Therefore: There are 3 subatomic particles: protons, neutrons and electrons. These are measured in...
-
Upload
lillian-june-foster -
Category
Documents
-
view
219 -
download
0
Transcript of Therefore: There are 3 subatomic particles: protons, neutrons and electrons. These are measured in...
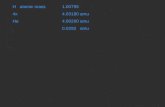
Therefore: • There are 3 subatomic particles: protons,
neutrons and electrons. These are measured in “atomic mass units” (amu) as their mass is so small.
SubatomicParticle Mass (amu) Location Charge
Proton ( p+ )1.673 x 10-27 kg
(1.0073 amu or 1 amu)
Neutron ( n0 )1.675 x 10-27 kg
(1.0087 amu or 1 amu)
Electron ( e- )9.1x 10-31 kg
(0.0005 amu or 0 amu)
Atomic Number and Mass Number
• Atomic Number = the number of protons–Unique to each element– In a neutral atom, the number of
protons equal the number of electrons.
• Mass Number equal to the total number of protons + neutrons in the nucleus of an atom.Ex) carbon-12
IsotopesAtoms that have the same number of protons but a different number of neutrons (mass.)
Isotopic NotationShorthand way of representing an isotope of an
element.
Ex) top number is the mass number (#p + #n)
bottom number is the atomic number (#p)
May also be written: chlorine-37 or Cl-37
The actual average atomic mass for all chlorine isotopes is 35.45 amu
3717 Cl
Isotopes of Hydrogena. hydrogen (hydrogen – 1) 1p+ 0n0 b. deuterium (hydrogen – 2) 1p+ 1n0 c. tritium (hydrogen – 3) 1p+ 2n0
11H21H31H
Isotope ProtonsNeutro
ns
Mass Numbe
r
Electrons
Isotopic
Notation
Carbon-12 6 6 12 6
Carbon-13 6 7 13 6
Carbon-14 6 8 14 6
Ions• Formed when an atom gains or loses an electron
a. Charge = # of protons - # of electrons Ex) Mg +2 = lost 2 electrons
# of protons: 12 # of electrons: 10 Charge: +2
Positively Charged ion - CATION
Ex) N-3 = gained 3 electrons # of protons: 7 # of electrons: 10
Charge: -3Negatively Charged ion - ANION
Isotope Protons Neutrons
Mass Number
Electrons
Isotopic Notatio
nCharge
Mg-25 12 13 25 10 +2
N-14 7 7 14 10 -3
Br-79 35 44 79 36 -1
79 135 Br
14 37 N
25 212 Mg
Atomic Mass:• The mass of an atom expressed in amu (atomic
mass units.)
• One amu is equal to 1/12 the mass of a carbon-12 atom.Average Atomic Mass:
• The weighted average of all an element’s isotopes.
• Mass Spectrometers are instruments used to measure masses of isotopes as well as their isotopic abundance.
• This is the number shown in the box on the Periodic Table.
• It is calculated by: (mass1 x %1) + (mass2 x %2) + …
Calculation of atomic mass
Magnesium has 3 naturally occurring isotopes:78.99% Mg-24, 10.00% Mg-25, and
11.01% Mg-26
Calculate the atomic mass of magnesium. (24 x 0.7899) = 18.9576 = 18.96+ (25 x 0.1000) = 2.500 =
2.500+ (26 x 0.1101) = 2.8626 = 2.863
24.323 24.32 amu
Calculation of atomic mass
Magnesium has 3 naturally occurring isotopes:78.99% is 23.98504 amu10.00% is 24.98584 amu11.01% is 25.98259 amu
Calculate the atomic mass of magnesium. (23.98504 amu x 0.7899) + (24.98584 amu x 0.1000) + (25.98259 amu x 0.1101)
24.31 amu
The MOLE
Atomic Mass - the mass of an atom, based on a C-12 atom, in atomic mass units (amu)
1 amu = 1.66 x 10-24g = 1/12 the mass of a C-12 atom
Example: atomic mass of Na = 23.0 amu
Atoms are too small to count or mass individually. It is easier to count many or mass many.
amu gram (atomic scale) (macroscopic scale)mole
Mole = amount of substance that contains 6.02 x 1023 particles (abbreviated: mol)
Avogadro’s Number = number of particles in a mole = 6.02 x 1023 particles
Particles can be atoms, ions, molecules, or formula units
Molar Mass = mass, in grams, per 1 mole of a substance
units = grams/mole (g/mol)
1 Mole = 6.02x1023 particles of substance
1 Mole = mass (g) of substance from PT
Nuclear Reactions Change the composition of an atom’s nucleus.
Protons & neutrons are called nucleons; atom is called the nuclide.
Nuclear vs. Chemical
1. Elements may be converted from one to another
2. Particles within the nucleus are involved.
3. Tremendous amounts of energy are released (million times that of chemical)
4. Rate of reaction is not influenced by external factors.
1. No new elements can be produced
2. Only electrons participate
3. Relatively small amounts of energy are absorbed or released
4. Rate of reaction depends upon factors such as temperature and pressure
Nuclear Radiation Penetrating Power
Ionizing Radiation is radiation with sufficient energy to change atoms and molecules into ions (can damage living tissue).
Nonionizing Radiation is radiation that does not have sufficient energy to ionize matter.
Types of Spontaneous Radiation:
A. Alpha Decay – spontaneous emission of alpha particle from the nucleus; from neutron-poor heavy nuclei
B. Beta Decay – spontaneous emission of beta particle from the nucleus; from neutron-rich nuclei
18177Ir226 222 4
88 86 2Ra Rn α 185 479 2Au _________ α
228 228 088 89 -1Ra Ac + β
131 0 13153 1 54I β Xe 14
714 06 1N C β
204 200 482 80 2Pb Hg + α
Characteristics of Subatomic Particles and Rays:1 amu = 1.6605x10-27kg
ParticleMass (amu)
Charge
SymbolStopped
byProton(Hydrogen nucleus)
1.00727647
Neutron1.00866490
Beta Particle (electron)
0.0005486
Alpha Particle
(Helium nucleus)
4.00150617
Gamma Ray (high energy
EMR)0
Positron(positively
charged electron)0.0005486
0
0
Nuclear Bombardment Reactions
14 4 18 17 17 2 9 8 1[ ]N He F O H
target nucleus
projectile new isotope (element)
ejected particleUnstable compound
nucleus
10 14 244 7 12 _____Be N Mg
2 3 41 1 2 _____H H He
238 247 192 99 0_____ 5U Es n
9 4 14 2 0_____Be He n
Nuclear Bombardment Reaction
Radioactive Decay of Strontium-90
What is the ½ Life of Strontium-90???
How long until no more Strontium-90 remains?