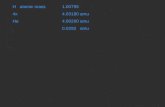
The Atom & Subatomic Particles. What we knew by 1932: 1.0087 amu (or 1 amu) 0 neutron 1 n 1.0073 amu...
-
Upload
philomena-alisha-jennings -
Category
Documents
-
view
227 -
download
4
Transcript of The Atom & Subatomic Particles. What we knew by 1932: 1.0087 amu (or 1 amu) 0 neutron 1 n 1.0073 amu...
What we knew by 1932:What we knew by 1932:
1.0087 amu1.0087 amu
(or 1 amu)(or 1 amu)
00neutron neutron 11nn
1.0073 amu1.0073 amu
(or 1 amu)(or 1 amu)
+1+1proton proton 11p or p or 11HH
0.0005486 amu0.0005486 amu
(or 0 amu)(or 0 amu)
-1-1electron electron 00ee
Relative MassRelative MassRelative ChargeRelative Charge
--11
11 11
00
Location of Subatomic ParticlesLocation of Subatomic Particles
• electrons located electrons located outside nucleusoutside nucleus
• protons & neutrons located protons & neutrons located inside nucleusinside nucleus
Atomic NumberAtomic Number
atomic number:atomic number: = size of nuclear charge= size of nuclear charge
= number of protons in nucleus= number of protons in nucleus determines identity of elementdetermines identity of element gives order to PTgives order to PT
For neutral atoms:For neutral atoms:atomic number = # protons = # electronsatomic number = # protons = # electrons
IsotopesIsotopes
atoms of same element can have different atoms of same element can have different mass numbersmass numbers
# protons must be the same so …# protons must be the same so …
# neutrons must be different# neutrons must be different
Dalton’s theory must be modified:Dalton’s theory must be modified:
the atom is divisiblethe atom is divisible
presence of subatomic particlespresence of subatomic particles
atoms of same element can be differentatoms of same element can be different
isotopes have different # neutronsisotopes have different # neutrons
NotationNotation
1212CC66
•left left superscriptsuperscript is is mass numbermass number
•left left subscriptsubscript is is atomic numberatomic number
NotationNotation
1212CC66
12 = # protons + neutrons12 = # protons + neutrons 6 = # protons6 = # protons
12 – 6 = 6 neutrons12 – 6 = 6 neutrons
How many neutrons in this How many neutrons in this atom?atom?
1717OO88
Mass number is ?Mass number is ?
Atomic number is ?Atomic number is ?
# of neutrons is ?# of neutrons is ?
# of electrons in atom is?# of electrons in atom is?
1717
88
17 – 8 17 – 8
88
= 9= 9
Mass number is ?Mass number is ?
Atomic number is ?Atomic number is ?
# of neutrons is ?# of neutrons is ?
# of electrons in atom is?# of electrons in atom is?
1515
NN77
1515
77
15 – 715 – 7
77
= 8= 8
Mass number is ?Mass number is ?
Atomic number is ?Atomic number is ?
# of neutrons is ?# of neutrons is ?
# of electrons in atom is?# of electrons in atom is?
FF 1919
99
19 – 9 = 1019 – 9 = 10
99
19
Other NotationsOther Notations
C-C-1212
# after symbol is # after symbol is mass numbermass number!!
must look up atomic numbermust look up atomic number
What’s the difference between What’s the difference between H-1, H-2, and H-3?H-1, H-2, and H-3?
All H’s have 1 protonAll H’s have 1 proton
1 proton, so it must have ? neutrons1 proton, so it must have ? neutrons
H-1: mass # = 1H-1: mass # = 1
1 proton, ? neutrons1 proton, ? neutrons
H-2: mass # = 2H-2: mass # = 2
1 proton, so it must have ? 1 proton, so it must have ? neutronsneutronsH-3: mass # = 3H-3: mass # = 3
00
11
22
Consider U-234, U-235, & U-238Consider U-234, U-235, & U-238
What’s the atomic number of U?What’s the atomic number of U? How many protons in U?How many protons in U? How many neutrons in U-234?How many neutrons in U-234? How many neutrons in U-235?How many neutrons in U-235? How many neutrons in U-238?How many neutrons in U-238? How many electrons in U?How many electrons in U?
9292
9292234 – 92 = 142234 – 92 = 142
235 – 92 = 143235 – 92 = 143
238 – 92 = 146238 – 92 = 146
9292
How many neutrons in Po-217?How many neutrons in Po-217?
What’s the atomic number?What’s the atomic number?
How many protons?How many protons?
So the neutrons are …So the neutrons are …
8484
8484
217 – 84 = 133 neutrons!217 – 84 = 133 neutrons!
IONSIONS
IONS = atoms that gained or lost IONS = atoms that gained or lost electronselectrons
protons and electrons aren't = anymore, so protons and electrons aren't = anymore, so ions are ions are not not neutral they carry a charge (+/-)neutral they carry a charge (+/-)
IONSIONS
Charge of ion: Charge of ion:
= # protons minus # electrons = # protons minus # electrons
(subtract the electrons since are negative)(subtract the electrons since are negative)
IONSIONS
If atom loses electrons, it has more (+) If atom loses electrons, it has more (+) protons than (-) electronsprotons than (-) electrons What kind of ion is it?What kind of ion is it?
If atom gains electrons, it has more (-) If atom gains electrons, it has more (-) electrons than (+) protonselectrons than (+) protons What kind of ion is it?What kind of ion is it?
positivepositive
negativenegative
Charge of IonsCharge of Ions
Ion chargeIon charge: : right superscriptright superscriptClCl-1-1 a chloride ion with a charge of -1a chloride ion with a charge of -1
NaNa+1+1 a sodium ion with a charge of +1a sodium ion with a charge of +1
OO-2-2 an oxygen ion with a charge of -2an oxygen ion with a charge of -2
If no right superscript, it’s understood to be zero and If no right superscript, it’s understood to be zero and therefore a neutral atomtherefore a neutral atom
Putting it all togetherPutting it all together
How many protons, neutrons, & electrons How many protons, neutrons, & electrons in each of the followingin each of the following
2323NaNa+1+1
11
2525MgMg+2+2
1212
3434SS-2-2
1616
6464ZnZn+2+2
3030
1919FF-1-1
99
1313NN-3-3
77
11 p, 12 n, 10 e11 p, 12 n, 10 e
12 p, 13 n, 10 e12 p, 13 n, 10 e
16 p, 18 n, 18 e16 p, 18 n, 18 e 9 p, 10 n, 10 9 p, 10 n, 10 ee
30 p, 34 n, 28 e30 p, 34 n, 28 e 7 p, 6 n, 10 7 p, 6 n, 10 ee
Lost Lost 1 e1 e--
Got Got 2 e-2 e-
Got Got 1 e-1 e-
Lost Lost 2 e-2 e-
Lost Lost 2 e-2 e-
Got Got 3 e-3 e-






































![Aasif quiz 10th_march[1] wekly forum uqc amu](https://static.fdocuments.net/doc/165x107/58864fa01a28ab32768b6fc7/aasif-quiz-10thmarch1-wekly-forum-uqc-amu.jpg)

