II. Limiting Reactants Stoichiometry – 3.7. A. Limiting Reactants b Available Ingredients 4 slices...
-
Upload
silas-robinson -
Category
Documents
-
view
216 -
download
0
Transcript of II. Limiting Reactants Stoichiometry – 3.7. A. Limiting Reactants b Available Ingredients 4 slices...

II. Limiting ReactantsII. Limiting Reactants
Stoichiometry – 3.7Stoichiometry – 3.7

A. Limiting ReactantsA. Limiting ReactantsA. Limiting ReactantsA. Limiting Reactants
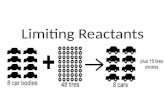
Available IngredientsAvailable Ingredients• 4 slices of bread• 1 jar of peanut butter• 1/2 jar of jelly
Limiting ReactantLimiting Reactant• bread
Excess ReactantsExcess Reactants• peanut butter and jelly

A. Limiting ReactantsA. Limiting ReactantsA. Limiting ReactantsA. Limiting Reactants
In a laboratory, usually one or In a laboratory, usually one or more of the reactants are present more of the reactants are present in in excessexcess. There is more than the . There is more than the exact amount required to reactexact amount required to react
Once one of the reactants is used Once one of the reactants is used up, up, no more productno more product can form can form

A. Limiting ReactantsA. Limiting ReactantsA. Limiting ReactantsA. Limiting Reactants
Limiting ReactantLimiting Reactant• used up in a reaction• Limits the amount of reactant that can combine
and determines amount of product• determines the amount of product that can form
Excess ReactantExcess Reactant• added to ensure that the other reactant is
completely used up• cheaper & easier to recycle

B. Limiting ReactantsB. Limiting ReactantsB. Limiting ReactantsB. Limiting Reactants
1. Write a balanced equation.
2. For each reactant, calculate the
amount of product formed.
3. Smaller answer indicates:
• limiting reactant
• amount of product

B. Limiting ReactantsB. Limiting ReactantsB. Limiting ReactantsB. Limiting Reactants
79.1 g of zinc react with 0.90 L of 2.5M HCl. Identify the limiting and excess reactants. How many liters of hydrogen are formed at STP?
Zn + 2HCl ZnCl2 + H2 79.1 g ? L0.90 L
2.5M

B. Limiting ReactantsB. Limiting ReactantsB. Limiting ReactantsB. Limiting Reactants
79.1g Zn
1 molZn
65.39g Zn
= 27.1 L H2
1 molH2
1 molZn
22.4 LH2
1 molH2
Zn + 2HCl ZnCl2 + H2 79.1 g ? L0.90 L
2.5M

B. Limiting ReactantsB. Limiting ReactantsB. Limiting ReactantsB. Limiting Reactants
22.4L H2
1 molH2
0.90L
2.5 molHCl
1 L= 25 L
H2
1 molH2
2 molHCl
Zn + 2HCl ZnCl2 + H2 79.1 g ? L0.90 L
2.5M

B. Limiting ReactantsB. Limiting ReactantsB. Limiting ReactantsB. Limiting Reactants
Zn: 27.1 L H2 HCl: 25 L H2
Limiting reactant: HCl
Excess reactant: Zn
Product Formed: 25 L H2
left over zinc

LIMITING REACTANTLIMITING REACTANTLIMITING REACTANTLIMITING REACTANT
Try Example Problem #2
• Method 1: Convert both reactants to product. See which is less.
• Method 2: Convert one reactant to another. See how much is needed.

LIMITING REACTANTLIMITING REACTANTLIMITING REACTANTLIMITING REACTANT
Problem #2:
• HF: limiting
• 4.0 mol excess SiO2

C. Percent YieldC. Percent YieldC. Percent YieldC. Percent Yield1. actual yield: measured amount of
product obtained from a reaction; measured in actual lab; less than theoretical yield due to experimental errors
2. theoretical yield: maximum amt. of product that could ideally be obtained from a given amount of reactant

C. Percent YieldC. Percent YieldC. Percent YieldC. Percent Yield
100yield ltheoretica
yield actualyield %
calculated w/stoich.
measured in lab

C. Percent YieldC. Percent YieldC. Percent YieldC. Percent Yield
When 45.8 g of K2CO3 react with excess
HCl, 46.3 g of KCl are formed. Calculate the theoretical and % yields of KCl.
K2CO3 + 2HCl 2KCl + H2O + CO2 45.8 g ? g
actual: 46.3 g

C. Percent YieldC. Percent YieldC. Percent YieldC. Percent Yield
45.8 gK2CO3
1 molK2CO3
138.21 gK2CO3
= 49.4g KCl
2 molKCl
1 molK2CO3
74.55g KCl
1 molKCl
K2CO3 + 2HCl 2KCl + H2O + CO2 45.8 g ? g
actual: 46.3 g
Theoretical Yield:

C. Percent YieldC. Percent YieldC. Percent YieldC. Percent Yield
Theoretical Yield = 49.4 g KCl
% Yield =46.3 g
49.4 g 100 =93.7%
K2CO3 + 2HCl 2KCl + H2O + CO2 45.8 g 49.4 g
actual: 46.3 g