3H2S• A) Which is the limiting reagent?• B) What is the mass of H2S which can be
formed from the limiting reagent?• C) How much excess reagent remains after the
reaction is complete?

Percentage Yield and Percentage Purity

Assumptions
• It is assumed in a chemical reaction that our products are always produced 100% pure. That means whatever we started with will all turn into our pure product
• So we make many assumptions• What we start with will all turn into product– Percentage yield
• What we make is 100% pure– Percentage purity

Not always the case
• We will get percentages of both• This is due to– Impure products– Reactants not measured exactly– Recovery of products is difficult– Human error in transfer of steps

Percentage yield
• The percent of what we actually get compared to what we expect
• The higher the better!• Percentage yield = actual yield / theoretical yield x
100%• Actual yield = the actual amount of product formed in
the experiment• Theoretical yield = the amount of product expected
from calculation• NOTE – No way to get below 0% or above 100%

Percentage Purity
• The measure of how much desired product is present in a mass of impure product
• Percentage purity = mass of desired product / mass of impure product x 100%
• The higher the better as it means our product is pure!• Very important in chemistry and in not so legal things
(breaking bad)• No way we can go below 0% or over 100%• 100% purity is very tough to achieve!

Steps to solving
• Do your calculation as if we are doing any stoichiometry calculation
• Assume everything is 100% percentage• Apply the percentage yield and purity
calculation at the very end.

Example
• When 45.8g of K2CO3 are reacted completed with excess HCl, 46.3g of KCl are produced. Water and carbon dioxide are also formed. Calculate the theoretical yield and the percent yield of KCl.
• K2CO3 + 2HCl → 2KCl + H2O + CO2 • Find our limiting first!

Example
• Consider the reaction below• 3Mg(OH)2 + 2H3PO4 → Mg3(PO4)2 + 6H2O
• Calculate the mass of Mg3(PO4)2 that will be formed from the reaction of 15.0g Mg(OH)2 that has a percentage purity of 85% with excess H3PO4
• Assume that the Mg(OH)2 is 100% pure first!

Example
• What mass of K2CO3 is produced when 1.50g of KO2 is reacted with an excess of CO2 according to the reaction
• 4KO2 + 2CO2 → 2K2CO3 + 3O2 • If our reaction has a percent yield of 76.0%?

Magic Triangle
• You can make magic triangles for both calculation
• %y = a/t• %p = d/i

Homework
• Page 133 #26-32 (odd)• Page 137 #33-38 (odd)• Limiting reagent worksheet• Stoichiometry Review worksheets