· The isotope with mass 62.93 amu must be more abundant because its mass is closer to 63.55 amu...
Transcript of · The isotope with mass 62.93 amu must be more abundant because its mass is closer to 63.55 amu...
AP® CHEMISTRY 2004 SCORING GUIDELINES
Copyright © 2004 by College Entrance Examination Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
16
Question 7
Use appropriate chemical principles to account for each of the following observations. In each part, your
response must include specific information about both substances.
(a) At 25°C and 1 atm, F2 is a gas, whereas I2 is a solid.
Both F2 and I2 are nonpolar, so the only intermolecular attractive forces are London dispersion forces. I2 is solid because the electrons in the I2 molecule occupy a larger volume and are more polarizable compared to the electrons in the F2 molecule. As a result, the dispersion forces are considerably stronger in I2 compared to F2.
1 point for indicating that both molecules have dispersion forces as IMFs 1 point for indicating that I2 molecules are more polarizable than F2 molecules
(b) The melting point of NaF is 993°C, whereas the melting point of CsCl is 645°C.
Both NaF and CsCl are ionic compounds with the same charges on the cations and anions. The ionic radius of Na+ is smaller than the ionic radius of Cs+ and the ionic radius of F! is smaller than the ionic radius of Cl!. Therefore, the ionic centers are closer in NaF than in CsCl. Melting occurs when the attraction between the cation and the anion are overcome due to thermal motion. Since the lattice energy is inversely proportional to the distance between the ion centers (Coulomb�’s Law), the compound with the smaller ions will have the stronger attractions and the higher melting point.
1 point for indicating that NaF and CsCl are both ionic compounds (or are composed of M+ and X! ions) 1 point for indicating that the strength of these forces is determined by the distance between the ionic centers (or the size of the ions)
(c) The shape of the ICl4�– ion is square planar, whereas the shape of the BF4�– ion is tetrahedral.
The central iodine atom in ICl4! has four bonding pairs and two lone pairs of electrons on the central iodine atom, so the molecular geometry is square planar. BF4
! has four bonding pairs and no lone pairs on the central boron atom, so the molecular geometry is tetrahedral.
2 points for indicating that ICl4! has two unshared electron pairs, but BF4
! has no unshared pairs
Note: 1 point earned if student gives incorrect numbers of unshared electron pairs but indicates that difference in number of unshared electron pairs determines difference in geometry.
AP® CHEMISTRY 2004 SCORING GUIDELINES
Copyright © 2004 by College Entrance Examination Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
17
Question 7 (cont�’d.)
(d) Ammonia, NH3 , is very soluble in water, whereas phosphine, PH3 , is only moderately soluble in water.
Ammonia has hydrogen-bonding intermolecular forces, whereas phosphine has dipole-dipole and/or dispersion intermolecular forces. Water also has hydrogen-bonding intermolecular attractive forces. Ammonia is more soluble in water than phosphine because ammonia molecules can hydrogen-bond with water molecules, whereas phosphine molecules cannot hydrogen-bond with water molecules.
1 point for indicating that NH3 can form hydrogen bonds but PH3 cannot 1 point for indicating that NH3 can form hydrogen bonds with water, but PH3 cannot
AP® CHEMISTRY 2005 SCORING GUIDELINES
Copyright © 2005 by College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
16
Question 7
Use principles of atomic structure, bonding, and/or intermolecular forces to respond to each of the following. Your responses must include specific information about all substances referred to in each question. (a) At a pressure of 1 atm, the boiling point of NH3(l) is 240 K, whereas the boiling point of NF3(l)
is 144 K. (i) Identify the intermolecular forces(s) in each substance.
NH3 has dispersion forces and hydrogen-bonding forces. NF3 has dispersion forces and dipole-dipole forces. (Credit earned for hydrogen-bonding and dipole-dipole forces)
One point is earned for the correct intermolecular attractive forces for both
NH3 and NF3.
(ii) Account for the difference in the boiling points of the substances.
The higher boiling point for NH3 is due to the greater strength of the hydrogen-bonding intermolecular attractiveforces among NH3 molecules compared to that of the dipole-dipole attractive forces among NF3 molecules.
One point is earned for correctly identifying NH3 as having stronger intermolecular forces
than NF3.
(b) The melting point of KCl(s) is 776°C, whereas the melting point of NaCl(s) is 801°C. (i) Identify the type of bonding in each substance.
Both KCl and NaCl have ionic bonds. One point is earned for naming ionic bonds as the bonds in
KCl and NaCl.
(ii) Account for the difference in the melting points of the substances.
The difference in the melting points is due to the different strengths of ionic bonding in the substances. The charges on the cations and anions are the same in both compounds, therefore the relative size ofthe ions is the determining factor. Since Na+ has a smaller ionic radius than K+, the lattice energy of NaCl is higher than that of KCl. Thus more energy is required to overcome the ionic forces in solid NaCl thanin solid KCl, and NaCl has the higher melting point.
One point is earned for a correct explanation of the cause of the difference in melting points of
KCl and NaCl.
AP® CHEMISTRY 2005 SCORING GUIDELINES
Copyright © 2005 by College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
17
Question 7 (continued) (c) As shown in the table below, the first ionization energies of Si , P, and Cl show a trend.
Element First Ionization Energy (kJ mol 1)
Si 786 P 1,012 Cl 1,251
(i) For each of the three elements, identify the quantum level (e.g., n = 1, n = 2, etc.) of the valence electrons
in the atom.
The valence electron is located in the n = 3 level for all three atoms.
One point is earned for the principal quantum level for all three elements.
(ii) Explain the reasons for the trend in first ionization energies.
Because the valence electrons in all three elements are shielded by the same number of inner core electrons and the nuclear charge increases going from Si to P to Cl, the valence electrons feel an increasing attraction to the nucleus going from Si to P to Cl. Valence electrons having a greater attraction to the nucleus, as in Cl , will be more difficult to remove, so Cl has the highest ionization energy. P has the second highest ionization energy, and Si has the lowest ionization energy.
One point is earned for explaining that greater ionization energy is due to increased nuclear charge.
Note: Explanations of the trend on the basis of effective nuclear charge are acceptable.
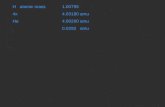
(d) A certain element has two stable isotopes. The mass of one of the isotopes is 62.93 amu and the mass of the
other isotope is 64.93 amu. (i) Identify the element. Justify your answer.
Copper. The relative average atomic mass is between the two isotopic masses given.
One point is earned for the element and the explanation.
(ii) Which isotope is more abundant? Justify your answer.
The isotope with mass 62.93 amu must be more abundant because its mass is closer to 63.55 amu (the relative weighted average atomic mass for copper) than is the mass of the other isotope.
One point is earned for the correct choice and explanation.
AP® CHEMISTRY 2006 SCORING GUIDELINES
© 2006 The College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
14
Question 7
7. Answer the following questions about the structures of ions that contain only sulfur and fluorine.
(a) The compounds SF4 and BF3 react to form an ionic compound according to the following equation.
SF4 + BF3 ! SF3BF4
(i) Draw a complete Lewis structure for the SF3+ cation in SF3BF4.
One point is earned for the correct Lewis structure (the structure must include lone pairs of electrons, which may be represented as dashes).
(ii) Identify the type of hybridization exhibited by sulfur in the SF3+ cation.
sp3
One point is earned for the correct hybridization.
(iii) Identify the geometry of the SF3+ cation that is consistent with the Lewis structure drawn
in part (a)(i).
Trigonal pyramidal
One point is earned for the correct shape.
(iv) Predict whether the F–S–F bond angle in the SF3+ cation is larger than, equal to, or smaller
than 109.5°. Justify your answer.
The F–S –F bond angle in the SF3+ cation is expected to be slightly
smaller than 109.5° because the repulsion between the nonbonding pair of electrons and the S –F bonding pairs of electrons “squeezes” the F–S –F bond angles together slightly.
One point is earned for stating that the angle is
smaller, with justification.
AP® CHEMISTRY 2006 SCORING GUIDELINES
© 2006 The College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
15
Question 7 (continued)
(b) The compounds SF4 and CsF react to form an ionic compound according to the following equation.
SF4 + CsF ! CsSF5
(i) Draw a complete Lewis structure for the SF5" anion in CsSF5.
One point is earned for the correct Lewis structure (the structure must include lone pairs of electrons, which
may be represented as dashes).
(ii) Identify the type of hybridization exhibited by sulfur in the SF5" anion.
sp3d2
One point is earned for the correct hybridization.
(iii) Identify the geometry of the SF5" anion that is consistent with the Lewis structure drawn
in part (b)(i).
Square pyramidal
One point is earned for the correct shape.
(iv) Identify the oxidation number of sulfur in the compound CsSF5.
+ 4
One point is earned for the correct oxidation number.
AP® CHEMISTRY 2006 SCORING GUIDELINES
© 2006 The College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
16
Question 8
8. Suppose that a stable element with atomic number 119, symbol Q , has been discovered.
(a) Write the ground-state electron configuration for Q , showing only the valence-shell electrons.
8s 1
One point is earned for the electron configuration.
(b) Would Q be a metal or a nonmetal? Explain in terms of electron configuration.
It would be a metal (OR an alkali metal). The valence electron would be held only loosely.
One point is earned for the correct answer and explanation, which must include reference to the
valence electron.
(c) On the basis of periodic trends, would Q have the largest atomic radius in its group or would it have the smallest? Explain in terms of electronic structure.
It would have the largest atomic radius in its group because its valence electron is in a higher principal shell.
One point is earned for the correct answer and explanation; the size must refer to number of
electron shells.
(d) What would be the most likely charge of the Q ion in stable ionic compounds?
+ 1 One point is earned for the correct charge.
(Must be consistent with configuration in part (a).)
(e) Write a balanced equation that would represent the reaction of Q with water.
2 Q(s) + 2 H2O(l) ! 2 Q+ (aq) + 2 OH "(aq) + H2(g)
One point is earned for H2 as a product.
One point is earned for balancing the equation.
(f) Assume that Q reacts to form a carbonate compound.
(i) Write the formula for the compound formed between Q and the carbonate ion, CO32" .
Q2CO3 One point is earned for the formula consistent
with the charge given in part (d).
(ii) Predict whether or not the compound would be soluble in water. Explain your reasoning.
It would be soluble in water because all alkali metal carbonates are soluble.
One point is earned for the answer consistent with the identification of Q.
AP® CHEMISTRY 2007 SCORING GUIDELINES
© 2007 The College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
Question 6
Answer the following questions, which pertain to binary compounds.
(a) In the box provided below, draw a complete Lewis electron-dot diagram for the IF3 molecule.
One point is earned for a correct Lewis diagram (can be done with dots or lines).
(b) On the basis of the Lewis electron-dot diagram that you drew in part (a), predict the molecular geometry of the IF3 molecule.
T-shaped One point is earned for the molecular geometry consistent with the Lewis diagram
in part (a).
(c) In the SO2 molecule, both of the bonds between sulfur and oxygen have the same length. Explain this observation, supporting your explanation by drawing in the box below a Lewis electron-dot diagram (or diagrams) for the SO2 molecule.
One point is earned for a correct diagram (can be done with dots or lines). One point is earned for some indication or discussion of resonance (but the point is not earned for a
description of resonance as a dynamic process).
OR
The bonds are the same length because they are both double bonds.
One point is earned for a correct diagram (can be done with dots or lines).
One point is earned for stating that both bonds are double bonds.
AP® CHEMISTRY 2007 SCORING GUIDELINES
© 2007 The College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
Question 6 (continued)
(d) On the basis of your Lewis electron-dot diagram(s) in part (c), identify the hybridization of the sulfur atom in the SO2 molecule.
sp2 One point is earned for hybridization consistent with part (c).
The reaction between SO2(g) and O2(g) to form SO3(g) is represented below.
2 SO2(g) + O2(g) !" 2 SO3(g)
The reaction is exothermic. The reaction is slow at 25°C; however, a catalyst will cause the reaction to proceed faster.
(e) Using the axes provided below, draw the complete potential-energy diagram for both the catalyzed and uncatalyzed reactions. Clearly label the curve that represents the catalyzed reaction.
One point is earned for an uncatalyzed reaction curve that must show that Ea > 0 and #H < 0.
One point is earned for a catalyzed reaction curve that must show Ea < uncatalyzed Ea , must be clearly labeled, and must begin and end at the same energies as the uncatalyzed curve.
AP® CHEMISTRY 2007 SCORING GUIDELINES
© 2007 The College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
Question 6 (continued)
(f) Predict how the ratio of the equilibrium pressures, 2
3
SO
SO
p
p, would change when the temperature of the
uncatalyzed reaction mixture is increased. Justify your prediction.
The ratio 2
3
SO
SO
p
p would increase as the temperature increases. Because
the reaction is exothermic (!H < 0), as the temperature is raised the
reaction shifts to the left.
One point is earned for the correct answer and explanation.
(g) How would the presence of a catalyst affect the change in the ratio described in part (f)? Explain.
The catalyst would not affect the value of the two equilibrium ratios but would increase the rate of the shifting of the system to the new equilibrium position. The catalyst does this by providing an alternate path with a lower activation energy.
One point is earned for the correct answer and explanation.
AP® CHEMISTRY 2007 SCORING GUIDELINES (Form B)
© 2007 The College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
Question 2
Answer the following problems about gases.
(a) The average atomic mass of naturally occurring neon is 20.18 amu. There are two common isotopes of
naturally occurring neon as indicated in the table below.
Isotope Mass (amu) Ne-20 19.99 Ne-22 21.99
(i) Using the information above, calculate the percent abundance of each isotope.
Let x represent the natural abundance of Ne-20.
19.99 x + 21.99(1! x) = 20.18 19.99 x + 21.99 ! 21.99 x = 20.18 19.99 x ! 21.99 x = 20.18 ! 21.99 !2 x = !1.81 x = 0.905 percent abundances are: Ne-20 = 90.5% Ne-22 = 9.5%
One point is earned for the correct answer.
(ii) Calculate the number of Ne-22 atoms in a 12.55 g sample of naturally occurring neon.
12.55 g Ne ! 1 mol Ne
20.18 g Ne !
0.095 mol Ne-221 mol Ne
! 236.022!10 Ne-22 atoms
1 mol Ne-22
= 3.6 ! 1022 Ne-22 atoms
One point is earned for the correct molar mass.
One point is earned for the correct fraction of
Ne-22 in Ne.
One point is earned for the number of atoms.
AP® CHEMISTRY 2007 SCORING GUIDELINES (Form B)
© 2007 The College Board. All rights reserved. Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
Question 2 (continued) (b) A major line in the emission spectrum of neon corresponds to a frequency of 4.34 ! 10!14 s"1. Calculate
the wavelength, in nanometers, of light that corresponds to this line.
=c "# =c"#
" = 8 1
14 1 9
3 0 10
4 34 10 10
. m s 1 nm
. s m
!
! !$ $
$ = 690 nm
One point is earned for the correct setup.
One point is earned for the answer.
(c) In the upper atmosphere, ozone molecules decompose as they absorb ultraviolet (UV) radiation, as shown
by the equation below. Ozone serves to block harmful ultraviolet radiation that comes from the Sun.
O3(g) UV O2(g) + O(g)
A molecule of O3(g) absorbs a photon with a frequency of 1.00 $ 1015 s!1.
(i) How much energy, in joules, does the O3(g) molecule absorb per photon?
=E h# = 6.63 ! 10!34 J s ! 1.00 ! 1015 s!1 = !$ 196.63 10 J per photon
One point is earned for the correct answer.
(ii) The minimum energy needed to break an oxygen-oxygen bond in ozone is 387 kJ mol!1. Does a photon with a frequency of 1.00 ! 1015 s!1 have enough energy to break this bond? Support your answer with a calculation.
!
!$ $$ $ =19 23
13
6.63 10 J 6.022 10 photons 1 kJ399 kJ mol
1 photon 1 mol 10 J
399 kJ mol!1 > 387 kJ mol!1, therefore the bond can be broken.
One point is earned for calculating the energy.
One point is earned for the
comparison of bond energies.
AP® CHEMISTRY 2008 SCORING GUIDELINES
© 2008 The College Board. All rights reserved. Visit the College Board on the Web: www.collegeboard.com.
Question 6
(a) Structures of the pyridine molecule and the benzene molecule are shown below. Pyridine is soluble in water, whereas benzene is not soluble in water. Account for the difference in solubility. You must discuss both of the substances in your answer.
Pyridine is polar (and capable of forming hydrogen bonds with water), while the nonpolar benzene is not capable of forming hydrogen bonds. Pyridine will dissolve in water because of the strong hydrogen bonds (or dipole-dipole intermolecular interactions) that exist between the lone pair of electrons on pyridine’s nitrogen atom and the solvent water molecules. No such strong intermolecular interaction can exist between benzene and water, so benzene is insoluble in water.
One point is earned for identifying a relevant structural difference between pyridine and benzene.
One point is earned for indicating that pyridine is soluble in water because pyridine can form strong dipole-dipole interactions (or hydrogen bonds) with water, while benzene cannot.
(b) Structures of the dimethyl ether molecule and the ethanol molecule are shown below. The normal boiling point of dimethyl ether is 250 K, whereas the normal boiling point of ethanol is 351 K. Account for the difference in boiling points. You must discuss both of the substances in your answer.
AP® CHEMISTRY 2008 SCORING GUIDELINES
© 2008 The College Board. All rights reserved. Visit the College Board on the Web: www.collegeboard.com.
Question 6 (continued)
The intermolecular forces of attraction among molecules of dimethyl ether consist of London (dispersion) forces and weak dipole-dipole interactions. In addition to London forces and dipole-dipole interactions that are comparable in strength to those in dimethyl ether, ethanol can form hydrogen bonds between the H of one molecule and the O of a nearby ethanol molecule. Hydrogen bonds are particularly strong intermolecular forces, so they require more energy to overcome during the boiling process. As a result, a higher temperature is needed to boil ethanol than is needed to boil dimethyl ether.
One point is earned for recognizing that ethanol molecules can form intermolecular hydrogen bonds, whereas dimethyl ether molecules do not form intermolecular hydrogen bonds.
One point is earned for recognizing that, compared to the energy required to overcome the weaker intermolecular forces in liquid dimethyl ether, more energy is required to overcome the stronger hydrogen bonds in liquid ethanol, leading to a higher boiling point.
(c) SO2 melts at 201 K, whereas SiO2 melts at 1,883 K. Account for the difference in melting points. You must discuss both of the substances in your answer.
In the solid phase, SO2 consists of discrete molecules
with dipole-dipole and London (dispersion) forcesamong the molecules. These forces are relatively weak and are easily overcome at a relatively low temperature, consistent with the low melting point of SO2 .
In solid SiO2 , a network of Si and O atoms, linked by
strong covalent bonds, exists. These covalent bonds are much stronger than typical intermolecular interactions, so very high temperatures are needed to overcome the covalent bonds in SiO2. This is consistent with the
very high melting point for SiO2.
One point is earned for recognizing that SO2 is a molecular solid with only
weak dipole-dipole and London forces among SO2 molecules.
One point is earned for recognizing that SiO2 is a covalent network solid, and that strong covalent bonds must be broken for SiO2 to melt.
AP® CHEMISTRY 2008 SCORING GUIDELINES
© 2008 The College Board. All rights reserved. Visit the College Board on the Web: www.collegeboard.com.
Question 6 (continued) (d) The normal boiling point of Cl2(l) (238 K) is higher than the normal boiling point of HCl(l) (188 K).
Account for the difference in normal boiling points based on the types of intermolecular forces in the substances. You must discuss both of the substances in your answer.
The intermolecular forces in liquid Cl2 are
London (dispersion) forces, whereas the intermolecular forces in liquid HCl consist of London forces and dipole-dipole interactions. Since the boiling point of Cl2 is
higher than the boiling point of HCl, the London forces among Cl2 molecules must be
greater than the London and dipole-dipole forces among HCl molecules. The greater strength of the London forces between Cl2molecules occurs because Cl2 has more
electrons than HCl, and the strength of the London interaction is proportional to the total number of electrons.
One point is earned for recognizing that the London forces among Cl2 molecules must be
larger than the intermolecular forces (London and dipole-dipole) among HCl molecules.
One point is earned for recognizing that the strength of the London forces among molecules is proportional to the total number of electrons in each molecule.