Regulation of Contraction in Striated Muscle
-
Upload
nguyenthuy -
Category
Documents
-
view
226 -
download
0
Transcript of Regulation of Contraction in Striated Muscle

Regulation of Contraction in Striated Muscle
A. M. GORDON, E. HOMSHER, AND M. REGNIER
Departments of Physiology and Biophysics and of Bioengineering, University of Washington, Seattle,
Washington; and Department of Physiology, University of California at Los Angeles, Los Angeles, California
I. Introduction 854II. Interacting Units in the Regulation of Contraction 856
A. Thin filament 856B. Thick filament 861C. Interaction surfaces of actin and myosin 864D. Cross-bridge cycle 864
III. Results of Structural, Biochemical, and Physiological Studies of Regulation 867A. Thin filament structural studies of regulation 867B. Biochemical studies of regulation 871C. Physiological studies on muscle fibers 873D. Regulation of reconstituted thin filaments in in vitro motility assays 898
IV. Models of Thin Filament Regulation 904V. Conclusions and Future Directions 910
A. Conclusions 910B. Future Research Directions 911
Gordon, A. M., E. Homsher, and M. Regnier. Regulation of Contraction in Striated Muscle. Physiol. Rev. 80:853–924, 2000—Ca21 regulation of contraction in vertebrate striated muscle is exerted primarily through effects onthe thin filament, which regulate strong cross-bridge binding to actin. Structural and biochemical studies suggest thatthe position of tropomyosin (Tm) and troponin (Tn) on the thin filament determines the interaction of myosin withthe binding sites on actin. These binding sites can be characterized as blocked (unable to bind to cross bridges),closed (able to weakly bind cross bridges), or open (able to bind cross bridges so that they subsequently isomerizeto become strongly bound and release ATP hydrolysis products). Flexibility of the Tm may allow variability in actin(A) affinity for myosin along the thin filament other than through a single 7 actin:1 tropomyosin:1 troponin (A7TmTn)regulatory unit. Tm position on the actin filament is regulated by the occupancy of NH-terminal Ca21 binding siteson TnC, conformational changes resulting from Ca21 binding, and changes in the interactions among Tn, Tm, andactin and as well as by strong S1 binding to actin. Ca21 binding to TnC enhances TnC-TnI interaction, weakens TnIattachment to its binding sites on 1–2 actins of the regulatory unit, increases Tm movement over the actin surface,and exposes myosin-binding sites on actin previously blocked by Tm. Adjacent Tm are coupled in their overlapregions where Tm movement is also controlled by interactions with TnT. TnT also interacts with TnC-TnI in aCa21-dependent manner. All these interactions may vary with the different protein isoforms. The movement of Tmover the actin surface increases the “open” probability of myosin binding sites on actins so that some are in the openconfiguration available for myosin binding and cross-bridge isomerization to strong binding, force-producing states.In skeletal muscle, strong binding of cycling cross bridges promotes additional Tm movement. This movementeffectively stabilizes Tm in the open position and allows cooperative activation of additional actins in that andpossibly neighboring A7TmTn regulatory units. The structural and biochemical findings support the physiologicalobservations of steady-state and transient mechanical behavior. Physiological studies suggest the following. 1) Ca21
binding to Tn/Tm exposes sites on actin to which myosin can bind. 2) Ca21 regulates the strong binding of MzADPzPi
to actin, which precedes the production of force (and/or shortening) and release of hydrolysis products. 3) Theinitial rate of force development depends mostly on the extent of Ca21 activation of the thin filament and myosinkinetic properties but depends little on the initial force level. 4) A small number of strongly attached cross bridgeswithin an A7TmTn regulatory unit can activate the actins in one unit and perhaps those in neighboring units. Thisresults in additional myosin binding and isomerization to strongly bound states and force production. 5) The ratesof the product release steps per se (as indicated by the unloaded shortening velocity) early in shortening are largelyindependent of the extent of thin filament activation ([Ca21]) beyond a given baseline level. However, with a greater
PHYSIOLOGICAL REVIEWS
Vol. 80, No. 2, April 2000Printed in U.S.A.
0031-9333/00 $15.00 Copyright © 2000 the American Physiological Society 853

extent of shortening, the rates depend on the activation level. 6) The cooperativity between neighboring regulatoryunits contributes to the activation by strong cross bridges of steady-state force but does not affect the rate of forcedevelopment. 7) Strongly attached, cycling cross bridges can delay relaxation in skeletal muscle in a cooperativemanner. 8) Strongly attached and cycling cross bridges can enhance Ca21 binding to cardiac TnC, but influenceskeletal TnC to a lesser extent. 9) Different Tn subunit isoforms can modulate the cross-bridge detachment rate asshown by studies with mutant regulatory proteins in myotubes and in in vitro motility assays. These results andconclusions suggest possible explanations for differences between skeletal and cardiac muscle regulation anddelineate the paths future research may take toward a better understanding of striated muscle regulation.
I. INTRODUCTION
A more complete understanding of the regulation ofstriated muscle contraction has come from the conver-gence of exciting new information from a number ofdifferent directions. New data have emerged about thestructure of actin, the thin filament, the myosin S1 head,possible sites of actin-myosin interaction, the regulatoryproteins, and the structural changes that generate fila-ment sliding and force production (see review by Cooke,Ref. 67). Correlations have been made between the stepsin the actomyosin ATPase cycle and the structuralchanges involved in the cross-bridge ATPase cycle, result-ing in a better understanding of the activation of myosinATPase by actin and the binding of different myosin-nucleotide states to actin (67). Structural studies haverevealed changes in the thin filament that accompanyactivation of contraction (see Refs. 475, 507). Recentbiochemical studies have better defined the steps beingregulated and provided a clearer description of the pos-sible modes of regulation (303, 455). Physiological studiesnow routinely use molecular techniques to better definethe modes of regulation in the striated muscle cell (331,333). Finally, in vitro motility techniques combining mo-lecular and biochemical studies along with physiologicalmeasurements of force and filament sliding offer newapproaches to the study of regulation of the cross-bridgecycle in striated muscle (148, 206).
The specific questions addressed in this review are asfollows.
1) What methods can be used to study regulation?What are their advantages and limitations?
2) Does Ca21 binding to troponin (Tn) C alone reg-ulate contraction or do strongly bound cross bridges con-tribute to thin filament activation? Does cross-bridgebinding to actin promote additional cross-bridge bindingto the thin filament? If cross-bridge binding further acti-vates the thin filament, which cross-bridge state(s) canproduce this activation?
3) Do the regulatory proteins merely regulate thenumber of cross bridges, or do they modify rate constantsof the transitions between various attached states of thecross bridge? Are any of the rate constants in the acto-myosin cycle controlled by thin filament regulatory pro-teins? Does troponin I block cross-bridge binding to one
or more actin monomers in the absence of Ca21? Whatfactors control the tropomyosin (Tm) position on the thinfilament? How flexible is Tm, and does the binding ofmyosin to actin vary within an A7TmTn unit?
4) Do regulatory proteins exert allosteric effects onthe interaction between cross bridges and the thin fila-ment?
5) What are the differences in the regulation of skel-etal and cardiac muscle, and how can the properties ofthe constituent protein isoforms be accounted for bythese differences?
We conclude that the major step controlled by Ca21
during activation is the strong binding of the MzADPzPi
species to actin with possibly some modulation by iso-forms or Ca21 of the kinetic steps in the cross-bridgecycle. Strong binding of MzADPzPi to actin is controlled byregulation of the effective actin concentration that varieswith the fraction of time the Tm spends in an “open”position. In the absence of Ca21, the weak electrostaticbinding of myosin to actin binding sites may be blocked atsome actins by TnI, but at other actins appear to be eitherunregulated or only poorly regulated. A major change inaffinity during activation occurs subsequent to a move-ment of Tm that uncovers strong hydrophobic bindingsites on actin by a modified steric blocking mechanism,blocking strong but not necessarily weak binding. In skel-etal muscle, the movements of Tm after Ca21 binding toTnC and the subsequent changes in Tn-Tm-actin interac-tion expose myosin-binding sites on actin, increasing theaffinity of actin for myosin. This increased affinity allowsweak binding to some actins and stronger binding toothers, presumably because of the flexibility of Tm. Theaffinity of all actins for myosin is increased when suffi-cient numbers of strongly attached cross bridges displace(or stabilize the displacement of) the Tm further thanoccurs with Ca21 binding alone, making still more sitesavailable for myosin binding to actin. One factor thatenhances the affinity of the actin filament for myosin,following Ca21 binding to TnC, is a structural change inactin occasioned by allosteric actions. A second factoraffecting myosin binding to actin is the distance of themyosin heads from actin binding sites, influenced bychanges in interfilament spacing with sarcomere length ormovement of myosin heads away from the thick filamenttoward the thin filament upon phosphorylation of thick
854 GORDON, HOMSHER, AND REGNIER Volume 80

filament components. This view of Ca21 activation of thethin filament may be thought of as “analogous to the effectof a ligand that binds to a ligand-gated ion channel andcause an increase in the channel open probability” (398).This is a helpful way to conceptualize the increased prob-ability of strong myosin binding to the thin filament,which is a result of Tm movement to increase the avail-ability of actins for strong binding by myosin, an activatedor open position. This Tm movement is in turn brought onby Ca21 binding to TnC and by strong cross-bridge bind-ing to actin to move Tm and/or stabilize it in this activatedor open position.
The range of Tm movement over actin’s surface andhence the extent of thin filament activation is controlledby Ca21 binding to TnC and strong cross-bridge binding toactin. First, Ca21 binding to TnC in one A7TmTn unitweakens TnI binding to actin and allows an azimuthal Tmmovement in the unit exposing myosin binding sites onsome or all of the seven actin monomers within the unit.Second, the TnT binding to Tm and the overlap of Tmfrom adjacent thin filament A7TmTn regulatory units al-lows coupling and propagation of Tm motion along thethin filament. The extent of this coupling, or cooperativeactivation, varies with the composition of the regulatoryprotein isoforms. Finally, cross-bridge binding contrib-utes to activation by stabilizing the Tm/Tn unit in anactivated position or allosterically modifying the actin.
The physiological studies of the regulation of forceand shortening in skinned muscle fibers and filamentsliding in the in vitro motility assay are the major focus ofthis review. The conclusions we draw from these studiesare as follows.
1) The force-pCa relationship can be understood asa Ca21 regulation of strong cross-bridge attachment withthe steep relationship between Ca21 and force requiringcooperative activation by strongly attached cross bridges.
2) The rate of force redevelopment (kTR) after rapidrelease and restretch in skinned fibers is determined bythe properties of the myosin at maximal Ca21 activation,but at submaximal Ca21 is best considered as a Ca21
regulation of the kinetics of activation of the thin filament.There is little evidence that either cooperativity betweenneighboring units along the thin filament or cooperativityof cross-bridge attachment within a regulatory unit influ-ences kTR.
3) The rapid activation of contraction by step in-creases in [Ca21] demonstrates that the rate of forcedevelopment depends primarily on [Ca21] and not on theinitial level of force. This is consistent with conclusionsfrom the kTR measurements that the activation rate re-sults primarily from the kinetics of Ca21 binding and thesubsequent conformational changes of the thin filament.
4) Measurements of the force transient followingrapid increases in [Pi] (kPi
) indicate that the Pi release stepand force-generating isomerization step in striated muscle
exhibit little Ca21 dependence, implying that they are notCa21 regulated. Therefore, the kinetic step regulated byCa21 is a strong cross-bridge attachment per se, beforeforce generation.
5) Measurements of unloaded shortening velocity inskinned and intact muscle fibers can be understood interms of the A. F. Huxley (215) cross-bridge model withdrag from negatively strained cross bridges balancing thepositive strain from force-generating cross bridges. Thereappears to be no intrinsic Ca21 regulation of the cross-bridge product release steps or detachment from actin,with the main Ca21 dependence being regulation ofstrong cross bridges binding. However, in skinned fibers,the shortening velocity over longer distances is Ca21 sen-sitive, and this may be due to a shortening-induced thinfilament deactivation. There appears to be little Ca21
dependence of the shortening velocity in intact muscle.Calcium modulates thin filament sliding speed in the invitro motility assay, but this is probably related to limita-tions on the number of cross bridges interacting with thethin filament.
6) Strongly bound cross bridges can activateskinned fibers in the absence of Ca21 with the steepnessof the relationship between [ATP] and force depending onthe muscle type, being greater in cardiac than skeletalmuscle. Rigor cross bridges [e.g., N-ethylmaleimide(NEM)-S1] do not appear to activate the thin filamentdirectly but appear to enhance its activation by Ca21.
7) Rigor cross bridges enhance Ca21 binding to TnC,while cycling cross bridges enhance Ca21 binding to TnCprimarily in cardiac muscle, with much less effect inskeletal muscle.
8) During relaxation, after myoplasmic [Ca21] hasbeen reduced to small values, the rate of force declineduring the “isometric” phase decreases with increases inthe initial force level in skeletal muscle. This result pro-vides evidence for sustained activation by strongly at-tached, cycling cross bridges in skeletal muscle.
9) During rapid shortening, the number of stronglyattached cross bridges falls, which probably accounts forthe shortening-induced deactivation observed in skeletalmuscle.
10) The in vitro motility assay provides an excitingnew technique for investigating the regulation of contrac-tion. Recent experiments support the conclusion thatCa21 regulates strong cross-bridge binding but also sug-gest that Ca21 could modulate another step in the cross-bridge cycle.
Thus the regulation of cross-bridge interactions togenerate force and shortening can be understood in termsof control of strong binding with possible secondary mod-ulation of cross-bridge kinetics. Differences in kineticrates can be understood in terms of intrinsic differencesin rates of transitions between actomyosin productsstates and differences in regulatory protein isoforms. The
April 2000 MUSCLE REGULATION 855

effective rates of attachment and detachment of force-generating cross bridges determine differences in maxi-mum rates. At submaximal levels of activation, kineticsare primarily controlled by the kinetics of Ca21 binding toTnC and the related transitions of the regulatory proteins.Differences in activation properties between muscle fibertypes can be understood in terms of differences betweenproperties of the regulatory protein isoforms. The cou-pling between regulatory units in cardiac muscle is stron-ger in the absence of Ca21, implying less flexibility of theTm-Tn system. Also in cardiac, but not in skeletal muscle,strong cross-bridge attachment promotes Ca21 binding,thus coupling Ca21 binding with cycling cross-bridge at-tachment and force generation.
II. INTERACTING UNITS IN THE REGULATION
OF CONTRACTION
In this section we review information on the proteinunits responsible for regulation of contractions. This willinclude the thin and thick filament, the proteins formingthese structures, and their interactions relevant to con-tractile regulation. We also discuss new information onthe actin and myosin structures and the interacting sur-faces between them to understand better the structuralbasis of regulation. Finally, we describe the cross-bridgecycle to specify the steps in this cycle being regulated.
A. Thin Filament
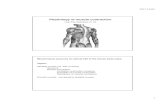
The main site for Ca21 regulation is the thin filament.Figure 1 is a diagram of the thin filament in striatedmuscle showing the three components: actin, Tm, and Tnwith the three Tn subunits. There have been a number ofreviews on the interaction between these components inregulation (77, 105, 154, 455) that can be referred to foradditional details. Tobacman’s review (455) contains aparticularly good discussion of the proteins and theirassembly into the thin filament.
1. Actin
Actin polymerizes spontaneously to form the back-bone of the thin filament, F-actin, which can be viewed aseither a two-stranded long-pitch helical structure or asingle short-pitch genetic helix structure. X-ray diffractionanalysis of crystals of actin-DNAse I (241), actin-gelsolin(305), and actin-profilin (406) shows that actin is com-posed of four subdomains. These subdomains surroundthe binding pocket for a divalent ion (Mg21 or Ca21) andnucleotide (ATP or ADP). This gives a structural basis forthe prominence of both divalent metals and nucleotide inthe actin polymerization and thin filament structure (93).The atomic model of the F-actin filament structure was
then derived from the structures of actin and X-ray dif-fraction patterns of oriented actin filament gels (203) andlater refined (281). This model shows the larger subdo-mains 3 and 4 are axially located with interactions acrossto the subdomains 3 and 4 of the actin in the secondstrand; the smaller subdomains 1 and 2 are located at theperiphery of the filament exposed to the solvent andavailable for interaction with myosin. In particular, sub-domain 1 contains both the NH2 and COOH termini ofactin and plays a prominent role in the interactions withmyosin (see scheme 1). Each actin makes contact withfour others, the preceding and following actins on thesame long-pitch helix and the two actins across the fila-ment on the other long-pitch helix (the preceding andfollowing actins on the short-pitch helix). The model ofthe thin filament (203) shows that each actin uses 10surface loops and 2 a-helices to make these interactions[see Milligan (317) for a description of these interactions].This atomic model of the actin filament is also used todefine the positioning of actin in the actin-Tm filamentand the fully regulated thin filament with Tm and Tn. Thisis discussed in section IIIA under the structure of theregulated thin filament.
2. Tm
Tm is an extended molecule ;42 nm long formed asa homodimer or heterodimer of two a-helical chains ar-ranged as a coiled coil. Stability of the coiled coil is
FIG. 1. A model of the molecular arrangement of troponin (Tn),tropomyosin (Tm), and actin in the skeletal muscle thin filament. Thevarious troponin subunits are indicated [TnC (red), TnT (yellow), andTnI (green)] as they lie along the two-stranded tropomyosin shown as ana- (brown) and b-heterodimer (orange) that in turn lies along an actin(gray) strand, spanning 7 G-actin monomers. Note that adjacent tropo-myosin molecules overlap head to tail with the NH2-terminal region ofthe highly asymmetrical TnT lying along the overlap region. The COOH-terminal region of TnT extends about one-third of the way along thetropomyosin (beyond Cys-190) and interacts with TnC and TnI, which inturn interacts with actin (see diagram in Fig. 3). (Figure courtesy of L.Smillie; adapted from S. Ebashi. Essays in Biochemistry, edited by P. N.Campbell and F. Dickens. Orlando, FL: Academic, 1974, vol. 10, p. 1–35;and C. Cohen. Sci. Am. 233: 36–45, 1975.]
856 GORDON, HOMSHER, AND REGNIER Volume 80

produced by hydrophobic interactions between nonpolarside chains contributed by amino acids in each chain.Each chain is 284 residues long and spans 7 actin mono-mers on each strand of the F-actin filament. The chainsare the products of at least two genes with variable ex-pression in different muscle types, mixed a and b in fastskeletal and cardiac muscle from smaller mammals andmore predominantly a,a in cardiac muscle from largermammals (455). Neighboring Tm overlap in a head-to-tailconfiguration with periodicity of 38.5 nm along the thinfilament.
Binding of Tm to F-actin is influenced by intrinsicinteractions between Tm and actin monomers, interac-tions between overlapping head-to-tail regions of contig-uous Tm molecules along the actin filament, and by otherproteins such as Tn and myosin that greatly increase thebinding. The intrinsic interactions may be through the 14quasi-equivalent repeats of neighboring regions ofcharged and uncharged side chains in the amino acidsequence of Tm (304). These can be divided into twoclasses of alternating sites (363), providing two possibletypes of binding to the actin filament that are electrostaticin nature. The X-ray images of Tm decorated F-actinfilaments (281) support the binding on the periphery ofthe F-actin filament, suggesting electrostatic binding. Thetightness of this binding and flexibility of Tm on the actinmay vary with the specific Tm isoforms making up thetwo strands. Isolated smooth muscle Tm is much moreflexible than skeletal Tm with only about one-third thepersistence length (55 nm compared with 150 nm, com-pared with the 42 nm length of Tm) (441). (Persistencelength is a measure of flexural rigidity of the molecule,being the arc length along the filament at which the angleof the tangent to the arc becomes uncorrelated in three-dimensional motion, a measure of the space constant forthe spread of bending along the molecule.) The flexibilityof cardiac Tm in actin (A)-Tm (as measured by fluores-cence anisotropy of a probe on Cys-190 on Tm) appears tobe more than for skeletal Tm in A-Tm (59). The tightnessof binding and flexibility may also vary along the strandbecause of the quasi-equivalent nature of the repeats andthe intrinsic flexibility of the Tm. In fact, some of therepeats may contribute little to the actin binding affinityof Tm as their deletion affects the affinity little as long asthe integral number of repeats and the coiled-coil struc-ture of the Tm is retained (193, 263). This implies that thehead-to-tail overlap between contiguous Tm providesmuch of the stability of the binding to F-actin. This isdemonstrated by the greatly reduced affinity of Tm fromwhich the overlap region has been deleted [the nonpoly-merizable Tm of Mak and Smillie (287)]. Troponin bindingextends from this overlap region to near the Tm Cys-190,one-third of the way from the COOH-terminal end of Tmtoward the NH2-terminal end (Figs. 1 and 3). S1 greatlyincreases the binding of Tm to F-actin (53, 86), decreasing
the flexibility of Tm (443) with the effect being greater forcardiac Tm than for skeletal Tm (59). Tn further stabilizesTm binding to F-actin and provides tethering sites con-trolled by Ca21 through troponin with some flexibility inbetween (363). In fact, the largest movements of Tm in theTm crystal occur near the COOH-terminal end (363).Movement of Tm over the surface of the thin filamentbrought on by Ca21 binding to troponin and myosin S1binding to actin are thought to be central to regulation asdiscussed in section IIIA.
3. Tn
Tn is composed of three, interacting subunits eachreceiving its identifying letter from the first identifiedproperty: troponin C (TnC) binds Ca21, troponin I (TnI)binds to actin and inhibits the actomyosin ATPase in aCa21-insensitive manner on a one-to-one basis with actin,and troponin T (TnT) links the Tn complex to Tm (158,287). It is known that the interactions among the Tnsubunits, Tm, and actin are Ca21 sensitive, allowing forCa21-induced conformational changes, modification ofthe average Tm position on the actin filament, and initia-tion of contraction. Figure 3 summarizes the interactionsbetween the subunits and the changes with Ca21.
A) TnC. TnC is the Ca21 sensor in skeletal and cardiacmuscle contractile regulation. Selective removal of TnCfrom skinned muscle preparations (334) prevents activa-tion by Ca21 (inhibiting force production) while reconsti-tution with native or recombinant TnC restores Ca21-sensitive contraction. TnC has two globular regions, anNH2 terminal and COOH terminal, connected by a longcentral helix. Each region contains two possible Ca21
binding sites of the E-F hand, helix-coil-helix type (Fig. 2).The COOH-terminal sites (III-IV) have high Ca21 affinity(;107 M21) and sufficient Mg21 affinity so that underintracellular, relaxed conditions, Mg21 is normally bound.These sites are termed structural sites because Mg21-Ca21 binding at these sites enhances TnC-TnI interactionand binding of TnC to the thin filament (519). The NH2-terminal sites (I-II) are the physiological Ca21 trigger siteswith lower affinity (;105 M21) and high selectivity ofCa21 over Mg21 (371). Substitution into skinned musclefibers of recombinant TnC deficient in Ca21 binding tothese two sites renders the fiber Ca21 insensitive (410,418). The NH2-terminal region in cardiac TnC (the TnCisoform in both cardiac and slow skeletal muscle) con-tains a single Ca21 binding site (II). In this cTnC, thepentagonal bipyramidal coordination at site I is notachieved because of amino acid substitutions at threecoordinating positions (leucine, alanine, and cysteine atthe X, Y, and 2Y positions in place of aspartic acids)(470). Elimination of Ca21 binding to cTnC site II rendersa cardiac or slow skeletal fiber insensitive to Ca21 (375).Reengineering site I of cTnC to bind Ca21 with site II still
April 2000 MUSCLE REGULATION 857

deficient does not restore Ca21 sensitivity, implying thatsite II Ca21 binding is most critical (432).
The structure of TnC has been solved in a number ofcases: X-ray crystallography of sTnC without (183, 428)and with Ca21 (213) and of the NH2-terminal fragmentwith Ca21 (425); NMR solution structures with and with-out Ca21 of NH2-terminal sTnC (130) and of sTnC (415),of cTnT (411), and of NH2-terminal human cTnC (419).TnC forms a dumbbell-like structure with the NH2- andCOOH-terminal regions separated by a long central helix(Fig. 2). The four Ca21 binding structures (3 for cardiacTnC) are composed of a helix-loop-helix region. For theCOOH-terminal Ca21 binding structures in skeletal TnCand cardiac TnC in the Ca21-bound state, the helicesflanking the binding loop are nearly perpendicular to oneanother forming an E-F hand structure (Fig. 2, left andright, seen most clearly in for helices A and B, C and D inthe NH2-terminal of Fig. 2, right). In the apo state lackingbound Ca21, the flanking helices are roughly parallel toone another (see the NH2-terminal region of sTnC in Fig.2, left). In the NH2-terminal without Ca21, this produces a
closed structure whereby the B-C helices and connectingloop are folded down along the central helix. Upon Ca21
binding in sTnC, as the B-C helices adopt a more perpen-dicular orientation, there is an opening of the structure,exposing hydrophobic amino acid side chains in the cen-tral helix that are thought to interact with sTnI (Fig. 2,right). Herzberg et al. (185) first proposed this model thathas now been verified for sTnC.
Somewhat surprisingly, the cardiac TnC NH2-termi-nal structure is closed both without and with Ca21 bind-ing (more resembling the sTnC apo structure; Fig. 2, left)(411). In fact, there may be little change in exposedhydrophobic surface on the NH2-terminal of cTnC onCa21 binding (419) or the change is less favorable and notnormally observed. This makes one question what drivesthe cTnC-cTnI interaction. Sykes et al. (276) suggest thatbinding of cTnC to cTnI induces the opening of the NH2-terminal of cTnC because the opening occurs with cTnCbinding to a cTnI peptide. The results of fluorescenceresonance energy transfer (FRET) studies of intramolec-ular distances in cTnC with cTnC-cTnI interaction aremixed with Putkey et al. (78), suggesting that TnC doesnot open with cTnI binding, whereas Cheung and cowork-ers (144) suggest that it does open. If after Ca21 bindingthe NH2-terminal cTnC was not open and required thecTnI binding to “force” open the structure, this bindingwould be less favorable and the conformational changepossibly slower. This could provide a major difference insequence of events during Ca21 regulation of cardiacmuscle compared with skeletal muscle. Another differ-ence is that the structural change, sensed by a fluorescentprobe, for saturated Ca21 binding is virtually complete forskeletal TnC, but not for cardiac TnC (84, 85) (see below).This could contribute, to some extent, to the lack of astructural change in cTnC detected by NMR upon Ca21
binding to the NH2-terminal site II. Nevertheless, cTnCcan reconstitute Ca21-activated force when reconstitutedinto skinned skeletal muscle fibers, although the maxi-mum may be less than achieved with native sTnC (63, 162,335).
Evidence that these structural changes in sTnC withCa21 binding occur and are important for regulationcomes from studies using recombinant TnC. In thesestudies either a disulfide cross-link has been engineeredinto the molecule to prevent the opening up of the NH2-terminal structure on Ca21 binding (156) or additional saltbridges engineered to make opening more difficult (125).In the first case, little activation is achieved with Ca21
when this TnC is substituted for the native TnC in skeletalmyofibrils, and in the latter case, much higher Ca21 isrequired to achieve activation.
A number of studies have been performed on thekinetics of Ca21 binding to TnC, isolated or in Tn. Thereare major differences between the skeletal and cardiacisoforms. In skeletal TnC with binding monitored either
FIG. 2. Left: ribbon representation of the crystal structure of turkeyTnC with Ca21 (solid circles) bound to the 2 COOH-terminal bindingsites. The NH2 terminal is identified, and the 8 lettered helices arecolored to show their orientation. [From Herzberg and James (183).]Right: ribbon representation of rabbit fast skeletal TnC with Ca21 (solidcircles) bound at both the 2 NH2- and 2 COOH-terminal binding sites.[From Houdusse et al. (213).] The lettered helices are colored to showorientation. Note the 2-lobe structure of both TnC and long connectinghelical stalk with each lobe containing two possible Ca21 binding sitesformed by 4 helices organized into 2 helix-loop-helix structures. WithoutCa21 binding, the helices are orientated more parallel to one another(see the A-B and C-D helices in the turkey TnC on the left). With Ca21
coordination, the helices are more perpendicular to one another in theso-called E-F hand configuration (see the A-B and C-D helices in therabbit fast skeletal TnC on the right). Note that with this change, the B-Chelices rotate up exposing part of the D helix of the central stalk.Herzberg et al. (184) hypothesized that this transition exposed hydro-phobic residues in the D helix enhancing TnC-TnI binding. Both struc-tures were downloaded from the Brookhaven Protein Data Bank anddisplayed using WebLab Viewer Pro.
858 GORDON, HOMSHER, AND REGNIER Volume 80

by an extrinsic fluorescent probe on TnC (233, 397) or byan intrinsic probe [Trp inserted at position 29 in a recom-binant TnC (234)], Ca21 binding to the NH2-terminal Ca21-specific sites appears to be diffusion limited (;108
M21zs21) while Ca21 dissociation is ;400–500 s21. Fur-thermore, using quin 2 fluorescence to monitor the free[Ca21], it was determined that the conformational changemonitored by the extrinsic/intrinsic TnC fluorescence oc-curred almost simultaneously with the Ca21 association/dissociation. Finally, the effective equilibrium constantfor the conformational change with Ca21 binding washigh enough so that virtually all TnC with bound Ca21 alsoexhibited the conformational change needed to interactwith the other Tn subunits to activate. In cardiac TnC,although Ca21 binding is probably diffusion limited (84,85), there is a measurable delay between Ca21 bindingand the conformational change measured by an extrinsicfluorescent probe (84, 85, 175) and also a delay betweenCa21 dissociation and the resulting conformationalchange (251). Furthermore, the conformational changesfor saturated Ca21 binding are not complete, implyingthat even at maximum Ca21 not all the cardiac TnC wouldbe in the activated conformation (84, 85). This could havegreat significance in terms of activation for cardiac mus-cle and is discussed in sections IIIA2 and VA.
B) TnI. TnI is the subunit that holds Tn together andonto actin by binding to actin, TnC and TnT, with many ofthese interactions regulated by Ca21 (Fig. 3). Isolated TnI,or a positively charged inhibitory peptide from TnI (96–116 of rabbit fast skeletal TnI) (442) bind to the NH2-terminal region of actin and inhibit the binding of myosin
and activation of the actomyosin ATPase in a one-to-onemanner. Additional residues on skeletal TnI (140–148),which show sequence homology and positive charge sim-ilarity to the inhibitory region of sTnI 96–116 (104), ap-pear to provide a second site of binding of sTnI to actin(463). These same sequences, potential actin bindingsites, appear in two similar regions of cTnI. They alsooccur in a third region of both sTnI and cTnI (360), but itis not clear which of these regions are the most importantfunctionally. This binding of TnI to actin is not responsi-ble for inhibiting actin directly, because TnI is onlypresent in a 1:7 ratio to actin, but it aids in anchoring theTn complex on the thin filament in the absence of Ca21.
A major step in Ca21-mediated interaction is the Ca21
modulation of TnI-TnC interaction. We focus on this in-teraction with skeletal muscle proteins and comment onlybriefly on the differences in cardiac TnC-TnI. There ismuch information on the sTnC-sTnI interaction, but nodefinitive structure, as crystallization of the full complexhas not been achieved. Neutron diffraction studies (341)and fluorescence studies (250, 271) of the complex sug-gest that they associate in an extended structure. Thisproposed structure is not like the wrap-around structureof the TnC homologous protein calmodulin interactingwith its target peptide, M-13 of myosin light-chain kinase(222, 306). It is more similar to the extended, antiparallelstructure of the essential light chain interacting with thelight chain-binding region of myosin S1 (379, 506). Thisstructure is also consistent with the hypothesis that thebinding of TnC to TnI occurs mainly in an antiparallelmanner (104).
To date, the only X-ray crystal structure is of sTnCwith the NH2-terminal fragment 1–47 of TnI bound at itsCOOH terminal (472). There is also an NMR structure ofcTnI (33–80) with the COOH terminal of cTnC (132). Thisbinding of TnC to TnI is dependent on the presence ofMg21 or Ca21 bound to sites III-IV of TnC and is thoughtto be the origin of the binding of TnC-TnI in relaxedmuscle. TnC can be selectively removed from skinnedmuscle fiber preparations by chelating divalent ions in alow-ionic-strength solution rendering the fibers Ca21 in-sensitive (71, 519). There is a Ca21-independent interac-tion of TnI with TnC, probably the central helix of TnCwith the NH2-terminal helix of TnI (104). However, theimportant Ca21-triggering interactions are probably be-tween the NH2-terminal domain of TnC including thehydrophobic region, exposed upon Ca21 binding to sitesI-II (47) with TnI residues 116–131 (463). Tripet et al.(463) hypothesized that this binding pulls the flanking TnIresidues 96–116 and 140–148 away from their actin bind-ing sites. The residues 96–116 of TnI could then bind tothe COOH-terminal domain of TnC. This is summarized inFigure 3. More complete information on the specific in-teractions awaits X-ray crystallographic or NMR studiesof the TnC-TnI complex.
FIG. 3. Diagram indicating the effect of Ca21 binding to TnC on theinteraction between the various thin filament proteins shown in Fig. 1. Ais actin, I is TnI, C is TnC, T1 is the NH2-terminal (1–158) portion of TnT,T2 is the COOH-terminal (159–259) portion of TnT, Tm is tropomyosinwith the NH2 and COOH terminals indicated in the head-to-tail overlapregion and the Cys-190 region indicated. Thicker lines imply strongerbinding, and thinner lines imply weaker binding. The state with Ca21
bound to the 2 NH2-terminal TnC triggering sites is shown on the right;the state without Ca21 bound to these 2 TnC sites is shown on the left.Note that Ca21 binding to TnC enhances the TnC-TnI and TnC-TnT2interactions and weakens the TnI-A interactions. This presumably al-lows the Tm to move on the surface of the actins opening up myosinbinding residues (see Fig. 5). [Modified from Heeley et al. (180).]
April 2000 MUSCLE REGULATION 859

The binding of TnI to TnC is strengthened greatly byCa21 through specific interactions at the NH2 terminal ofTnC (223). Thus Ca21 binding to TnC may switch TnIaway from multiple binding sites on actin to multiplebinding sites on TnC (471). These Ca21-dependentchanges in TnC-TnI interactions weaken the binding toTnI to actin. In fact, Ca21 abolishes the binding of isolatedTnI-TnC to actin (371), and there is also much evidencefor this in thin filaments during regulation. Studies usingFRET between fluorescent probes on TnI and actin andTnC and TnI show clearly that Ca21 induces a closerapproximation of TnC-TnI and an increased distance be-tween TnI and actin (138). This further supports the con-cept that TnI-actin binding acts as a Ca21-sensitive an-chor(s) of Tn-Tm to actin. To fully understand this Tn-Tm-actin interaction, we must describe the role of TnT.
C) TnT. TnT appears to be the structural “glue” thatholds the Tn-Tm-actin complex together (Figs. 1 and 3) asit binds to Tm, TnI, TnC, and actin [see Perry (361) andTobacman (455) for comprehensive reviews of these in-teractions]. In this position, it serves a number of roles inCa21 regulation. It acts not only to assist in binding TnC-TnI to Tm-actin and Tm to actin, but in cooperative acti-vation of the thin filament. TnT is a highly asymmetricmolecule [;18.5 nm long for skeletal (116) and longer forcardiac TnT (455), compared with 40 nm for Tm]. Theglobular COOH-terminal region (TnT2) interacts withTnC, TnI, and Tm while the extended NH2-terminal region(TnT1) lies along the COOH-terminal region of Tm includ-ing the region of overlap with the neighboring Tm NH2
terminus (287, 497). In this position, TnT is in a position toinfluence the flexibility of Tm, since the Tm overlap re-gion is responsible for much of the affinity of Tm for actinand Tm shows its greatest flexibility in this region (363).TnT has many isoforms with a hypervariable region anda number of alternative spliced variants (30, 416). Withthese diverse functions, it is not surprising that differ-ent isoforms may give rise to variable properties thatare just beginning to be explored. We first consider thebasic interaction before speculating on the role of thevariants.
The TnT interaction with TnC-TnI-Tm both increasesthe inhibition of actomyosin ATPase in the absence ofCa21 and increases the stimulation in the presence ofCa21 (104, 158, 362, 373, 471). The binding to TnI-TnCrequires the COOH-terminal region, TnT2 (359), but thecooperative activation requires TnT1 (403). This cooper-ativity occurs through the interaction of TnT1 with Tm inthe region where neighboring Tm overlap and possiblywith actin (358). This interaction of TnT1 plus additionalneighboring TnT residues with Tm and actin enhances theactomyosin ATPase compared with that for actin alone(289). TnT is also important in the binding of the Tmcomplex to the actin filament (358). Binding of TnT to Tmand the actin filament, occurring through TnT1, is Ca21
insensitive (358). There appears to be binding alsothrough TnT2, but Ca21 strengthens the TnC-TnT2 inter-action thereby weakening the binding of TnT2 to Tm andactin (373). Because the NH2-terminal region of TnT2 maybe inhibitory to the actomyosin ATPase (289), the Ca21-mediated increased interaction of TnC-TnT2 may aid inactivating the thin filament. This provides a second sitefor Ca21 to regulate the positioning of Tm on the thinfilament (in addition to the Ca21-mediated decrease inTnI-actin binding discussed above). However, the actionof Ca21 does not cause a large decrease in the affinity ofthe Tm-Tn complex for actin as Wegner and Walsh (490)saw only a two- to threefold decrease and Rosenfeld andTaylor (397) a sixfold decrease in skeletal muscle. Thisdecrease is functionally significant in skeletal muscle asCa21 (with Tn) increases the size of the cooperativity unitalong the thin filament (297, 389). In contrast, Dahiya et al.(74) observed little change in the binding of Tn-Tm toactin with Ca21 in cardiac muscle. This difference inproperties between skeletal and cardiac muscle may playa role in the functionally important differences in regula-tion between the two preparations. Not surprisingly, iso-forms of TnT, differing in this region of interaction ofTnT2 with TnC, confer different Ca21 sensitivities to ac-tivation of myofilament ATPase and skinned fiber tension(372).
The binding of TnT to TnI is not affected strongly byCa21, although there may be changes in the regions of TnIinteracting with TnT (463). Modification of this interac-tion can modify the Ca21 sensitivity of control of theacto-S1-ATPase by the reconstituted regulatory system(232). This is not surprising because of the multiple inter-actions between these regulatory subunits, several ofwhich are regulated by Ca21 as discussed above. It wassuggested by Tripet et al. (463) that one component of thisinteraction was due to the NH2-terminal region of TnI.Another component of this TnI-TnT2 interaction appearsto involve the presence of heptad repeats in both TnI andTnT, implying that the interaction may involve a-helicalcoiled coils (423).
The importance of TnT as well as TnI and Tm inregulation is clearly highlighted by the mutations in theseproteins that are shown to cause familial hypertrophiccardiac myopathy (21, 248, 345, 361). Thus TnT throughits interactions with TnC-TnI-Tm and actin helps deter-mine the position of Tm on the thin filament and theeffects of the regulatory system on actin, both importantin regulation. Therefore, the differences in TnT isoformsbetween muscle types and during development lead toaltered Ca21 sensitivity (337, 391, 402, 454, 457), enhancedactivation of the ATPase (104, 157, 279, 373), and possiblechanges in cooperativity, important functional differencesin the regulation of contraction.
860 GORDON, HOMSHER, AND REGNIER Volume 80

B. Thick Filament
The thick filament is the bipolar polymer of the motorprotein myosin that interacts with actin to produce forceand sarcomere/muscle shortening. It also contains otherproteins such as C, H, X, and M proteins and the largeelastic protein titin. The motor protein, myosin II, is com-posed of two heavy chains with molecular masses of;200 kDa each and four light chains, two each of so-called essential and regulatory light chains (ELC and RLC,respectively), of molecular mass ;20 kDa. The heavychains form a parallel two-chain coiled-coil structure overmost of their length except for the large, globular NH2-terminal regions, termed heads or S1 (subfragment 1).One pair of light chains bind to each S1. The coiled-coilregion of the myosin, termed the myosin rod, forms fila-ments due to interactions between pseudo-repeats of op-positely charged amino acids in the rod regions with themolecules shifted by a regular interval along the filamentof ;14.3 nm. With this stagger, the two heads, paired S1regions, project outward from the filament at regularintervals. In all thick filaments, these projections are he-lically arranged with about a 14.3-nm repeat. In vertebratestriated muscles, there is a three-stranded structure with14.3 nm between paired S1 head projections or 43 nmbetween heads projecting in one direction. In the longitu-dinal center of the thick filament, the molecules are ar-ranged in a head-to-tail configuration to give the wholefilament a bipolar structure.
The S1 head structure projecting from the backboneof the thick filament interacts with actin to generate forceand filament sliding. The structure of the S1 has beendetermined by Rayment et al. (379) and is shown in Figure4. The location of the S1 projections from the thick fila-ment may depend on the nucleotide state of S1 (505). S1heads with ADPzPi appear to lie down on the surface ofthe thick filament, whereas ATP and rigor heads may bemore disoriented moving away from the surface (288). Onthe surface, the heads lie on helical ridges. This is likelyan antiparallel arrangement with the paired S1 heads fromone myosin projecting in opposite directions interactingwith the S1 head from the next myosin, possibly in adimer (342). Presumably any change in the S1 shape dueto a change in bound nucleotide or changes in the lightchains may disrupt this interaction and release the S1head from the ordered structure on the backbone, allow-ing it to move toward the actin filament. This was firstnoticed with changes in temperature (505) but presum-ably also occurs with more physiological changes such aseither RLC or C-protein phosphorylation (see below).
Other proteins have been shown to play an importantrole in the thick filament structure, although they are notmotor proteins. C protein, identified by Offer et al. (340),forms seven or eight stripes across the A band in thesarcomere shown by antibody labeling. It bundles the
myosin molecules together in the filament during devel-opment (407). The X and H proteins may perform func-tions similar to that of C protein as the proportion of thesethree similar proteins varies somewhat reciprocally be-tween different fiber types (422). The large elastic mole-cule titin may play a major structural role in the sarco-mere contributing to the passive and possible activeelasticity as well as sarcomere stability (482). The M-lineproteins provide struts connecting the thick filamentstogether at the center of the A band in the sarcomere.
What role do these proteins of the thick filament playin regulating actin-myosin interaction in vertebrate stri-ated muscle? We first consider the myosin light chains.For a discussion of the properties of the myosin lightchains in regulation, see the reviews by Sweeney andco-workers (431, 435) and Trybus (464). In smooth mus-cle, phosphorylation of the RLC is essential for contrac-tion (464). Phosphorylation of the RLC in vertebrate stri-ated muscle is not essential for contraction, but itenhances the force and force redevelopment rate at lowlevels of Ca21 activation (309, 436, 437). Studies bySweeney et al. (438) using RLC mutants demonstratedthat phosphorylation was acting primarily to neutralizepositively charged amino acids toward the NH2 terminalof the RLC from the phosphorylatable serine. Levine et al.(272) showed that phosphorylation of the RLC affects thestructure of rabbit skeletal muscle thick filaments, disor-dering the myosin S1 heads, presumably by moving themaway from the thick filament surface toward the thinfilament. Whether this disorder was caused by phosphor-ylation alone or by an effect on the S1 nucleotide state isnot clear, but phosphorylation produced the thick fila-ment structural change which, as discussed in sectionsIIIC1 and IIIC2, promotes cross-bridge attachment andactivation at lower Ca21 levels.
In some invertebrate muscles, direct Ca21 binding tothe myosin is responsible for Ca21 activation (446). Thisoccurs through Ca21 binding to the ELC with the coordi-nation stabilized by interactions of the ELC with both theRLC and the myosin heavy chain in the light chain bindingregion (506). This type of binding does not occur with thevertebrate ELC, but the RLC chain of vertebrate striatedmuscle can bind Ca21 and Mg21 and probably binds Mg21
under resting conditions (13, 204). Early results suggestedthat this site exchanged Ca21 for Mg21 too slowly to beresponsible for primary regulation but could modulateregulation under steady-state Ca21 activation (13).
A modulation of thick filament structure associatedwith activation has been suggested by the long-recognized;1% increase in the repeat spacing between myosin crossbridges in the thick filament that appeared to precedeforce development (220, 364). What role structural changeplays in activation and whether it is caused by Ca21
binding or some other event remain to be determined.A role for Ca21 in regulating both force and the rate
April 2000 MUSCLE REGULATION 861

FIG. 4. A and B: intermolecular interactions in the rigor actomyosin complex. In A, peptide backbone of twoconsecutive long pitch actin monomers interacting with the myosin S1 structure is shown as the peptide backbone withlight chains indicated (LC). For a description of the procedures for docking the actin and S1 structures, see Milligan(317). In B, the S1 has been rotated about a vertical axis to expose the surface of contact with the actin. Residuesconstituting the main hydrophobic binding site are shown in blue. The region of actin to which this binds is also shownin blue on the actin structure at the left. The lysine-rich loop of the myosin S1, residues 626–647, which is absent in theX-ray structure, is shown as green spheres as are their probable electrostatic interaction sites on actin, 1–4, 24, and 25.The myosin S1 loop 404–415 and the presumed contact sites on actin (residues 324–334) are shown in purple. The
862 GORDON, HOMSHER, AND REGNIER Volume 80

of force redevelopment was suggested by the data ofMetzger and Moss (312) in skinned rabbit skeletal musclefibers. Partial extraction of the RLC both increased theforce and rate constant of force redevelopment at lowCa21 (312, 356) and decreased the maximum shorteningvelocity at high Ca21 (200). In contrast, Szczesna et al.(445), extracting most of the RLC using a different extrac-tion technique, found that the extraction decreased boththe force at low Ca21 and the rate of force development.The reason for the discrepancy is not clear. Metzger andMoss (312) further suggested that the RLC inhibits cross-bridge attachment and that this inhibition was relieved byCa21 binding to the RLC. They also hypothesized that theeffect was exerted through the RLC and not TnC, sincethe shift in Ca21 sensitivity produced by elevated Mg21
could be inhibited by partial extraction of the RLC, butnot by extraction of TnC (312). The effect of Mg21 iscomplex, however, as they later showed that partial ex-traction of the RLC has opposite effects on the force-pCarelationship at high and low Mg21. To investigate the roleof Ca21 binding to the RLC, native RLC was extractedfrom skinned rabbit skeletal muscle fibers and replacedwith a non-Ca21 binding mutant (a recombinant chickenRLC with a point mutation decreasing Ca21 binding). Inthese fibers, there was only partial reconstitution of forceand Ca21 sensitivity, the rate of force redevelopment wasdecreased, and the rate of relaxation was increased. Thisimplies that the RLC is important in controlling the myo-sin and cross-bridge kinetics but did not provide a uniquetest of their hypothesis about the role of Ca21 binding tothe RLC (79).
It has been known for some time that the RLC mod-ulates acto-myosin ATPase activity, since its extractiondecreases the ATPase activity (290) and myosin light-chain composition affects the ATPase (431, 464). There-fore, changes in the RLC should affect cross-bridge bind-ing as well as modulation of contraction. Extraction of theRLC disorders myosin head structure, moving them awayfrom the backbone of the thick filament (273) to an evengreater extent than phosphorylation of the RLC (272).Thus it is not surprising that RLC extraction can affect
force (see sect. IIIC1) and the rate constant for forceredevelopment (see sect. IIIC2) in a similar manner to RLCphosphorylation. In contrast, Ca21 binding to the RLCdoes not appear to disorder the myosin head structure ofthe thick filament (272), making it unlikely that Ca21
exerts a disordering action through the RLC.The other proteins of the thick filament, C protein in
particular, may modulate Ca21 activation [see Winegrad(501) for a recent review]. Studies from the Moss labora-tory (198–200) have shown that partial extraction of the Cprotein increases the Ca21-activated force at low Ca21 inboth skeletal and cardiac preparations, much as doesmyosin RLC phosphorylation, and increases the speed ofshortening at low Ca21 in skeletal muscle fibers. Theypropose that C protein constrains the motion of the my-osin heads to keep them close to the surface of the thickfilament (200, 331). Extraction of C protein might thenallow the myosin heads to move out toward the actinfilament, increasing their effective concentration and in-creasing cross-bridge attachment at any Ca21. Phosphor-ylation of C protein is associated with increases in cardiaccontractility (170, 171), but there are results that do notsupport this conclusion (501). The confusion in resultsmay originate from the difficulties in phosphorylating Cprotein and not other proteins that can affect regulation.There is evidence that phosphorylation of C protein incardiac muscle increases the ATPase activity (298) bypromoting movement of heads away from the surface ofthe thick filament (199, 331, 491). There is also a sugges-tion that the effects of phosphorylation of C protein havemore effect on the activity of a-myosin (V1 myosin) thanon b-myosin (V3 myosin) (492) and on the movement ofthe myosin heads away from the thick filament. Theysuggest that phosphorylation of C protein not only movesthe myosin head away from the thick filament backbonebut may order it, decreasing its flexibility (492). Theyhypothesize that the increased proximity to the thin fila-ment increases the rate of myosin attachment to actin andthereby force. Furthermore, they hypothesize that thedecreased flexibility increases the rate of detachment ofmyosin from actin and therefore could increase the speed
FIG. 4. (Continued) secondary binding site, myosin residues 567–578 and actin 95–100 on the (n 2 1)th actin areindicated in red. [Modified from Milligan (317).] C–E: G-actin monomer with shadow of Tm show position in (C) for zeroCa21 (C), for high Ca21 (D), and for the rigor S1 bound state (E). Space-filling model of a single G-actin in the orientationshown in A with the comparable coloring to the myosin interaction sites on actin indicated in A and B. Actin residues1–4, 24–28 are colored green; 144–148, 340–346 blue; 332–336 purple; and 93–96 red. Actin residues within the shadowof Tm in the three positions shown by Vibert et al. (475) are shown as gray. These actin residues in C and D wereestimated from the diagrams of Vibert et al. (475) with the assistance of W. Lehman. The actin residues within theshadow of Tm in the rigor position (E) were estimated by shifting Tm 106 shift from the high Ca21 position. Note in C
in the absence of Ca21, only the electrostatic binding sites, 1–4, 24–28, (green) are not “covered.” In the presence of highCa21 (D), all of the sites except the residues hydrophobic residues 332–336 (purple) are exposed. In the presence of rigorS1 binding (E), none of the indicated myosin binding sites on actin is covered. Thus, in the absence of Ca21, only weakbinding sites are exposed. In response to Ca21 binding to TnC, Tm moves to reveal additional binding sites. With bindingof S1, Tm is moved to a position exposing all the myosin binding sites. This is a static picture of Tm in fixed tissue andis not a complete picture of the dynamic Tm. The flexibility of Tm may be sufficient for it to move over the actin surfaceas much as from D to E just with thermal fluctuations (363).
April 2000 MUSCLE REGULATION 863

of shortening (492). This is speculative but provides atestable model. Cardiac C protein should be able to bemodified more by phosphorylation than skeletal muscle Cprotein because cardiac C protein has three or four phos-phorylatable sites compared with only one for skeletal Cprotein. Thus the state of phosphorylation of C proteinmay modify the thick filament structure and modulateCa21 activation of contraction.
Finally, titin has been thought of as playing a mostlypassive role in the sarcomere, keeping filaments alignedand providing passive stiffness. Recent studies have sug-gested that this view needs to be revised as titin maychange its properties and its interaction with the otherfilaments in response to low levels of Ca21 and therebymodify the properties of the resting or active sarcomere(426, 482). The importance of these changes remains to beclarified.
C. Interaction Surfaces of Actin and Myosin
Knowledge of the structural relationships of the actinand myosin molecules has advanced rapidly with X-raysolution of their protein structures (203, 241, 378–380,417). Integration of these structures with electron micro-graphic (EM) reconstructions and chemical mapping sug-gests that four sites produce the interaction associatedwith contraction [see Fig. 4, based on Milligan and co-workers (317, 319, 378)]. There are two sets of ionicinteractions. The first involves negative charges of subdo-main 1 of the nth actin from the Z line (amino acids 1–4and 24/25) with the lysine-rich positively charged myosin50k/20k loop (626–647), and the second involves residues95–100 or 93–95 of subdomain 1 of the (n21)th actintoward the Z line with myosin residues 567–578 (317). Thestrong binding site of rigor interaction, present at the endof the power stroke, is the interaction of a hydrophobicmyosin amino acid sequence 529–558 (with contributionsfrom 647–659) with hydrophobic actin residues 341–354and 144–148 of the nth actin and residues 40–42 of the(n21)th actin (data not shown). A second strong actomy-osin binding site involves the unresolved structure of themyosin 404–415 loop interacting with actin 332–334 pro-line residues at the junction of actin subdomains 1 and 3.This is an important site because the myosin cardiac
R403Q mutation is associated with the manifestations offamilial hypertrophic cardiomyopathy (FHC), a reductionin the actomyosin ATPase, and an inhibition of the in vitromotility sliding speed (73, 137, 439).
It has been suggested that myosin binds to actin firstwith the ionic binding prompting docking of actin and my-osin (378). As strong binding occurs, there may be a changeof the cleft between the upper and lower halves of the50-kDa domain, which opens a passage way for the releaseof phosphate through the “back door” (or 50 kDa cleft side)of the nucleotide binding site (377, 378, 514). The result ofthe cleft change is hypothesized to be conveyed to the20-kDa region, stiffened by the ELC (379, 468), moving theend of the 20-kDa lever arm ;7 nm (114, 378, 379). As theneck region rotates, movement of Ser-243 or Arg-245 opensa pathway for Pi escape from its binding site (514). Thismechanism has been called the “swinging lever arm” modelof cross-bridge action (202). Recent studies have tested thisidea by extending the 20-kDa level arm region by insertingrepeating 20-kDa segments. These studies have shown, aspredicted by the model, that the in vitro sliding speed in-creases in proportion to the length of the extended leverregion (6, 466, 468).
D. Cross-Bridge Cycle
The ATPase reaction mechanism that powers thisswinging lever involves the reaction sequence shown inthe abbreviated scheme 1 [see Cooke (67) for a reviewand more complete scheme]. For myosin alone, the reac-tion sequence is given in scheme 1 by steps 19, 39, and 59.Here the rate of ATP binding to myosin and actomyosin is;1 3 106 M21zs21 (for steps 1 or 19 which include theformation of a collision complex followed by isomeriza-tion to a strongly bound form of ATP), and the cleavageby myosin (step 39) is relatively rapid, 125 s21 at 20°C (seeTable 1). For myosin alone, product release occurs firstwith Pi release (at a rate-limiting value of 0.5 s21 at 20°C)followed by the temperature-sensitive release of ADP(291). For the cross bridge and its interaction with actin,the reaction mechanism is given by the sequence of steps
1–8. This mechanism first involves rapid ATP binding toform actomyosinzATP (AMzATP) followed by the rapiddissociation to actin and myosin-ATP (MzATP) in step 2.
864 GORDON, HOMSHER, AND REGNIER Volume 80

TABLE 1. Rate constants for ATPase reaction steps from solution biochemistry (acto-S1, acto-HMM, and
myofibrils) and skinned muscle fibers (rabbit psoas fibers)
Rate Constant
Condition Preparation
Temperature,°C pH
Ionicstrength,
mMActo-S1
Acto-HMM Myofibrils Fibers
k11, M21 z s213 106†
ATP binding 5 7 66 0.9 (286) 0.6 (286)10 7 36 1.5 (286)13 7 200 0.2–1‡ (142)20 7 50 2.7 (286) 3.0 (286)20 7 150–200 1.0 (235) 1.1 (382) 1.6‡ (44)22 7 9 6 (44)
k139 1 k239, s21
ATP cleavage (myosin) 10 7 200 49 (382) 55 (207)10 7 36 40 (286) 40 (286)13 7 200 60‡ (111)15 7 100 55 (64)20 7 36 95 (286)20 7 66 53 (286)20 7 100 125 (235)20 8 150 250 (14)22 7 9 45 (44)25 7 200 168 (382)
K3, no unitsCleavage equilibrium 10 7 36–200 1.5 (286, 382) 1.2 (286)
13 7 200 5‡ (111)20 7 36 2.4 (176) 2.8 (176)20 8 150 9 (14)22 7 9 1.5 (44)
k13 1 k23, s21
ATP cleavage(Acto-S1) 10 7 3 0.9 (496)
20 7 3 3.1 (496)30 7 3 5.6 (496)
k15 2 k17, s21
Pi release rate 10 7 200 30‡ (75), 100§ (207)10 7 3 36 (496)10 7 36 15 (286)12 7 200 16‡ (176)20 7 200 31‡ (176)
k15 2 k17, s21
Pi release 20 7 3 77 (496)20 7 36 40 (286)20 7 66 27 (286)22 7 9 45 (44)
s21 ADP release 20 7 10–50 200 (449), 325 (44)20 7.1 200 13–46‡ (76), 400§ (76)
kcat, s21
Steady-stateATPase 4–5 7.4 16 0.8 (451)
5 7–7.4 150–200 1.7 (35), 0.8* (35) 1‡ (35), 5.8 (176)10 7 3 0.8 (182)10 7 9 2 (44)10 7 36 6.5 (286)10 7–7.4 150 3.4 (182), 1.3* (182) 0.8‡ (352)
12–13 7 200 1.9‡ (110), 16‡ (176)5.1‡ (177), 18.5§ (177)
15 7 9 4.3 (44)15 7 65–200 5 (424), 5.1 (182) 5.1 (140)
†1.8, †2.2* (182)1.8‡ (140)
20 7 3 2.5 (496)20 7 36 22 (286)20 7 66 12 (286)20 7 50–200 8.2 (182), 3.5* (182) 3.2‡ (489)20 7–7.4 50–165 22 (64), 15* (182)22 7 9 14.5 (44)22 7 20–200 20 (414) 31‡ (176)
HMM, heavy meromyosin. For preparation values, reference numbers are given in parentheses. * Chemically cross-linked. † Given valuesshould be multiplied by 106. ‡ Isometric contraction. § Shortening contraction.
April 2000 MUSCLE REGULATION 865

Next there is a fast (and reversible) cleavage of ATP onthe myosin head (forming MzADPzPi, step 39). At very lowionic strength (,10 mM) and high concentrations of actin,ATP is cleaved in solution by the AM complex withoutsignificant dissociation. The rate of ATP cleavage by theAM complex is .30 times slower than that by myosinalone and is the rate-limiting step for actomyosin ATPaseat low ionic strength (see Table 1) (495). Furthermore, theequilibrium constant for ATP cleavage by AMzATP is ,1(495). At physiological ionic strengths, however, the rateof dissociation of the AMzATP complex to A and MzATP isso rapid and complete that virtually all cleavage occurs onthe dissociated myosin. Thus we omitted ATP cleavage byAM in scheme 1. The rate of Pi release from AMzADPzPi is.50 times faster than that from MzADPzPi (see Table 1).Under physiological activating conditions, there is a rapidreassociation (step 4) of actin and MzADPzPi forming aweakly bound A-MzADPzPi state that then isomerizes (step
5) to a more strongly bound AMzADPzPi state (a transitionprobably regulated by Ca21) (see sects. IIIC2, IIIC4, andIIID). The strongly bound AMzADPzPi has been hypothe-sized to isomerize to produce force and AM**zADPzPi
(lever arm motion, step 6) and may be stabilized in thestrong binding force-exerting form by the release of Pi instep 7 (75). Here we represent the force generation and Pi
release as separate steps based on single fiber experi-ments (134, 243, 316). However, it has also been suggestedthat strong actin binding, force generation, and Pi releaseoccur in the same step (202, 351, 377). Whether forcegeneration occurs at step 6 or is contemporaneous to step
7 (with the omission of step 6) and the formation of thestrong binding state remains unresolved. Nevertheless,the transition from the weakly bound A-MzADPzPi to thestrongly bound AM**zADP involves a large change in freeenergy (350, 496) and is the transition during which forceis generated. The rate of Pi release from A-MzADPzPi toAM**zADP and Pi is represented in Table 1 as a combina-tion of steps 5, 6, and 7. An irreversible isomerization ofthe AM**zADP state to an AM*zADP (not shown in scheme1 and which may be ATPase rate-limiting under isometricconditions) then occurs (414). This isomerization is fol-lowed by a rapid release of ADP (187, 449). These twosteps are represented by step 8 in Table 1. Rate constantsfor many of these transitions have been estimated fromsolution biochemistry and skinned fiber studies (see Ta-ble 1). Finally, the steady-state rate of ATP hydrolysis perS1 head (kcat) under various conditions is given in Table 1.
Studies of the transient kinetics of isolated myofibrilsand in single skinned muscle fibers (using caged sub-strates, caged products, and a fluorescent Pi binding pro-tein) have been performed (44, 75, 76, 107, 110, 142, 176,286). Results from solution biochemistry and more struc-tured systems are in good agreement. The rates of ATPbinding to AM, AMzATP dissociation to A-MzATP, and ATPcleavage as well as the equilibrium constant for ATP
cleavage are all similar for the three preparations of ac-tomyosin in solution, actomyosin in myofibrils and fibers(110, 111, 142, 209) (Table 1). The rates of product releaseare more difficult to study in solution biochemistry be-cause the rates of product release from unconstrainedacto-S1 or acto-heavy meromyosin (HMM) are fast (495).In the muscle fiber, strain is applied to the cross bridgeand is thought to slow the rates of product release (70,207, 286). This conclusion follows from the fact the kcat
for actomyosin in solution is greater than fivefold greaterthan that in fibers contracting under isometric conditions(286) (see Table 1). Even during rapid shortening in mus-cle fibers, the measured rates of ATP hydrolysis are ap-proximately one-half those measured in solution. Further-more, the rate of high-energy phosphate hydrolysis (kcat)in intact muscles is increased during shortening and isinhibited during eccentric contractions (contractions dur-ing which a contracting muscle is forcibly lengthenedproducing a very large force) (503). These results, to-gether with the rapid ATP hydrolysis following its bindingto actomyosin, suggest that the rate of product release inthe muscle fiber is the rate-limiting step of the cross-bridge mechanism. Actomyosin interaction has been con-strained in acto-S1 and myofibrillar preparations usingcross-linking agents and gives results roughly comparableto those in isometrically contracting fibers (see Table 1).
The isomerization of the weak binding A-MzADPzPi
state (formed in the rapid reassociation of actin andMzADPzPi) to form a more strongly bound AMzADPzPi
state is probably the transition regulated by Ca21 (286).This conclusion is supported by the study of muscle fibertransients produced by the photogeneration of Pi fromcaged Pi. These studies showed that the rate of Pi releasefrom strongly bound AMzADPzPi in isometrically contract-ing muscle fibers at 10°C is 30 s21 (75, 316, 495). This rateis far greater than the isometric steady-state ATPase rate(1–2 s21) and implies that the rate-limiting step, at leastunder isometric conditions, occurs after the release of Pi.During shortening, similar experiments show that the rateof Pi release increases to .100 s21, implying that a stepimmediately before, immediately after, or the Pi-releasestep itself, is strain dependent (207). A comparison of kcat
to the Pi release rate is problematic because kcat is theproduct of the [AMzADPzPi] times the rate constant for Pi
release. Thus the increased kcat during shortening mightbe explained by an increase in the fractional occupancyAMzADPzPi. However, this requires an increase in thenumber of strongly bound cross bridges, and measure-ments show that during shortening the number of stronglybound cross bridges falls to approximately one-third ofthe isometric value (118, 239, 240). Finally, the swinging-lever cross-bridge model, with a cross-bridge throw of 10nm and a maximum shortening velocity of ;2 musclelengthszhalf-sarcomere21zs21 implies a duration of cross-
866 GORDON, HOMSHER, AND REGNIER Volume 80

bridge attachment of ,5 ms (duration of steps 4–8 and 1
and 2) per ATP hydrolysis cycle.The development of a fluorescent phosphate binding
protein (FPBP), which binds Pi at rates of ;1,000 s21 witha Michaelis constant (Km) of ,1 mM, permitted measure-ment of the time course of Pi release from strongly boundAMzADPzPi in acto-S1 solutions, myofibrils, and skinnedmuscle fibers (16, 44, 109, 176, 177, 495). In solution at lowionic strength and 10°C, the rate of Pi release fromAMzADPzPi is in excellent agreement with the measure-ments in isometrically contracting muscle fibers (30 s21)(75, 495). A significant discrepancy exists in studies usingthe FPBP in fibers. In fibers containing the FPBP, pCa at5, into which ATP is photogenerated, steady-state rate ofPi release is not achieved over the first 3 or 4 cross-bridgebridge turnovers, i.e., the rate of ATP hydrolysis is 3–10times greater than that measured using enzymatic meth-ods, freeze-clamping methods, or acto-S1 solutions in thesteady state (see Table 1) (176–178). To account for thedifference, the value for k28 for at least the first fourcross-bridge turnovers using FPBP must be much largerthan is seen in more conventional ATPase measurements(see Table 1). Measurements of Pi release using the FPBPare made under conditions (,1 mM Pi) radically differentfrom more physiological (Pi ;1 mM) conditions. Effortsshould be made to engineer a FPBP whose Km for Pi is inphysiological or submillimolar range. In rapidly shorten-ing fibers at 10–12°C, the steady-state rate of ATP hydro-lysis is near 20 s21 (177), whereas the Pi (75) and ADP(76) release rates exceed 100 s21. These results suggestthat for minimally strained cross bridges, the rate-limitingstep for ATP hydrolysis occurs before the release of Pi
itself (286). At present, there are no probes for fibersto directly monitor the weak to strong cross-bridgebinding transition (A-MzADPzPi to AMzADPzPi) or forthe AM**zADP to AMzADP isomerization limiting ADPrelease.
The question remains as to which of the steps in theactomyosin ATPase reaction are regulated by troponin/tropomyosin and Ca21. Kinetic studies show that therate-limiting step of the myosin ATPase cycle is the re-lease of hydrolysis products (285) or an isomerizationafter ATP cleavage but before Pi release, and that foractomyosin ATPase the rate-limiting step occurs after Pi
release in the isometric contraction. These observations,in addition to those to be discussed, imply that the controlof the muscle ATPase rate by regulatory proteins andcalcium must occur between steps 4 and 5 in the mech-anism shown in scheme 1, between A-MzADPzPi attach-ment and an isomerization preceding Pi release. Twohypotheses have been advanced to account for the regu-lation of actomyosin ATPase. The first is a steric blockingmodel, which postulates that through Ca21 binding to Tn,Tm is positioned on the thin filament so that the strongbinding of myosin to actin, which has been prevented in
the absence of Ca21, is now allowed. The second is akinetic regulation model that postulates that Ca21 bindingto Tn regulates the rate of the weak to strong cross-bridgetransition. Both models are discussed below, the stericblocking model in section IIIA and the kinetic model insection IIIB.
III. RESULTS OF STRUCTURAL, BIOCHEMICAL,
AND PHYSIOLOGICAL STUDIES
OF REGULATION
A. Thin Filament Structural Studies of Regulation
1. Steric blocking
Structural studies on regulation of the actin-myosininteraction in vertebrate striated thin filaments have fo-cused for some time on the position of Tm in controllingthis interaction. As discussed in section IIA, early studiesshowed that Tm was located along the actin thin filamentin a position to influence this interaction (322) and sug-gested that Tm might sterically block the interaction ofmyosin S1 with actin. The position of tropomyosin wasidentified through three-dimensional image reconstruc-tions of electron micrographs of isolated thin filaments ofactin, actin-Tm, or regulated thin filaments (see Amos,Ref. 5) and through X-ray diffraction studies of muscles ororiented thin filaments. X-ray diffraction studies (172, 219,347), measuring changes in the intensity of the secondactin layer line upon activation in whole muscle wereinterpreted as showing a movement of Tm on the periph-ery of the thin filament to a position in the groove of theactin long helix. Later studies questioned whether thischange in the second actin layer line was due entirely tomovement of Tm or whether there was some componentdue to changes in Tn position or in actin structure (seeSquire and Morris, Ref. 421). The earlier investigators(172, 219, 347) hypothesized that upon activation Tmmoved from a position that blocked actin and myosininteraction to one that allowed it. The binding of Ca21 toTn was hypothesized to initiate this movement (194), thusoriginated the steric blocking model of regulation. Thetime course of the change in the actin layer line wasconsistent with this hypothesis as Kress et al. (254)showed that, during the rising phase of activation, inten-sity increases in the second actin layer line preceded therise in cross-bridge attachment signaled by the increase inintensity of the I(1,1) X-ray reflection or the increase inmuscle force. Furthermore, because these changes oc-curred at sarcomere lengths at which thick and thin fila-ment overlap was absent, they suggested that the move-ment was independent of the behavior of the cross bridge.
Three-dimensional image reconstructions of thin fil-aments using electron micrographs of fixed and nega-
April 2000 MUSCLE REGULATION 867

tively stained or quick-frozen specimens have confirmedand extended these studies. Early attempts at reconstruc-tions gave differing results depending on whether S1 bind-ing to actin was taken as tangential or “end-on” (see Amosfor a review, Ref. 5). More definitive studies of frozen,hydrated filaments showed upon S1 binding that Tmmoved from a position that might block S1 attachment toone in which it would not interfere with S1 binding (318).These electron micrographs set the stage for the morerecent interpretations of images of regulated thin fila-ments with and without Ca21 and with rigor S1 binding(see sect. IIIA2).
The interpretation of both the X-ray and EM measure-ments (see Squire and Morris, Ref. 421) have been ques-tioned because they do not take into consideration thepresence of Tn and possible changes in its structure withCa21. In addition, these studies do not consider changeswithin actin accompanying Ca21 or cross-bridge bindingto thin filaments, and there is evidence that these changesin actin occur (see Squire and Morris, Ref. 421).
The simple, steric blocking view of regulation wascalled into question through later studies. Biochemicalstudies of regulation indicated that there may be regula-tion of a kinetic step rather than a regulation of binding(see sect. IIIB). These studies also suggested that attachedcross bridges might activate the thin filament [see Cha-lovich (55) for a review]. More recent biochemical studies(303) suggest that there may be three states of the thinfilament, not just two, implying a more complex activationmechanism. Physiological studies measuring stiffness inskinned fibers at low [Ca21] and low ionic strength im-plied that actin and myosin can bind even at rest (39), i.e.,under conditions where the second actin layer line wasnot affected. Thus, if there was steric regulation, it wasnot absolute as some interaction occurred even withoutCa21 binding to troponin. Further impetus for additionalstructural studies came with the solution of the crystalstructure of actin (241), enabling Lorenz et al. (282) tocompute how actin monomers were arranged in the thinfilament. The crystal structure of the S1 head of myosin(378) allowed for approximations of the interaction inter-faces of actin and myosin referred to in section IIC of thisreview and indicated in Figure 4. Thus examination ofspecific actin-myosin interactions at the amino acid levelcould begin. This was a prerequisite for determining theposition (or positions) of Tm with respect to this interac-tion interface.
2. Three-state dynamic steric model
Recent X-ray diffraction studies of skinned fibers andoriented filaments and EM reconstruction studies of iso-lated thin filaments have shown positions of Tm support-ive of a three-state [blocked, closed, and open (303)]model of thin filament regulation. This interpretation is
consistent with biochemical studies discussed in sectionIIIB. In addition, fluorescent and electron paramagneticresonance (EPR) studies of Tm position have providedadditional information. We consider first the results of theEM and X-ray techniques. The conclusions of the EMstudies and the published X-ray diffraction studies are ingood agreement (201). Three-dimensional image recon-structions of electron micrographs of isolated, fixed, neg-atively stained thin filaments from Limulus and frog stri-ated muscle (see Refs. 264, 265, 475) or quick-frozenfilaments (507) revealed that in the absence of Ca21, Tmoccupies a position that blocks most of the potentialbinding sites for myosin on actin (see Fig. 4C) (hydropho-bic residues 340–346 and 144–148 colored blue, and 332–336 colored magenta). In the absence of Ca21, the onlysites available for weak electrostatic interactions withmyosin are the negatively charges sites in residues 1–4(colored green), and 93–95 (colored red) in subdomain 1of actin (see Fig. 4C). Binding of S1 to these sites wouldalso direct S1 binding to additional sites on the thinfilament when available. In this state, the thin filamentwould be in the “off” position. Further inhibition of S1binding would occur through competition with TnI forbinding to the NH2-terminal region of one to two actins inthe A7TmTn structural regulatory unit. Thus there is goodevidence for steric regulation of strong cross-bridge at-tachment by Ca21, Tn, and Tm.
After Ca21 binding to Tn, Vibert et al. (475) and Xu etal. (507) found that Tm moved ;25° around on the sur-face of the actin filament (Fig. 4D) from its position in theabsence of Ca21. This movement occurred with bothchemically fixed and frozen-hydrated specimens (507).The Tm movement exposed several additional potentialbinding sites (24–28 colored green and 340–346 coloredblue in subdomain 1 of actin, and 144–148 colored blue atthe junction between subdomains 1 and 3 of actin),thereby promoting stronger hydrophobic binding of actinto myosin sequences 529–558 and 647–659. However, Tmcontinued to block myosin’s access to the potential bind-ing sites (residues 332–336 colored magenta in Fig. 4D).Tm might be stabilized in this position by electrostaticinteraction between the seven pseudo-repeat sequencesin Tm and actin residues located in actin subdomains 3and 4 (281). This position could be equivalent to theMcKillop and Geeves “closed” position (303) (see discus-sion later and in sect. IV). Upon the addition of S1, in theabsence of MgATP and Ca21, Tm appeared to move an-other 10° around the actin filament exposing additionalmyosin binding sites, specifically residues 332–336 (seeFig. 4E). This effect was seen with two or more S1 boundper seven actin regulatory units. At lower concentrationsof S1 in the absence of MgATP, they saw evidence ofcooperative spread of the Tm shift along the thin filament.The shift in Tm was clear at a distance of 3 Tm from thesite of rigor S1 binding, less at 7–12 Tm, and negligible at
868 GORDON, HOMSHER, AND REGNIER Volume 80

.12 Tm lengths (with 26 Tm/thin filament). This Tmposition in the presence of S1 in rigor might be equivalentto the open position of McKillop and Geeves (303). Thusthere is structural evidence for three activation states ofthe thin filament: off, partially on, and on to compare withthe biochemical evidence for three activation states[blocked, closed, and open in the McKillop and Geevesnomenclature (303)]. They suggest that “full switch-on ofthe thin filaments by reversal of steric-blocking requiresboth Ca21 and the strong binding of myosin heads, actingin sequence” (475) based on their structural studies usingrigor S1 binding. Subsequent studies, using interaction ofnative thick and thin filaments in the presence of MgATP,found little evidence for shifting of Tm beyond the -Ca21
position by the relatively few cycling cross bridgespresent in these studies (72). However, it is not knownhow much significant strong cross-bridge binding waspresent under these conditions.
Similar conclusions on activation by strongly at-tached, rigor cross bridges were reached by Poole et al.(367) and Lorenz et al. (281) using X-ray diffraction ofskinned rabbit skeletal muscle fibers and oriented, regu-lated actin-Tm filaments [see Holmes (201) for a morecomplete discussion]. In their work, changes in the inten-sity of the second actin layer line were interpreted aschanges in Tm position. In the absence of Ca21, weak,electrostatic attachment could occur, but what theytermed “stereospecific weak binding” could not occur.Upon Ca21 binding to Tn, Tm, which is at a radius of 3.9nm on the actin filament, moves by 25–30° to a positionclose to the position it occupies in the absence of Tn(281). Upon the attachment of rigor cross bridges at over-lap of thick and thin filaments in the sarcomere, Tm was“observed” to have moved further to the “fully activated”position. These observations agree with the EM imagereconstructions of Lehman et al. (475) above and suggesta three-state steric model, comparable to the three-statemodel of McKillop and Geeves (303) proposed from bio-chemical studies.
Although there seems to be equivalence between thethree structural states and the three biochemical states ofMcKillop and Geeves (303), the agreement may be super-ficial. McKillop and Geeves (303) suggest that in the pres-ence of rigor cross bridges all actin thin filament sites arein the open conformation (available to myosin for strongbinding), but in the presence of high Ca21, only ;25% ofall actin thin filament sites are in the open conformationwith 75% in the closed conformation (available only forweak myosin binding). If one assumes that all actins inthe A7TmTn regulatory unit are in the same conformationbecause of the Tm position [as do McKillop and Geeves(303)], this would imply a distribution of Tm positions inthe presence of Ca21, with 75% in the partially activatedposition and 25% in the rigor, fully activated position. Thiswas not observed in the EM studies of chemically fixed
filaments (475), but it is consistent with the X-ray diffrac-tion data that measure the average Tm position. Thispoint will be returned to in the following paragraph.
The existence of three states of Tm on the thinfilament is different from the two equilibrium states forTm binding to actin, the equilibrium determined by Ca21
and S1 binding first suggested by Phillips et al. (363). Thequestion is whether in fact there is a third state particu-larly in the absence of rigor cross bridges. The structuralstudies do suggest three states, but as discussed by Squireet al. (420), quoting earlier comments of Phillips et al.(363) and Cohen and Vibert (66), the thin filament is adynamic, breathing structure. Tm has so much flexibilitythat it is incorrect to describe its position as fixed. Thusboth the X-ray and EM images of the Tm position do notgive a complete picture of the true Tm position. In thecase of X-ray diffraction, the estimated Tm position wouldbe a time-averaged one. Because the EM images are offixed tissue, the Tm might be more likely to be fixedcloser to its equilibrium position. However, the EM recon-structions are from thin filament images containing anumber of A7TmTn structural units and thus average theposition of Tm over a number of regulatory units. Inaddition, the reconstruction technique uses helical aver-aging with nonuniformities such as Tm-Tm overlap andTn contributing some, but probably only a small amountto the reconstructed image (420). Thus neither the X-raynor the EM images show the dynamics of Tm and, as such,do not give an accurate picture of regulation. The Tmposition with Ca21 binding might be particularly dynamicbecause the Ca21 binding to TnC is transient and thestructural changes in TnC and the other regulatory pro-teins as a result of Ca21 binding may not be complete (84,85). Thus, even if the Tm position with the maximalconformational change with Ca21 binding was shifted byexactly the same amount as with rigor S1 binding, thetime-averaged Tm position with Ca21 binding would bebetween the “off” and “on” positions. The Tm position,determined by S1 rigor binding to actin, would be lessdynamic because the S1 rigor binding is very tight andonly very slowly reversible compared with cycling cross-bridge binding. If the average Tm position with Ca21
bound to TnC was in fact at the position shown by Vibertet al. (475) for the fixed filaments, the thermal fluctuationsof Tm observed in the crystal structure of Tm by Phillipset al. (363) (up to 0.8 nm between the middle and COOH-terminal end) would be sufficient to give an angularchange greater than the angular separation between theCa21-bound and rigor Tm states.
The flexibility of Tm on the thin filament was shownby Szczesna and Fajer (443) to be nearly as flexible as insolution using a maleimide spin-label on Cys-190 of Tmreconstituted into fibers from which myosin, Tm, and Tnhad been extracted. The mobility of the labeled domain ofTm was decreased little by binding to actin and was
April 2000 MUSCLE REGULATION 869

changed little by Tn in the presence and absence of Ca21
but was stabilized by rigor S1 binding. Ca21 binding to Tnincreased the disorder of labeled domain, but the changesare small, indicating weak association of this Tm domainwith actin. Thus, in the presence of Ca21, Tm may beflexible enough so that at any time some of the actins ina A7TmTn regulatory unit might be in the state with allmyosin binding sites on actin available (see Fig. 4E),whereas others might be in a closed or possibly blockedstate (Fig. 4, C and D). Whether this occurs or not de-pends on the flexibility of the Tm in association with theTn subunits and the effects of Ca21 binding to TnC andthe binding of S1. Tm flexibility will be returned to insection IV on modeling. The data of Tregear et al. (462),showing that there are preferred target zones for myosinbinding to actin in activated insect flight muscle, indicatethat there within the regulatory unit there may be actinswith different myosin affinities. These differences in my-osin binding to actin could be because of Tm flexibility orbecause of constraints on myosin attachments to actin inthe highly ordered sarcomeres of insect flight muscle.
To relate the structural observations to the regula-tion of muscle contraction, we need to know the Tmposition with cycling cross bridges rather than with rigorcross bridges. The X-ray data of Kress et al. (254) showthat the changes in the second actin layer line occur withmuscles stretched to beyond thick and thin filament over-lap and that the changes in intensity can be as large asthose with filament overlap. To the extent that this layerline indicates the Tm position, this would suggest that thechanges in Tm position with Ca21 are similar to thosewith Ca21 plus cycling cross bridges, but there are datafrom Maeda (368, 369) that suggest otherwise. Thus theX-ray data do not settle the argument conclusively.
Another method of measuring the Tm position on theactin filament has been to use fluorescence probes. Lehrerand Ishii (225–227, 269), using isolated thin filaments,measured changes in fluorescence of a variety of labels onTm (primarily on Cys-190) or changes in energy transferbetween a tryptophan on actin and a probe on Tm (269) tostudy Tm movement. Their results imply that there is littlechange in the Tm position as deduced from fluorescencechanges until myosin S1 binds in a rigor link. Later studiesfrom this laboratory (12) show that Ca21 binding doesshift the Tm position in a manner consistent with theX-ray and EM results.
If in fact it is correct to interpret the EM and X-raydiffraction data in terms of three states of thin filamentactivation which differ in the specific actin binding sitesavailable to myosin, these three states should correspondto three different myosin binding affinities. One wouldexpect that the actual affinity would depend on the spe-cific nucleotide state of the myosin and possibly also oncross-bridge strain. There would also be the question ofwhether a myosin once bound to the Ca21 induced “par-
tially on” position of Tm, could then undergo an isomer-ization and push the Tm out of the way to induce the moretightly bound “on” state. This has not been ruled out onenergetic considerations but seems less likely to be thecomplete explanation because of the experiments ofSwartz and co-workers (430, 516, 517) on S1 and S1zADPbinding to the overlap and nonoverlap regions in myofi-brillar sarcomeres discussed in section IIIC6. The differenteffective affinities of the actin in the three structuralstates are again similar to the affinities for the threeblocked, closed, and open states of McKillop and Geeves(303), but again there is the lack of perfect equivalencewith the partially on Ca21-bound structural state repre-senting a mixed biochemical state of 75% closed and 25%open.
Thus the structural studies of Tm position on theactin filaments imply three states of activation of the thinfilament determined by the dynamic position of the flex-ible Tm. Ca21 controls the Tm transition from allowingweak to somewhat stronger myosin binding and low or noforce, but the strongest myosin binding occurs after Tmhas shifted to the position where it does not block any ofthe myosin binding sites on actin. This shift in Tm may becaused by or stabilized by the myosin binding.
There are others (55) who interpret these changes inthe thin filament in terms of an allosteric mechanism ofregulation. The following evidence supports such an in-terpretation. The spin-label (443) and fluorescence [seeChalovich (55)] studies show changes in the thin filamentor actin structure with S1 binding. In addition, biochem-ical studies show an enhanced S1 ATPase with the thinfilament over that of actin alone in the presence of rigorS1 binding (32) that they termed “potentiation.” Others(500) observed that this was not a true potentiation sincethe maximum binding velocity (Vmax) and binding con-stant of the S1 ATPase were not increased with regulatedfilaments activated by Ca21 and strongly attached crossbridges (NEM-S1) over that seen with actin filamentsalone. What was observed was an increase in the ATPasefor low S1 concentration for the regulated filaments acti-vated by Ca21 and cross bridges over that for actin alone(as in Fig. 5B) without a change in the maximum ATPase.However, Tm binding alters actin structure (281), andCa21 binding increases thin filament flexibility (224, 512).Actin may be modified in response to Ca21, so one clearlycannot treat the regulatory proteins as purely modifyingthe binding of S1 to actin alone. One must also considerthe possibility of direct interaction of Tm with S1. S1increases the binding of Tm to actin either by some directinteraction or an effect mediated through actin. In addi-tion, there could be changes in actin brought on by Ca21
binding to Tn and interactions through Tm or stronglyattached cross bridges through Tn-Tm. Thus, in additionto the shift of Tm and changes in myosin binding surface,one must consider strongly the possibilities of molecular
870 GORDON, HOMSHER, AND REGNIER Volume 80

changes in actin. The movement of Tm in response toCa21 binding and cross-bridge attachment is the majorstructural change and is the focus of the analysis pre-sented in this review. Following the suggestion that thinfilament activation is analogous to the ability of a ligand toincrease the open probability of a ligand-gated channel(398), it could be said that the Tm movement leads to anincreased probability of actin sites being open to strongbinding by myosin.
B. Biochemical Studies of Regulation
The biochemical studies of regulation have been wellreviewed by Chalovich (55) and Tobacman (455). Twoimportant conclusions come from these studies, with sup-porting evidence reviewed by Chalovich (55). The first isthat Ca21 regulates actomyosin ATPase by controlling akinetic transition (specifically the rate of the transition ofthe cross-bridge binding to actin from a weak to a strongbinding form) and not by controlling the binding of myo-sin to actin per se. The second is that the strong bindingof myosin to actin can modulate this transition through itseffect on the thin filament. The first conclusion (that therate of the weak to strong transition is regulated) origi-nated with the observation that at low ionic strength (18mM) in the presence of regulatory proteins, [Ca21] regu-lated the acto-S1 ATPase rate with the expected hyper-bolic relationship between regulated actin concentrationand ATPase rate. However, over this same range of actinconcentration, there was no change in the fraction of S1bound to actin (at 1 mM S1 and 100 mM actin, ;50% of theS1 bound to actin/Tm/Tn in the absence and presence ofCa21), suggesting that myosin binding to actin was notbeing regulated by Ca21 (57). This observation could notbe explained by the simple steric blocking model (thatCa21 controls access of the cross bridge to the thin fila-ment in a switchlike on or off mechanism) because thesteric blocking model would predict that the ATPase ratewould be proportional to the fraction of S1 bound toactin. The model for the actomyosin ATPase reactionmechanism at that time involved S1zADPzPi binding toactin to form the weakly bound A-S1zADPzPi followed bythe release of Pi to create a strongly bound A-S1zADPstate. Furthermore, because the rate of ADP release toform the A-S1 state and the binding of ATP and dissocia-tion of the A-S1zATP to A and S1zATP are very fast, theonly conclusion consistent with these data was thatTn/Tm controlled the rate of the transition from theweakly bound A-S1zADPzPi state to the strongly boundA-S1zADP state. With this understanding of the steps in-volved, it was hypothesized that Ca21 regulated the phos-phate release rate (which was thought to be the weak tostrong cross-bridge transition) (56, 57). Thus there was“kinetic” regulation of the actomyosin ATPase by Ca21
(55). Tests of this model using caged Pi to generate phos-phate transients showed that the processes associatedwith force production and Pi release are little, if at all,affected by [Ca21] (316, 479) (see sect. IIIC4). However, ifadditional steps exist between the weakly boundAMzADPzPi and strongly bound AMzADP complex, thenregulation of their equilibrium or rate constants couldalso produce the observed behavior. Supporting evidencefor kinetic regulation came at the same time from skinnedmuscle fiber data demonstrating that the rate of forcedevelopment (kTR) increases with [Ca21] while the origi-nal steric blocking model predicted an independence ofkTR from [Ca21] (35) (see sect. IIIC2 for other interpreta-tions of this data). The kinetic model holds that the Ca21
binding to Tn allows the thin filament to undergo a tran-sition that is affected by strong cross-bridge binding. Asecond aspect of this model is that the strong cross-bridgebinding turns on the thin filament in a graded, cooperativefashion.
Many studies have pointed to a role for strong bind-ing of myosin to the regulated skeletal thin filaments inregulating myosin binding and actin-activated ATPase ac-tivity. The studies of Trybus and Taylor (465) and Greeneand Eisenberg (160) showed that binding of S1 or S1zADPto regulated actin was highly cooperative in the absenceof Ca21 but that the cooperativity decreased greatly in thepresence of Ca21. Both discussed this effect in terms ofthe regulated filament being switched to an “active” or onstate of increased S1 affinity. The change in fluorescenceof a fluorophore attached to TnI (159, 465) or Tm (269)monitors changes in the thin filament structure thought tobe associated with this increased affinity. These changesoccurred when about one-half of the actin binding siteswere occupied with S1 (or S1zADP). Thus the strongbinding rigor S1 or S1zADP was hypothesized to switchthe actins in the regulated unit of the thin filament from alow to a high affinity for S1. Weaker binding forms of S1(S1zATP, S1zADPzPi, or N,N9-p-phenylenedimaleimide(pPDM)-S1] do not have this effect (56; see also Chalov-ich, Ref. 55).
Similar studies on activation of S1 ATPase by regu-lated actin came to similar conclusions. They are inher-ently more difficult to interpret because of the need tohave some MgATP to measure acto-S1 ATPase activity.The studies of Bremel and Weber (32), investigating ATP-ase of S1 and regulated filaments in the presence of lowMgATP, demonstrated that even in the absence of Ca21,strong S1 rigorlike binding activates the ATPase. Thestoichiometry of this activation was similar to the switch-ing of the regulated thin filament from low to high myosinaffinity discussed above, ;50% of actin sites occupied(31). This work was supported by many other studies thatdemonstrate activation of the ATPase by strong-bindingS1 species such as NEM-S1 (161) but little activation byweaker binding forms such as pPDM-S1 (161). This
April 2000 MUSCLE REGULATION 871

seemed to imply that strongly bound cross bridges, inaddition to enhancing the binding of myosin to the regu-lated filament in the absence of Ca21, also enhanced theATPase activity. In the presence of Ca21 and a low S1-to-actin ratio, the addition of strong binding S1 (NEM-S1) toregulated acto-S1 increases the ATPase activity. The reg-ulated acto-S1 has a higher Vmax than in the presence ofCa21 alone, but the Vmax does not exceed that observedusing unregulated actin for a given S1 concentration un-der their low ionic strength conditions (500). This impliesthat when the ratio of S1 to actin is small, Ca21 cannotcompletely activate the regulated filament and that addingstrong-binding NEM-S1 enhances the ATPase for a givenS1-to-actin ratio. With NEM-S1, there is no increase in theATPase Vmax over that seen with actin alone or regulatedplus Ca21 and high S1 concentration so there is not a true“potentiation.” What occurs is that the relative affinity ofactin for S1 is enhanced by the presence of the regulatoryproteins plus Ca21 and NEM-S1. The increased affinitywill increase the activation for a fixed S1 concentration inbiochemical studies or in the filament lattice in the fiber.Two questions then arise: 1) Do long-lasting, strong-bind-ing states exist in the fiber? 2) Does the occupancy of
actin binding sites by myosin in the fiber approach thatrequired for activation in the biochemical studies? Thesequestions are discussed in section IIIC1.
The studies of Lehrer and Morris (270) of the S1ATPase activity in the presence of skeletal actin, actin-Tm, actin-Tm-Tn 6Ca21 as a function the [S1] have servedas a model for the role of strong cross-bridge activation ofthe thin filament during normal cross-bridge cycling.Their studies were interpreted as showing that, comparedwith S1 ATPase with actin alone, the ATPase activity withactin-Tm-Tn 1Ca21 was inhibited at low [S1] and poten-tiated at high [S1]. Thus, during cross-bridge cycling, itwas assumed that even in the presence of Ca21, a suffi-cient number of S1 cross bridges must be strongly boundto move the Tm to a position where the actin in theregulated filament could bind freely to S1 and activate theATPase. Figure 5A illustrates the S1 ATPase as a functionof the [S1] (for [S1] .50% of the [actin]) for a number ofdifferent types of actin filaments. As can be seen, theATPase for S1 with actin-Tm-Tn-Ca21 is always greaterthan for actin alone for all [S1] showing no inhibition. Incontrast, their Figure 3 shows it always below the curvefor all [S1] (not shown here). In Figure 5A (their Fig. 1),
FIG. 5. Effect of Tm, Tn, and Ca21 on the S1 ATPase measured as a function of [S1] using isolated skeletal (A) orcardiac (B) proteins. A: S1 ATPase with actin alone (1A), with actin-tropomyosin (1ATm), with actin, tropomyosin, andtroponin 6Ca21 (1ATmTn-Ca21 and 1ATmTn2Ca21). The ATPase is expressed as the absolute amount (in nmol) ofATP hydrolyzed per second corrected for the amount hydrolyzed by S1 alone. Note that the ATPase of S1 with theregulated ATmTn filaments without Ca21 (0.5 mM EGTA) is lower than the ATPase with actin alone, indicating that thefilaments are regulated. With Ca21, the S1 ATPase with regulated actin, ATmTn, depends on the S1 concentration beingslightly above that for actin alone up to an S1 concentration of ;4 mM but increasing more steeply above that S1concentration. In these experiments, there was 3.2 mM F-actin, 0.79 mM Tm, 0.77 mM Tn, 0.05 M NaCl, 5 mM Mg21, and0.05 mM Ca21, pH 7.9, at 23°C. Thus the S1 ATPase rate for regulated actin 1 Ca21 is enhanced over that for F-actin aloneat any S1 concentration and is cooperatively increased for an S1 concentration approximately equal to the F-actinconcentration used. However, the saturating maximum binding velocity (Vmax) is the same as that for F-actin alone sothat there is no potentiation of Vmax (500). [From Lehrer and Geeves (267), with data from Lehrer and Morris (270).] B:ATPase for skeletal S1 with cardiac regulated filaments in the presence of Ca21 (pCa 4.8) as a function of [S1] is shown.ATPase is expressed as the change in [MgATP] per second (in mM). Note that as [S1] is increased from a very low valueup to ;3 mM, the ATPase increases linearly, but that above 3 mM the ATPase also increases linearly, but at a higher rate.ATPase is enhanced by increased [S1] in the presence of the regulatory proteins and Ca21. The conditions for theirexperiment were 20 mM imidazole (pH 7.3), 3.5 mM MgCl2, 7 mM KCl, 5 mM skeletal F-actin, 1.5 mM cardiac Tm, 1 mMcardiac Tn, and 25°C. [From Butters et al. (46).]
872 GORDON, HOMSHER, AND REGNIER Volume 80

there are no data points for [S1] below 50% of [actin]. Infact, the ATPase activity/S1 with regulated filaments plusCa21 appears to be constant up to [S1] 5 [actin] abovewhich it increases. This is very similar to the ATPase datafor the cardiac regulatory proteins measured by Tobac-man (46) shown in Figure 5B. The data of Tobacman (46)are particularly significant because they are taken at verylow [S1], ,0.02 of the [actin]. Over the range of [S1] up to3–4 S1/7 actins (;50%), the ATPase/S1 is constant. Abovethis, the ATPase/S1 is higher, which could be called po-tentiation, but in fact, the change is not in the Vmax, but inthe apparent binding constant, the S1 binding. Becausethe ATPase/S1 is constant down to very low [S1], there isno cooperative activation by strong binding cross bridgesrequired for actin-activated S1 ATPase of regulated thinfilaments. There are sufficient actin sites available in theactin-Tm-Tn unit in the presence of Ca21 for S1 to bindand go through the cross-bridge cycle without the partic-ipation of any neighbors. If there were a requirement forthe participation of neighbors, then the ATPase/S1 wouldnot be constant but would depend on [S1]. Above 3–4 S1/7actin, there is an apparent potentiation. This implies thatCa21 binding is sufficient to activate for low S1 but thatcooperative binding of S1 can further activate at higher[S1]. This is discussed in section III, A, B, and C1, in thecontext of what occurs physiologically in the sarcomere.The behavior seen in Figure 5B with one ATPase/S1 seenbelow a particular [S1] and a higher ATPase/S1 above this[S1] is what would be expected of the McKillop andGeeves (303) model (discussed next). In their model, afraction of the actin sites would be in the open configu-ration for strong S1 binding in the presence of Ca21, buteven more would be open in the presence of stronglybound neighboring S1.
Many of the recent biochemical studies and modelsof regulation have come from Geeves and colleagues (133,136, 179, 297, 303). These studies evaluated both thekinetics of and steady-state binding of S1 to regulated thinfilaments. Light scattering was used to measure total(weak 1 strong) S1 binding and fluorescence of pyrenelabeled actin to measure strong binding of S1 to actin.Furthermore, Geeves and Lehrer (136) combined studiesof myosin binding to the regulated filament with measure-ments of changes in Tm position on actin (indicating thinfilament activation) using pyrene-labeled Tm (226). Theirstudies show that the binding of actin to myosin occurs inat least two steps; the formation of what they term an “Astate” consisting of actin weakly bound to an S1 having astrongly bound nucleotide, followed by an “R state” withactin strongly bound to an S1 with a weakly bound nu-cleotide. Isomerization of the A state to form the R stateis required to accelerate the S1 ATPase and generate forcein muscle [see Geeves (133) for a review]. Using equilib-rium binding and kinetic measurements of binding of S1to regulated thin filaments, McKillop and Geeves (303)
proposed a three-state model for activation of the thinfilament. In this model, the thin filament could occupy ablocked state that is unable to bind S1, a closed state thatcan only bind S1 relatively weakly (the A state) or an openstate in which S1 can bind and undergo the isomerizationto the more strongly bound R state. They further charac-terized the Ca21 and ionic strength dependence of theequilibrium constants for the various thin filament andmyosin binding states (179, 303). In more recent studies,they have extended this analysis to include cooperativeactivation of the thin filament using labeled Tm (136, 297).In the presence of Ca21, the cooperative unit appears toextend for more than 7 actins, up to 14 indicating moreflexibility of Tm in regulation expansion of the coopera-tive unit with loosened binding of Tn to actin. Thesestudies are discussed in more detail in section IV, describ-ing models of regulation, but provide a good frameworkfor discussing the physiological studies.
In summary, it is difficult to establish from the bio-chemical studies alone whether Ca21 is regulating strongattachment in a steric manner or regulating a kinetic step.A major problem is that solution biochemistry measuresonly ATPase rates and binding of long-lived cross-bridgestates. The following sections will conclude from fiberstudies and in vitro motility assays that the main stepregulated is strong cross-bridge attachment, but that therealso may be some regulation of cross-bridge kinetics.
C. Physiological Studies on Muscle Fibers
The control of contraction is studied in muscle fiberswhose organized sarcomeric structure allows measure-ment of physiological variables such as force, the rate offorce development, and shortening. Although biochemicalstudies are needed to define the possible molecular inter-actions, they must be reconciled with the results of mus-cle fiber studies where there are geometric and mechan-ical constraints imposed on the relationships between theproteins within the sarcomere. The sarcomeric structurecan either limit or enhance interactions depending onwhether the geometry separates or brings the interactantstogether and whether one or several of these interactionsare dependent on strain. Early studies using intact musclecells demonstrated the importance of Ca21 in the activa-tion of contraction [see Ebashi and Endo (87) for a re-view], but more detailed understanding of the mechanismof regulation commenced with studies of skinned musclepreparations first described by Natori (338). Skinningmuscle fibers allow control of the intracellular environ-ment, free of the sarcolemmal barrier, and allows physi-ological tests of models of control arising from biochem-ical studies. They also allow exchange or partialreplacement of some of the regulatory proteins [TnC, TnI,TnT, and MRLC; see Moss (331)] to examine roles of
April 2000 MUSCLE REGULATION 873

protein isoforms in regulation and to test models of mo-lecular interactions during regulation. Furthermore, theyallow measurement of Ca21 binding through changes instructural labels (fluorescence or EPR) on regulatory pro-teins (TnC, TnI) exchanged into skinned preparations orthrough measurements of 45Ca binding. Findings fromthese physiological studies are discussed along with theirimplications for regulation of contraction.
The central measurement for studying regulation inthe skinned fiber has been steady-state force as a functionof [Ca21] because it is relatively easy and assesses howCa21 controls force-generating cross-bridge attachmentunder a variety of experimental conditions. A secondarymeasure has been muscle fiber stiffness assessed eitherwith rapid length changes, steps, ramps, or sinusoids. Thisstiffness measurement provides information about thefraction of cross bridges attached when properly cor-rected for filament compliance and nonuniform sarco-meric properties. By varying the rate of change of length,information can be obtained about the rate of attachmentand detachment of cross bridges in both resting and ac-tive muscle. Using this technique, Brenner et al. (39)concluded that in both the relaxed and active musclecross bridges can attach and detach rapidly. At low[Ca21], low ionic strength, and low temperatures, 65–90%of cross bridges are attached (39). This decreases to justa few percent at more physiological ionic strengths andtemperatures. Such cross bridges have high rates of at-tachment/detachment and very weak binding and gener-ate no active force, but are detected by stiffness measure-ments.
Before discussing the results of physiological studiesof contractile regulation, we first consider the constraintimposed by the sarcomeric structure on the number ofactins with which myosin can interact. A simple calcula-tion derives the appropriate numbers given the density ofthick filaments (500/mm2), the number of myosins/halfthick filament (300), the ratio of thin to thick filaments (2),the overlap of thin and thick filaments (0.75 mm/h), andthe number of actins per 38-nm thin filament repeat (14,7/Tm).1 This calculation implies that in rigor (assuming
attachment of all cross bridges), one S1 head binds per 1.8actin monomers, or 3.9 S1 heads per A7TmTn regulatoryunit. Steric considerations suggest that in isometric con-tractions not all S1 heads can bind. Measurements suggestthat 20–40% of all S1 heads bind to the thin filamentduring a maximal isometric contraction (67, 280). If so,only 0.75–1.5 S1 heads are bound per regulatory unitunder fully activated isometric conditions. During un-loaded shortening, when the number of attached andcycling cross bridges falls to less than one-third of theisometric number [assuming stiffness is proportional tothe number of attached cross bridges (117, 240)], only 1S1 can be attached for every 15–30 actins, ;1 S1 for every21 regulatory units. This number also assumes that bothS1 heads of a myosin molecule can attach at the sametime. If only one S1 per myosin can attach, these numberswill all be decreased by a factor of two. These numbersshould be remembered when discussing cooperative ac-tivation by attached cross bridges during isometric andunloaded shortening contractions.
As discussed in section IIIB, a major question fromthe biochemical studies is whether the number of force-generating cross bridges or the kinetics of the transitionsbetween cross-bridge states are controlled by Ca21. Toanswer this question, the physiological studies must in-clude kinetic measurements investigating how Ca21 con-trols steady-state shortening and the pre-steady-state ki-netics of force development. Control of shortening isdiscussed in section IIIC5. Several techniques are nowavailable to study Ca21 regulation of pre-steady-state ki-netics. These involve mechanical or chemical perturba-tions of the skinned fiber and observations of the kineticsof the return to steady state. Some time ago, Brenner (34,35) devised a clever mechanical procedure for detachingmost of the strongly attached cross bridges and studyingthe rate constant for reattachment and tension redevel-opment. This method and its implications for regulationare discussed in section IIIC2. A more recent and powerfulapproach involves rapid chemical changes in skinned fi-bers by the use of “caged” compounds that are diffusedinto the skinned fibers in an inactive form and releasedrapidly to an active form by photolysis of the cagedcompound. This approach has been used to produce stepchanges in Ca21, a Ca21 chelator, Pi, MgATP, and MgADP
1 Electron microscopic and X-ray diffraction studies show thatmyosin molecules are arranged in groups of three every 14.3 nm alongthe thick filament. Each half thick filament has 0.75 mm [(1.65 mm/thickfilament2 0.15 mm bare zone)/2] of cross bridges/half-sarcomere so thatthere are 52 crowns of myosin molecules/half-sarcomere. Thus ;300 S1cross bridges per half thick filament may attach to the thin filaments. Inskeletal muscle fibers, there are ;500 thick filaments/mm2 of myofibril.Thus maximally 1.5 3 105 cross bridges can form per half-sarcomere foreach square micron of cross-sectional area. At maximal overlap, there is0.75 mm thin filament overlap per half-sarcomere/thin filament. Eachthin filament contains 14 actin molecules/38-nm thin filament length or370 actin molecules/mm thin filament. Thus 280 actin molecules areavailable for attachment per thin filament per half-sarcomere at maximaloverlap. There are 2 thin filaments per thick filament or 1,000 thinfilaments/mm2 muscle. Consequently, per half-sarcomere, there are 2.83 105 actin molecules/mm2. If maximally 1.5 3 105 cross bridges z h21 z
mm22 can form, one S1 head will be bound for every 1.8 G-actinmolecules. If only 20–40% of all S1 heads are bound to the thin filamentsduring maximal isometric contraction (67), there will be 1.5–3.0 S1heads bound for every 38 nm of a single actin strand, i.e., maximally0.75–1.5 S1 heads bound/regulated strand that has 7 actin moleculesavailable. During rapid, unloaded shortening, the total number of crossbridges attached and cycling at any one time falls to less than one-thirdof the number attached in the isometric case (assuming stiffness isproportional to the number of cross bridges attached, Refs. 117, 118,240). This corresponds to one S1 attached every 15–30 G-actin mono-meres, or one S1 head per two plus regulatory units.
874 GORDON, HOMSHER, AND REGNIER Volume 80

(209, 283). We discuss, in sections IIIC3, IIIC4, and IIIC8,the results of the first three of these techniques and theimplications of the pre-steady-state kinetics for the mech-anism of regulation of contraction.
1. Control of isometric force: force-pCa relationship
The initiation of isometric force by elevated Ca21 ina skinned muscle fiber was an early demonstration thatCa21 activates contraction (87, 181, 338). The steady-staterelationship between [Ca21] and force was shown to besteeper than that expected if force was just proportionalto Ca21 binding (181, 237). This relationship was first fitby Donaldson and Kerrick (83) with the Hill equation(189), F 5 F0 [Ca21]n/(Kn 1 [Ca21]n) (originally used todescribe the binding of O2 to hemoglobin). This has be-come the equation of choice to fit these data. In thisequation, the size of the Hill coefficient n, often called nH,is related to the number of interacting sites. For muscle,such an association has not been shown, but the equationis widely used to characterize the relationship betweenCa21 and force. In this form, K is the [Ca21] producinghalf-maximum force, here called the Ca21 sensitivity. Be-cause most investigators use pCa 5 2log10[Ca21], theform of the Hill equation often used to fit the data is
F 5F0
@1 1 10n~pCa1/22pCa!#
The pCa1/2 gives a measure of the [Ca21] required toactivate the muscle, the Ca21 sensitivity of activation, butis not necessarily a direct measure of the Ca21 affinity ofthe Ca21 binding sites giving rise to activation, as dis-cussed below. The pCa1/2 also varies greatly with condi-tions.
The Hill nH gives a measure of the cooperativity ofCa21 activation. If Ca21 binding to a single site controlsforce in a one-to-one basis, then n 5 1. If as in skeletalTnC, Ca21 binds to two sites, n can be up to 1.2 (155) ifthe sites are independent, and binding to both sites isrequired for activation. For control of isometric force, n isalways .1, varying from ;2 to 5–6 in skeletal and cardiacmuscle (23, 24). The value of n depends on the experi-mental conditions and how the data are analyzed. If forcedata from a number of fibers are averaged at each pCa andthen the average force is plotted versus pCa, differencesin Ca21 sensitivity between fibers can result in an appar-ently lower n for the ensemble than for any individualfiber. It is better to fit the Hill equation to the data fromeach fiber and average the resultant Hill nH and pCa1/2
values. With such a steep force-pCa curve, small changesin conditions can produce large changes in nH. Thus careshould be taken in interpreting changes in nH. Someinvestigators (see Moss, Ref. 331) find that a single Hillequation does not fit the data because the slope of the
curve may be greater for low [Ca21] than for high [Ca21]so that two Hill equations give a better fit to the data.However, most investigators find that the data are ade-quately fit by a single Hill equation (23, 513).
A) FACTORS AFFECTING CA21
SENSITIVITY. A number of di-verse factors are known to increase the Ca21 sensitivity(i.e., decrease pCa1/2) of the force-pCa relationship [seeYates (513) for an extensive review and Table 2]. Theseinclude increases in pH (81, 102, 396) or sarcomere length(101, 103); decreases in ionic strength (113, 146, 247, 452),lattice spacing (294, 296, 300), Pi (315), MgATP (141), and[Mg21] (82, 83); phosphorylation of the myosin RLC (309,437); or addition of NEM-S1 (429) or MgADP (128, 195).The effect of lattice spacing on Ca21 sensitivity posesproblems in many of the studies of the force-Ca21 rela-tionship, since force generation per se can decrease thelattice spacing (43). Some researchers minimize changesby preshrinking the lattice to the in vivo spacing with 4%dextran T-500 (296).
The factors listed in Table 2 alter activation by dif-ferent mechanisms. For example, changes in pH (100, 348,396) and changes in ions such as K1 (113) alter Ca21
binding to TnC. Elevated [Mg21] does not appear to alterthe force-pCa curve by direct competition with Ca21 forbinding to TnC because sites I and II have very low affinityfor Mg21 (371), and Mg21 does not shift the fluorescenceof a probe on TnC incorporated into the thin filamentupon Ca21 binding to these sites (513, 520). However,Mg21 may modify interactions within the thin filamentregulatory proteins (513). Decreases in sarcomere lengthand increases in lattice spacing decrease Ca21 binding incardiac (196) but not skeletal muscle (124, 357) eventhough in skeletal muscle, Ca21 sensitivity still varies withsarcomere length. Thus some of these effects are ex-plained by changes in Ca21 binding, but others apparentlyoperate through different mechanisms. Increases in sar-comere length, decreases in lattice spacing, and phos-phorylation of the myosin RLC (272) all either decrease
TABLE 2. Factors increasing Ca21 sensitivity
of activation of contraction
Factor
Acting Through
Enhanced Ca21
bindingEnhanced myosin
binding
Increased pH XDecreased [Mg21] XIncreased sarcomere length X (in CM) XDecreased lattice spacing X (in CM) XDecreased ionic strength X XDecreased [K1] XDecreased [Pi] XMRLC phosphorylation XDecreased [MgATP] ? XIncreased [MgADP] ? X
CM, cardiac muscle; MRLC, myosin regulatory light chain.
April 2000 MUSCLE REGULATION 875

the distance between the filaments or decrease the dis-tance from the myosin S1 to actin. Decreases in interfila-ment distances could affect the Donnan potentials at thesurfaces of the filaments and possibly the effective [Ca21]because of the 21 charge on Ca21 (96). This does notseem to be important in the skinned fiber measurementsbecause the fluorescence of a probe on TnC sensitive toCa21 binding, exchanged into skinned skeletal musclefibers, demonstrates the same fluorescence-pCa relation-ship at long and short sarcomere lengths (shorter andlonger interfilament separations) (163). The sarcomerelength, lattice spacing, and myosin RLC phosphorylationeffects on Ca21 sensitivity would more likely result froman increased effective myosin [S1], increased S1 bindingto actin, and force production for a given level of Ca21
binding. Similarly, decreases in ionic strength, MgATP,and Pi; increases in MgADP; or substituting 2-deoxy-ATP(dATP) for ATP (383) will also increase the myosin bind-ing to actin and thus the cross-bridge attachment andforce for a given level of activation. In the case of dATP,both force and stiffness are enhanced at all submaximalpCa for skeletal and cardiac muscle, but only in cardiacmuscle are the maximum force and stiffness enhanced(384). This suggests an additional action of strongcross-bridge attachment accompanying enhanced Ca21
sensitivity.Another effect of increases in strong cross-bridge
attachment will be to increase the activating effect ofstrongly attached cross bridges within the individual reg-ulatory unit and in neighboring regulatory units. This isdescribed in section IIIB and later under factors that con-trol nH. This increased activation by strongly attachedcross bridges will result in additional cross bridges at-tached for a given Ca21 concentration, an apparent in-crease in Ca21 sensitivity. Thus changes in Ca21 sensitiv-ity can be understood in terms of changes either in Ca21
binding and the resulting increase in the open probabilityof actin for myosin binding or in the binding of myosin S1to actin at a given level of Ca21 activation of the thinfilament.
In addition to direct effects on TnC Ca21 binding ormyosin-actin interaction, Ca21 sensitivity can be modifiedthrough changes in the other Tn subunits. Phosphoryla-tion of TnI in cardiac muscle at the two NH2-terminal Sersites (Ser-22 and -23) decreases Ca21 sensitivity by de-creasing Ca21 binding to the Tn complex (395) throughmodulation of the TnC-TnI interaction (278). Changes inTnT also have major effects on Ca21 sensitivity duringdevelopment or in pathological conditions such as FHC asdiscussed in the TnT part of section IIA. Because of TnT’srole as a glue holding the regulatory complex together(interacting with Tm, TnI, and TnC), it is not surprisingthat changes in TnT will affect the ease of movement Tmin response to Ca21 binding and to myosin cross-bridgeattachment.
B) THE STEEPNESS OF THE FORCE-PCA RELATIONSHIP. Thesteepness of the force-pCa relationship (nH) provides in-formation about the departure from independent activa-tion of the force-generating units or about the interactionbetween units in the thin filament. This will be termedcooperative activation of the thin filament. Skinned skel-etal and cardiac muscle measurements have yielded nH
values as large as 6 (23, 25), implying great cooperativity.In addition, these values may underestimate actual/realcooperativity due to sarcomere shortening or the meth-ods used to average the data. Possible sources for coop-erativity include the following: 1) coupling between Ca21
binding at the two NH2-terminal sites on skeletal TnC; 2)coupling between Ca21 binding sites along the thin fila-ment; 3) cross bridge-induced increase in Ca21 binding inthat regulatory unit or neighboring regulatory units; and4) cross bridge-induced movement of Tm to activate thethin filament in a direct or allosteric manner in that reg-ulatory unit and neighboring regulatory units.
The first possible source of cooperativity involvesCa21 binding to each TnC. In fast skeletal muscle, wherethere are two NH2-terminal low-affinity Ca21 bindingsites, nH could be as great as 1.2 (155) due to this coop-erativity, and less in cardiac muscle whose TnC has onlyone functional Ca21 trigger site. However, nH is verymuch greater than 1.2 in both skeletal and cardiac muscleso that this is not the major source of cooperativity forskeletal muscle. Substitution of skeletal TnC for cardiacTnC in cardiac preparations increases nH (11), supportingthe idea that some cooperativity exists within the NH2-terminal sites of skeletal TnC.
A second source of cooperativity is coupling betweenCa21 binding sites along the thin filament, i.e., calciumbinding to one site enhances Ca21 binding to adjacent Tnsites along the thin filament. This coupling could occurthrough head-to-tail interactions between neighboringTm. Fluorescent probe studies have demonstrated thatthere is some cooperativity in Ca21 binding along the thinfilament (nH of up to 1.5) (155, 163, 459), but this is notsufficient to completely account for the high nH values.Partial extraction of TnC greatly decreases nH (26, 334),implying that cooperativity along the thin filament is im-portant, but this does not necessarily indicate that thiscooperativity is through Ca21 binding. Extracting TnCleaves TnI attached to actin and the remaining Tn-Tmcomplex in place. Brandt et al. (26) suggested that sincethey could see changes in nH with as little as one TnCextracted per thin filament, there was cooperative inter-action of all the Tn along the thin filament. In contrast, theresults of Moss et al. (332) suggest that the activating unitis about three A7TmTn units long, since maximum Ca21-independent tension was achieved when only one-third ofthe Tn along the whole thin filament was extracted. Thisis a somewhat artificial estimate, since removing Tn af-fects both Tm binding to actin and its interactions in the
876 GORDON, HOMSHER, AND REGNIER Volume 80

thin filament (see sect. IIA). Further evidence of the co-operativity between neighboring Tn comes from the stud-ies of Tobacman and co-workers (46). They measured theATPase activity of S1 using maximally Ca21-activatedcardiac thin filaments made from mixtures of the wild-type cTnC and a cardiac TnC mutant (CMBII) (375) lack-ing Ca21 binding at the single NH2-terminal Ca21 triggersite. The ATPase for a fixed S1-to-actin ratio increased ina curvilinear (quadratic) manner with the fraction of func-tional Ca21 binding units at high [Ca21]. They interpretedthis to mean that adjacent TnC must bind Ca21 to allow S1to bind and hydrolyze ATP in the presence of these reg-ulated actin filaments. Moss et al. (334) observed a similarcurvilinear relationship between total TnC and force dur-ing maximal Ca21 activation in skinned skeletal fiberswith partially extracted TnC. However, in that study, theTnI-TnT subunits of Tn were still present along the thinfilament, which could affect the cooperativity. Recently,Regnier et al. (387) and Morris et al. (328) extracted nativeTnC from skinned skeletal fibers and replaced it withmixtures of native TnC and a mutant TnC unable to bindCa21 to the regulatory site(s). They found the relationshipbetween force and number of activatable units (i.e., thefraction of native TnC in the mixture of native and mutantTnC) at high Ca21 is approximately linear. It is not clearwhether the difference between this linear relationshipand the curvilinear relationship of Butters et al. (46) isdue to the differences between skeletal and cardiac thinfilament regulation or to differences in the experimentaltechniques used. The implication is that Ca21 binding toadjacent Tn sites may be more important for completeactivation in cardiac muscle than in skeletal muscle.
A third possible source of cooperativity is the effectof strongly bound cross bridges, acting through Tm andTn, to increase Ca21 binding to the thin filament andthereby increase activation. There is good evidence thatthis occurs in the presence of rigor cross bridges in bothskeletal (32, 50, 122) and cardiac muscle (196). However,the important physiological question is whether stronglybound, cycling cross bridges affect Ca21 binding to TnC.There is good evidence that Ca21 binding to TnC is in-creased by strongly bound, cycling cross bridges in car-diac muscle (196), but not in actively contracting skeletalmuscle (124). In cardiac muscle, Tobacman and co-work-ers (46) suggested that Ca21 binding to one TnC facilitatesthe binding of S1 to an actin in the same unit, which inturn facilitates Ca21 binding to the next TnC to “turn on”that unit. There is no compelling evidence for such aphenomenon in skeletal muscle, but there is some evi-dence that cycling cross bridges may affect the TnC struc-ture in skeletal thin filaments (4, 163, 275), although thiseffect is probably small (292). Thus this form of cooper-ative activation may be important in cardiac muscle butmost likely does not play a large role in skeletal muscle
activation. This point is considered again in section IIIC9
on shortening-induced deactivation.A fourth possible source of cooperativity is a direct
activation of the thin filament by strongly attached crossbridges, independent of a change in Ca21 binding. Asdiscussed in section IV, strongly attached cross bridgesmay enhance the binding of cross bridges to that unit orto neighboring actin-Tm units in an allosteric or a gradedmanner. This mechanism may facilitate additional cross-bridge attachment subsequent to Ca21 binding to Tn andTm movement (see sect. IIIA). Additional discussion ofactivation by strong cross-bridge binding is found in sec-tion IIIC6. Rigor cross bridges activate skinned skeletalfibers (393) and skinned skeletal and cardiac muscle prep-arations (307). Furthermore, strongly bound noncyclingcross-bridge attachments (NEM-S1) can activate the ATP-ase activity of S1 and regulated thin filament in biochem-ical preparations (20, 500) and enhance the Ca21 sensitiv-ity of contraction in skinned muscle preparations (429)(see sect. IIIB1). In the presence of NEM-S1, the nH forCa21 activation is greatly diminished, decreasing the co-operative feedback of the endogenous cross bridges(429). However, under physiological conditions, the rigorstate is short lived, and noncycling cross bridges do notexist. The important question, therefore, is can cross-bridge states, other than the rigor state, cooperativelyactivate muscle? As discussed in section IIIC6, crossbridges attached in the AMzADP state may activate thethin filament. As discussed in section IIIB, cycling crossbridges increase the S1 ATPase of Ca21-activated isolatedthin filaments (155) (Fig. 5). In addition, the nH for theCa21 dependence of the S1 ATPase with regulated fila-ments is greater than that for TnC structural changes(155). In skinned fibers, the nH for force is much greaterthan the nH for TnC structural changes [fluorescentprobes (4, 163, 292)]. Both results indicate that there isgreater cooperativity in activation than can be accountedfor from Ca21 binding to TnC. Thus the cooperativitymust occur by a mechanism other than enhanced Ca21
binding. Furthermore, procedures that decrease strongcross-bridge attachment and force, such as treatmentwith 2,3-butanedione-2-monoxime (BDM), N-phenylmale-imide (NPM), or mutant TnC species with low Ca21 bind-ing, decrease Ca21 sensitivity and nH. Thus cooperativeactivation by cycling cross bridges contributes to activa-tion in both skeletal and cardiac muscle but may be moresignificant in cardiac muscle because the activation due toCa21 binding may be less complete, as discussed in sec-tion IVA.
The above discussion has focused primarily on datafrom skinned fibers, where the [Ca21] is controlled withbuffers and the steady-state force-pCa relationship mea-sured. The steady-state force-pCa relationship has alsobeen measured in intact cardiac and skeletal muscle prep-arations, monitoring intracellular [Ca21] with Ca21 indi-
April 2000 MUSCLE REGULATION 877

cators and achieving relatively steady electrical activationusing a variety of techniques. This was first accomplishedin cardiac muscle (515), yielding high nH values oftengreater than those observed in otherwise similar skinnedmuscle preparation (131). In these studies, there was nocontrol of sarcomere length or lattice spacing so thatshortening during activation could influence the observedCa21 sensitivity (see discussion in section IIIC9). Similarlylarge nH values have been obtained for intact skeletalmuscle fibers (324, 493) equal to the highest reportedvalues for skinned skeletal muscle fibers. There are un-certainties about the measurements in intact fibers suchas the calibration of the intracellular Ca21 indicators, theassumptions of a steady state of force and [Ca21], and theeffects of sarcomere shortening on Ca21 sensitivity. Nev-ertheless, it is clear that cooperative mechanisms of Ca21
activation are also present in intact skeletal and cardiacmuscle cells.
In summary, cooperative activation varies with mus-cle type and is likely related to differences in regulatoryprotein isoforms and myosins. In cardiac muscle, theevidence shows that cooperative, increased Ca21 bindingcaused by myosin attachment to the thin filament is im-portant for thin filament activation. This cooperativitymay be through the mechanism suggested by Tobacmanand co-workers (46), whereby the binding of Ca21 to acardiac TnC allows myosin attachment in one regulatoryunit which, in turn, increases Ca21 affinity of the nearestTnC along the thin filament. In skeletal muscle, coopera-tive activation appears to be more through the stronglyattached, cycling cross bridges in one regulatory unit,through Tm movement or an allosteric mechanism, in-creasing cross-bridge attachment in neighboring unitswithout significantly enhancing Ca21 binding to TnC. Forboth cardiac and skeletal muscle however, Ca21 binds toTnC to begin the activation process and allow cross-bridge attachment. These strongly attached cross bridgescan enhance activation through additional movement ofTm to open up more strong myosin binding sites on actinor through an allosteric mechanism (see section IV onmodeling). This gives rise to the steep force-pCa relation-ship. Thus the measured force-pCa relationships in skel-etal and cardiac muscle support this two-step activationscheme proposed by Vibert et al. (475) and McKillop andGeeves (303).
2. Control of force redevelopment:
force-kTR relationship
In the previous section we concluded that Ca21 bind-ing to TnC, through Tn-Tm, controlled steady-state forceby controlling the initial cross-bridge attachment. More-over, strong cross-bridge binding promotes additionalcross-bridge binding in skeletal muscle and possibly car-diac muscle. Strong cross-bridge binding does strengthen
Ca21 binding in cardiac muscle, providing further activa-tion. As discussed above, an important, unanswered ques-tion is whether control of contraction occurs via regula-tion of the number of force-generating cross bridges orthe kinetics of the transitions between cross-bridgestates. To gain additional insight, the effects of [Ca21] oncross-bridge kinetics must be measured. In this sectionwe discuss Ca21 control of the pre-steady-state kinetics offorce. In subsequent sections, we consider other physio-logical measures of contractile regulation.
In 1986, Brenner and Eisenberg (40) developed aclever mechanical procedure for estimating the rate con-stant of cross-bridge attachment and force generation. Inthis technique, a single skinned muscle fiber, generatingsteady-state isometric force at a particular pCa, is allowedto shorten for a brief period under unloaded conditions.The muscle fiber is then restretched to its original sarco-mere length (see Fig. 6A). In this technique, force alwaysredevelops at the same length, eliminating any sarcomerelength dependence of force production. It is assumed thatthe unloaded shortening and the rapid restretch detachesmost of the strongly bound cross bridges, forcing mostcross bridges into weakly bound states. After the rapidrestretch of the fiber, the rate of force redevelopmentmonitors the approach to the isometric steady-state dis-tribution of force-generating cross bridges (35). The as-sumption that the period of rapid shortening detachesattached cross bridges is justified by the greatly reducedstiffness and I1,1/I1,0 X-ray equatorial reflection ratio (33)observed during this maneuver. A further reduction in thenumber of strongly attached cross bridges during therapid restretch was also suggested by the fact that theforce and stiffness immediately after restretch often fallto very low values. However, in many cases, the forceimmediately following the restretch does not fall to theresting level but has a value of up to 50% of isometricforce (see Fig. 6A) (45). This value may represent theforce produced by the stretch of cross bridges that rapidlyreattach just before the restretch. Finally, it is assumedthat the release and restretch do not affect thin filamentactivation. Evidence supporting this assumption comesfrom fluorescent probe studies of TnI incorporated intoskinned skeletal muscle fibers (41). This is discussedfurther in section IIIC9 on shortening-induced deactiva-tion.
After the shortening/restretch procedure, force risesfollowing a single exponential time course to ;95% of theisometric force when the sarcomere length of the centralpart of the fiber is held constant by length feedback (34,309). The rate constant for tension redevelopment istermed kTR. In studies without sarcomere length control(63, 429), kTR is reduced somewhat (;30%), and there isoften a small departure from a single exponential at high[Ca21]. In the latter case, a rate constant is calculatedfrom the time to redevelop one-half of the maximum force
878 GORDON, HOMSHER, AND REGNIER Volume 80

(63, 309). Brenner (34) showed that the kTR- pCa relation-ship is usually shifted to the right of the force-pCa rela-tionship, indicating a difference in the Ca21 sensitivity ofthe steady-state force and kTR. kTR is often plotted as afunction of the steady-state force at each [Ca21] (see Fig.6B), to isolate control of kTR through the number offorce-generating cross bridges (presumably proportionalto force) from control of the kinetics of cross-bridgecycling. The shape of the kTR-force relationship and themaximum kTR rate vary greatly among different muscles.When the maximum kTR is plotted against the unloadedshortening velocity (Vu) for a number of muscles anddifferent conditions, there is a roughly linear relationship
over a 10-fold range of shortening velocities (see Fig. 6C).Given the linear relationship between shortening velocityand actomyosin ATPase (15), this result implies that kTR isdirectly related to the steady-state cross-bridge turnoverrate. Increasing the cross-bridge cycling rate by substitut-ing 2-deoxy-ATP for ATP as the contractile substrate in-creases the maximum kTR, while decreasing the cyclingrate by lowering the [ATP] decreases the maximum kTR
(383). In faster mammalian skeletal fibers, kTR increasesup to 15 times with increasing force ([Ca21]) and is con-caved upward (see Fig. 6B). It was shown first by Brenner(35) that, in rabbit psoas skinned muscle fibers, the kTR-force relationship is highly curvilinear, increasing from
FIG. 6. Measurement of the rate constant for force redevelopment (kTR) (A), as a function of the steady-state forcelevel (B), and the correlation of maximum kTR and maximum unloaded shortening velocity for different muscles anddifferent conditions (C). A: protocol for measuring kTR, a plot of force vs. time, is shown. After force has reached a steadystate at different pCa values (indicated on right), the fiber is shortened by ;20% of the fiber length (Lf) at a rate of 4Lf /sfor ;50 ms and restretched to the original length. The rate constant for force redevelopment is estimated from the timeto half-maximum redeveloped force. Note how the rate of force redevelopment slows with increased pCa (decreasedCa21). B: rate constant kTR, determined as in A as the average of a number of fibers, is plotted as a function of the relativesteady-state force at each pCa. Note the lack of change in kTR with increased force at low force (low Ca21) and the rapidincrease with elevated Ca21 for forces .50% of maximum. C: maximum kTR (s21) is plotted as a function of maximumunloaded shortening velocity [in muscle lengths (ML)/s for different muscles and different conditions]. Triangles are datafrom rabbit or rat soleus muscles; circles are data from rabbit psoas muscles; open circle is datum from psoas at 10°Cin deoxy-ATP (381). Unloaded shortening velocity (Vu) was computed from values measured at 10 or 15°C in soleus andpsoas muscles and extrapolated to 5, 10, 15, or 20°C using a Q10 of 2.5 (503). The solid line is a least-squares linearregression line to the data (r2 5 0.93). [Data from Brenner (35), Metzger and Moss (310, 311), Millar and Homsher (316),and Regnier and Homsher (381).]
April 2000 MUSCLE REGULATION 879

;1–1.5 s21 at low force to 4–5 s21 at maximum force at5°C, and increasing from ;2 s21 to 15 s21 at 15°C. Sub-sequently, this same sort of curvilinear relationship wasseen by other workers in the same rabbit psoas fibers(309, 437), in mammalian slow-twitch (type I) fibers (310),in frog myofibrils (23), and in cardiac trabeculae (344,504). The main differences are in the absolute magnitudeof the minimum and maximum rates and the shape of thekTR-force relationship. In slower mammalian skeletal fi-bers, maximal kTR is slower and the increase in kTR withincreasing force is much less (310). In cardiac cells, kTR
increases less with [Ca21] than in skeletal muscles (166,344, 504). Additionally, increasing [Pi] reduces force butincreases kTR in skeletal muscle (386, 476), but depressesboth force and kTR in cardiac muscle (448). Thus therelationship is a complex one, but important because itgives information about the pre-steady-state kinetics.
In his initial papers on kTR measurements, Brenner(see Ref. 36 for an excellent review of this subject) inter-preted force redevelopment following the release andrestretch of muscle as the transition of cross bridges fromthe weak to the strongly bound force-generating state.Because cross bridges can attach weakly to the thin fila-ment in the absence of Ca21, Brenner et al. (42) con-cluded (as discussed in sect. IIIA) that the original stericblocking mechanism (172, 219, 347) is not strictly correct.Furthermore, although only 5–10% of the cross bridgesare weakly attached at any one time at the ionic strengthconditions existing in resting muscle cells (42, 404, 405),all the cross bridges could potentially make the weak tostrong transition to generate force (since they all wouldbe in rapid equilibrium with the weak-binding state). ThusBrenner (35) suggested that Ca21 controls this transitionbetween the weak and strongly bound states. To describethe transition from the weakly attached to strongly at-tached, force-exerting state, Brenner (35) used the A. F.Huxley (215) formalism as modified by Kushmerick andKrasner (259) with an attachment rate constant fapp anddetachment rate constant gapp (see actomyosin ATPasediagram, scheme 1). The subscript “apparent” is usedbecause both rate constants combine the rate constantsof a number of steps in the actin-myosin ATPase cycleinvolving attachment to actin, various actin-myosin nucle-otide states, and detachment. From measurement of theATPase rate and stiffness of the fiber as a function of pCa(and force), Brenner and Eisenberg (40) estimated theabsolute values of fapp and gapp. He concluded that gapp
was not a function of [Ca21] but that fapp was. Subsequentinvestigators using this model have come to similar con-clusions (310, 437).
The important question is what aspect of the weaklyto strongly bound transition does Ca21 control? There aretwo competing hypotheses. The one possibility is thatCa21 affects a kinetic parameter of the power stroke ofthe attached cross bridge and thereby influences the tran-
sition from weak to strong attachment. This would be atrue Ca21 regulation of cross-bridge kinetics. A second isthat Ca21 affects the kinetics of thin filament activationthrough the kinetics of Ca21 binding to Tn and the sub-sequent conformational changes in the thin filament thatallows strong cross-bridge binding with no effects on thepower stroke per se. Both are consistent with the initialstudies of Brenner (35).
In the first hypothesis, the direct effect of Ca21 on thecross-bridge power stroke could be through a Ca21 de-pendence of one of the steps in the ATPase cycle such asPi release (57). However, as discussed in section IIIC4, theforce-generating and Pi release steps exhibit little or nodependence on Ca21 (316, 386, 479). Still, a direct effecton the myosin cross-bridge could result from Ca21 bind-ing to the myosin RLC as suggested by Metzger and Moss(312). As discussed in section IIB, the RLC has a Ca21
binding site which, under resting intracellular [Mg21] con-ditions, would be saturated with Mg21. Evidence for RLCcontrol of contraction was provided by Metzger and Moss(312), who showed that removal of the RLC results in asmall enhancement of kTR, at any level of Ca21 activation.These studies imply that the RLC influences the magni-tude of kTR but probably not its Ca21 dependence. ThatCa21 binding to the RLC is not necessary for maximumkTR is demonstrated by the observation that a maximumkTR can be achieved even in the absence of Ca21 when anoxidized cardiac TnC (aTnC) is substituted for the nativesTnC (167). aTnC activates skeletal muscle in the absenceof Ca21 when substituted for native sTnC. This demon-strates that the Ca21 activation of kTR is through the thinfilament, not the myosin directly (63). Finally, in vitromotility measurements of the sliding speed of unregulatedF-actin filaments, propelled by rabbit skeletal HMM with afull complement of light chains, show that the speed isindependent of Ca21 (148, 206). These observations sug-gest that Ca21 is not directly regulating myosin in verte-brate skeletal and cardiac muscle.
The second hypothesis is that the Ca21 or forcedependence of kTR is exerted through effects on thinfilament activation. This hypothesis was first stated ex-plicitly by Landesberg and Seidman (261) but was impliedearlier by Brenner (35). Landesberg and Seidman (261)(see sect. IV) showed that a nonlinear kTR-force curve (34)(Fig. 6B) could be obtained using a simple model thatcouples the kinetics of Ca21 binding to TnC to the kineticsof cross-bridge transitions between weakly and stronglybound, force-generating states (see Fig. 8). The rate con-stants for the cross-bridge transitions, fapp and gapp, donot depend on Ca21 but must be the same order ofmagnitude or much smaller than the rate of thin filamentactivation. The rate of Ca21 binding to the thin filament ishigh at saturating Ca21, but low at low [Ca21], since thebinding rate is proportional to [Ca21]. Furthermore, evenif the binding rate is high, in this model Ca21 binding to
880 GORDON, HOMSHER, AND REGNIER Volume 80

TnC regulates the rate of transition into the force-gener-ating state because the overall rate of force developmentis a product of the probability of the thin filament beingactivated {[Ca21]*Keq/(1 2 [Ca21]*Keq) in a simple modelwith a single Ca21 binding site} and fapp (see Hancock etal., Ref. 165). Support for this kind of model is convincingand comes from experiments (shown in Fig. 8B and de-scribed also in sect. IV) in which the kinetics and affinityof Ca21 binding to TnC are changed using drugs or TnCisoform substitution. Calmidazolium, which decreases therate of Ca21 dissociation from TnC [3- to 4-fold in solution(234)], has no effect on maximal kTR but increases kTR atlow levels of Ca21 (385), as predicted by this model (383).Substituting aTnC (for native TnC) gives the maximumkTR independent of Ca21 (63). Substitution of an NH2-terminal deletion mutant of skeletal TnC, which has agreatly increased rate of Ca21 dissociation from TnC [2- to3-fold in solution for native TnC (392)], shifts the force-pCa by ;0.55–0.77 pCa units and causes similar shifts inthe kTR-pCa relationship (58, 388) but has little or noeffect on the kTR-force relationship. These studies indi-cate that Ca21 activation of the thin filament can be amajor factor determining the kTR-force relationship, alongwith the kinetics of the cross-bridge attachment/detach-ment.
In recent studies, Moss and co-workers (301) haveshown that changes in sarcomere length shift the kTR-force relationship such that during submaximal activationkTR is elevated at longer sarcomere lengths. McDonald etal. (301) demonstrated that most of this effect was due tothe decrease in filament lattice spacing that accompaniedthe increased sarcomere length. The force for submaxi-mal Ca21 is also enhanced by decreasing the distance thatthe myosin head has to reach out to attach to actin (294,300). Both of these results could be explained by anincrease in fapp as the effective myosin concentration nearactin binding sites is increased.
If the kTR-force relationship is determined by theCa21 activation of the thin filament along with the kineticsof cross-bridge attachment/detachment, we need to ques-tion what role cooperative thin filament activation bystrongly attached cross bridges. The basic shape of thiscurve for skeletal muscle concaved upward, increasingsteeply with force (and thus strong cross-bridge attach-ment) for forces .60% open probability (Po) (Fig. 6B)suggests that cooperative activation depending on force/cross-bridge attachment may be important. The Landes-berg and Seidman (261) model shows that this shape canbe achieved without cooperative activation by stronglyattached cross bridges, but does not rule it out. If it werepresent, it would involve activation of the thin filament bystrongly attached cross bridges acting through movementof Tm (sect. IIIA) or allosterically through actin, whichwould facilitate attachment of additional cross bridgeseither in that regulatory unit or neighboring units (see
sect. IIIC1B). Perhaps when enough force-generating crossbridges are attached, the rate of strong attachment byadditional cross bridges would be increased. Such posi-tive feedback cannot be invoked where the curve is flat(165) or even sloped in the opposite direction in high Pi incardiac muscle (448). The most compelling evidence thatactivation by strongly attached cross bridges can play arole in the kTR-force relationship is the data of Swartz andMoss (429) who showed that adding noncycling stronglyattached cross bridges (NEM-S1) can enhance the kTR tothe maximum value at low levels of Ca21. At these levelsof NEM-S1, there was neither activation of force in theabsence of Ca21 nor decreased force at maximal Ca21.However, this result does not establish a role for cyclingcross bridges in determining the kTR-force relationship inunmodified skinned fibers.
If strongly attached cross bridges contribute to en-hancing the redevelopment of force, the cooperativitymust be within a regulatory unit and not due to interac-tions between neighboring units. Metzger and Moss (311)partially extracted the TnC to decrease the effect ofstrongly attached cross bridges to activate neighboringunits (28, 307; see sects. IIIC1 and IIIC6). They found thatpartial extraction of TnC, which decreased greatly the HillnH slope of the force-pCa relationship and the maximalforce, had no effect on either maximum or submaximumkTR. These studies have been extended, in skinned fastskeletal fibers reconstituted with the mixtures of cardiacTnC and the cardiac TnC mutant lacking Ca21 binding atsite II (CMBII) (328); the results show that maximum kTR
was little affected by mixtures that reduced maximumforce to 20% of control maximum force (Fmax). Similarstudies in skeletal muscle fibers gave similar results. Mix-tures of native/wild-type sTnC and mutant sTnC (deficientin Ca21 binding to sites I and II) were reconstituted intoskinned skeletal muscle fibers and found to affect maxi-mal kTR little, even at 10% of control Fmax (387).
Further evidence that there does not need to becooperative activation between neighboring units comesfrom the aTnC studies (oxidized cTnC which activates inthe absence of Ca21; see above) (63). Full activation ofkTR was achieved by extraction of native TnC and substi-tution with sufficient aTnC to activate only 20% of themaximum force in the absence of Ca21. This implied thatneighboring regulatory units did not have to be activatedto achieve maximum kTR. Of course, activation by aTnC isdifferent from that achieved by normal Ca21 binding toTnC in that Ca21 binding is reversible and the structuralchange from Ca21 binding may not be complete.
Therefore, it seems that the kTR-force relationship isnot determined by cooperative activation between neigh-boring thin filament regulatory units, and thus the thinfilament unit controlling kTR cannot be much larger thanthe basic A7TmTn regulatory unit.
The role of cooperative activation within a regulatory
April 2000 MUSCLE REGULATION 881

unit remains unclear; however, evidence from the kTR
measurement itself (34) suggests that cooperative activa-tion by strongly attached cross bridges, even within oneregulatory unit, is not required for redevelopment offorce. Because the release-restretch procedure shouldleave few strongly attached cross bridges, if they requiredfor cooperative activation, then a few strongly attachedcross bridges must form before others could attach. Thiswould result in a lag in the rise of force during redevel-opment. However, such a lag has not been observed.
In conclusion, the dependence of kTR, the rate con-stant for force redevelopment after the shortening andrestretch protocol, on activation and force is best ex-plained at this time by changes in activation of the thinfilament and changes in the rate of strong cross-bridgeattachment as a result of additional thin filament activa-tion. There is probably little influence of cooperativitybetween regulatory units along the thin filament on kTR,but possibly some influence of cooperative activation bystrongly attached cross bridges within each regulatoryunit.
3. Pre-steady-state kinetics of Ca21 activation (kCa)
Another technique for investigating pre-steady-statecross-bridge kinetics is the use of rapid step changes in[Ca21]. In intact fibers, electrical stimulation causes rapidCa21 release from the sarcoplasmic reticulum across thewhole muscle cell because of the rapid inward conduc-tion of the electrical signal by the muscle transversetubule system. This produces nearly synchronous releaseof Ca21 across the muscle cell. A technique to achieverapid, synchronous activation in skinned fibers is therapid release of Ca21 from a chelator whose affinity forCa21 declines greatly upon photolysis, a so-called cagedCa21. The chelator should be selective for binding Ca21
over Mg21, with a large change in Ca21 affinity uponphotolysis elevating [Ca21] from 1027 to 1025 M in the fi-ber within fractions of a millisecond. Several compoundssatisfy these criteria with variable success. The firstgroup, the Nitr series, developed by Tsien and colleagues(1), is 1,2-bis(2-aminophenoxy)ethane-N,N,N9,N9-tetraace-tic acid-related compounds. They are highly selective forCa21 over Mg21 but have low quantum efficiency so thatrelatively intense radiation is required to release sufficientCa21 for maximal activation. The second calcium chela-tor, DM Nitrophen (an EDTA derivative), developed byKaplan and Ellis-Davies (242), has a high affinity for bothCa21 and Mg21, a higher quantum efficiency, and under-goes a large affinity change upon photolysis. The third,nitrophenyl EGTA (NP EGTA), also developed by Ellis-Davies and Kaplan (98, 99), selectively binds Ca21 overMg21, has a higher quantum efficiency, and has a largechange in affinity upon photolysis. However, because un-photolyzed NP EGTA has such a high affinity for Ca21,
any unphotolyzed NP EGTA after photolysis binds Ca21
that has just been released. This produces a transientspike in [Ca21] upon photolysis of some of NP EGTAcomplexed with Ca21 rather than the desired step in-crease. This behavior must be considered in interpretingthe results (99).
Several studies used these compounds to investigatethe pre-steady-state kinetics of regulation of contractionby Ca21. These include studies by Wahr and Rall (478),Araujo and Walker (7), Ashley et al. (10) and Patel et al.(356) in skeletal muscle, and by Araujo and Walker (7)and Palmer and Kentish (344) in cardiac muscle. Wahrand Rall’s (478) study in skinned frog skeletal muscle(478) using DM Nitrophen and Nitr-5 is the most compre-hensive. They found that the rise in force resulting fromlaser photolytic release of Ca21 could be described by twoexponentials, a fast initial rise followed by a slower ap-proach to the maximum isometric force. The fast risealmost matched the rapid rise in an intact fiber stimulatedelectrically at the same temperature. The slower rise maystem from sarcomeric nonuniformities in the fiber, as theexperiments did not use sarcomere length control, but itcould also be a property of activation. The slow rise is notseen in the intact fiber and was not caused by the partic-ular caged compound, as it was seen with both Nitr-5 andDM Nitrophen. We will call the fast rate constant kCa.Their measured kCa increased with increasing final [Ca21]and increasing final steady-state force and produced akCa-force relationship similar to the kTR-force relationshipshown for skinned rabbit skeletal muscle in Figure 6B.The value of kCa at maximal Ca21 activation did notdepend on the initial force level and varied by increasingthe preflash [Ca21] or by decreasing force to ,40% ofmaximum by either partial extraction of TnC or pretreat-ing the fiber with 0.2 mM vanadate during activation.
In similar experiments using rabbit psoas skinnedskeletal muscle fibers, Araujo and Walker (7) observed asingle rate constant for the force increase after flashactivation in Nitr-5, Nitr-7, or NP EGTA. Working at ahigher temperature (15°C rather than 10°C), they found asimilar increasing relationship between the final steadyforce and kCa, with kCa increasing from ;1 to 16 s21 fromlow to maximum force. Measurements of kTR under sim-ilar conditions showed an increase from ;2 to 22 s21 overthe same force range (309). Thus the kTR and kCa valueswere similar under comparable conditions. BecauseAraujo and Walker’s equipment (7) was limited to releas-ing only enough Ca21 to partially activate the fiber, it wasnecessary to photolyze caged Ca21 in solutions of ele-vated [Ca21] to achieve final forces over the full range offiber activation. For final Ca21 levels that gave maximumforce, they observed that the kCa had little dependence onthe initial force level. Unfortunately, their method of vary-ing the initial force level results in final Ca21 levels thatalso vary so that while final force was maximum for all
882 GORDON, HOMSHER, AND REGNIER Volume 80

Ca21 activations, the final Ca21 was not. Wahr and Rall(478) found that kCa did not depend on the initial force, onthe number of force-generating cross bridges attached atthe time of activation, or on the final force, but only on thefinal [Ca21]. This suggested that they were studying therate constant of Ca21 activation of the thin filament. Patelet al. (356) also found that kCa did not depend on the finalforce when force was varied by TnC extraction, but kCa
did depend on the final [Ca21]. Furthermore, Araujo andWalker (7) and Patel et al. (356) found that a decrease in[Mg21] increased the kCa, more at low-intermediate forcelevels and less at higher force. The origin of the Mg21
effect was unclear and could be due to an effect on TnC,on Tm binding, or on the myosin RLC. Patel et al. (356)attributed the effect to the RLC because partial extractionof the RLC decreased the Ca21 dependence of the kCa. Asdiscussed in section IIIC2 and later in section IV, the RLCextraction may directly affect cross-bridge kinetics, andnot Ca21 regulation. Ashley et al. (10) also found that kCa
(they actually measured the half-life) increased with thefinal [Ca21] and final steady force in frog skeletal muscle,but unlike the data of Wahr and Rall (478) and Araujo andWalker (7), they found that kCa saturated at higher [Ca21].This difference may be due to variations in data analysisor experimental conditions.
The studies in cardiac muscle gave similar results.Araujo and Walker (7) observed that kCa in skinned ratventricular myocytes increased from 1 to 4 s21 as forcewas increased from low to near maximum. This is slightlylower than the 4–10 s21 range of kTR values reported bythe same laboratory under comparable conditions forskinned rat ventricular trabeculae (504). As in their skel-etal muscle data, kCa was independent of the initial forcelevel. Palmer and Kentish (344) measured kCa, kTR, andthe rate constant for relaxation (sect. IIIC8) in both rat andguinea pig cardiac muscle. Rat cardiac muscle has muchfaster cross-bridge kinetics than guinea pig cardiac mus-cle (473), but the Ca21 sensitivity is similar (344). In bothpreparations, kCa increased with increasing [Ca21] andfinal force, and maximum kCa at 22°C was about the sameas the maximum kTR and rate of relaxation for that tissue.In the case of guinea pig myocardium, the kTR varied lesswith force than for rat myocardium. The relative values ofmaximum kCa, kTR, and rate constant of relaxation for thetwo muscles varied with the actomyosin ATPase rate ofthe myosin from that tissue, suggesting that these maxi-mum rates are determined by the cross-bridge cyclingrates.
The conclusions of these studies are that the rateconstants for kCa and relaxation vary with the final [Ca21]and vary little with the initial or final force level per se.Thus there is little evidence that strongly attached crossbridges play any role in the kinetics of Ca21 activationmeasured by kCa. However, although the slower rate ob-served by Wahr and Rall (478) at higher force levels might
be due to nonuniformities of sarcomeres during contrac-tion, it could also be caused by cooperative activation bystrong attached cross bridges. However, there is littleevidence that interactions between neighboring regula-tory units play an important role in activation, as extrac-tion of TnC had little effect on the maximal rate (356,478); thus any cooperativity would need to be within aregulatory unit. The TnC extraction experiments need tobe expanded to include substitution of TnC lacking NH2-terminal Ca21 binding to strengthen these conclusions.Thus the kinetics of activation, measured from the pri-mary rate constant kCa, appear to be dominated by acti-vation through Ca21 binding to TnC.
The observation that the kCa does not appear tosaturate (7, 478; but see Ashley et al., Ref. 10) suggeststhat it may not be determined solely by the rate of Ca21
binding to TnC and the resulting conformationalchange in TnC and interaction with TnI (see Refs. 84,85, 175, 397). The rate of the conformational change inTnC with Ca21 should saturate. However, it is not clearthat the investigators increased the final [Ca21] suffi-ciently to test for true saturation. In any case, the datafit well with the simple modeling of activation used todescribe the kTR-force relationship (165; see sect. IV)whereby Ca21 regulates thin filament activation and notcross-bridge kinetics.
The same conclusions can be drawn for the studieson cardiac muscle. Comparison of the kCa and kTR ratesindicates that the maximums are similar for the sametissue at the same temperature, but the dependence onforce may be different. The data for guinea pig cardiacmuscle show less dependence of kTR on force than kCa onforce. As shown by the modeling of Hancock et al. (165),this is possible if strong cross-bridge attachment in-creases Ca21 binding to TnC. Thus the kCa data supportthe conclusions that Ca21 regulates strong attachment ofcross bridges and has little or no direct effect on cross-bridge kinetics, and there is little cooperative activationby strongly attached cross bridges to determine the initialrate of force development.
4. Kinetics of force relaxation with step increases in
Pi (kPi)
Subsequent to MzADPzPi cross-bridge binding to ac-tin, the products of hydrolysis are released in an orderedfashion, i.e., Pi first, followed by ADP (286). Studies of thekinetics of isolated actin and myosin in solution (or sol-uble fragments of myosin, HMM or S-1) have shown thatthe release of Pi from newly formed AMzADPzPi is rapid(36 s21 at 10°C and 77 s21 at 20°C) (495) compared withthe steady-state ATPase rate (2–3 and 12–22 s21, respec-tively) (see Table 1). The release of ADP in these uncon-strained cross bridges is .200 s21 (412, 450). In solution,there is no strain on the actomyosin interaction of the
April 2000 MUSCLE REGULATION 883

AMzADPzPi complex. However, in muscles contractingunder isometric, loaded isotonic or eccentric conditions,there are relatively large positive forces applied to thecross bridge, and in this case, the release of Pi and ADPare significantly slowed. This conclusion is based on mea-surements of muscle mechanics (76, 186), the time courseof ATP hydrolysis of muscles contracting under theseconditions (110, 111), and the time course of Pi formationand release using a Pi monitoring system (176, 177) (seeTable 1). Likewise, in rapidly shortening muscle, eventhough the rate of ATP hydrolysis is faster than that seenin the isometric case, cross bridges still bear force and therate of product release is slower (,20 s21) than that seenin isolated unloaded proteins (205, 209, 258). Studies ofthe free energy change associated with product releasehave shown that a large amount of free energy is releasedwhen Pi dissociates from the AMzADPzPi system (ca. 240kJ/mol depending on the [Pi]). It was suggested that therelease of Pi is directly associated with the power strokeitself (351, 378, 379, 496). Furthermore, it has been shownin numerous studies that the force exerted by an isomet-rically contracting skeletal muscle is inhibited as the in-tracellular [Pi] increases (69, 76, 186, 246, 295, 316, 350,370). Over 10 years ago Pate and Cooke (350) reportedthat the isometric force declines linearly with the log [Pi].This result could be interpreted to mean that the powerstroke (that portion of the cross-bridge cycle that gener-ates force and/or shortening) is produced by the dissoci-ation of Pi; i.e., Pi release and force generation are syn-chronous events. In their kinetic studies, Chalovich et al.(57) found that calcium regulation of contraction could beexplained if Ca21 regulated either the rate of Pi releasefrom the AMzADPzPi complex or a rapid equilibrium stepbefore the release. This interpretation is consistent withthe fact that at 10–15°C, as the [Pi] in the fiber is in-creased, kTR increases above its value of ;15 s21 at ,1mM Pi and increases asymptotically three- to fourfold as[Pi] is increased to 30 mM (313, 386, 476).
If the release of Pi from the cross bridge is directlycoupled to the power stroke, then the photogeneration of[Pi] from caged Pi in the filament lattice during isometriccontraction should produce an exponential decline inforce whose rate should increase linearly with [Pi]. Testsof this hypothesis have shown that the rate of the tensiondecline increases as [Pi] is increased (8, 75, 315, 479).However, plots of the rate of the Pi transient, kPi
, are notlinearly dependent on [Pi] but exhibit saturation kinetics.These experiments were most simply interpreted asshowing that before the release of Pi, a cross bridgeundergoes a force-generating isomerization from a non-force exerting AMzADPzPi state to one, AM*zADPzPi, whichexerts force (75, 316). This force-generating isomerizationis followed closely by the release of Pi. A similar conclu-sion has been reached independently in sinusoidal analy-sis studies of force (244, 245) and in pressure-jump stud-
ies of contracting skeletal muscle fibers (119, 120) atvarious [Pi]. These groups suggested that during the nor-mal cross-bridge cycle, immediately after the force-gener-ating isomerization, Pi was released from the AM*zADPzPi
state in a rapid equilibrium reaction, which, because of itslarge free energy change, shifts the cross-bridge distribu-tion toward those exerting force and thus stabilizes theforce-exerting cross bridges. A simple modification of theHuxley (75) model of the cross bridge is able to accountfor the observed behavior. The rate of the force-generat-ing isomerization under isometric conditions (at 10°C) is;30 s21 at 1 mM Pi, nearly twice as fast as the kTR
measured in the same fiber under the same conditions.This implies that the two measurements are not observingthe same transition (315, 386). Luo et al. (284) offeredanother interpretation of the caged Pi studies. They ar-gued that the force-generating step should be much fasterthan that observed and correctly pointed out that signifi-cant compliance in the fiber system would slow the ob-served kPi
compared with that actually occurring at thelevel of the cross bridge. However, the behavior of thetension transient according to their hypothesis, even withthe added compliance, would exhibit multiexponentialbehavior, which was not observed. Furthermore, if Pi
release is directly coupled to force generation, isometricforce should decline linearly with log [Pi] as reported byPate and Cooke (350). If the force generation occurs in astep immediately preceding the release of Pi, the declinein force will exhibit an S-shaped relation with log [Pi]. Thedata in Millar and Homsher (315) suggest such behavior.Additional experiments are needed to resolve this ques-tion.
In slow-twitch muscle fibers, photogeneration of Pi
produces an exponential fall in force similar to that ob-served in fast skeletal muscle (316). The amplitude of thePi transient is smaller than in fast-twitch skeletal muscle(in agreement with the effects of [Pi] on force, Ref. 316),but kPi
is .10 fold slower (1 s21). The Pi release mayactually be the rate-limiting step in the ATPase cycleunder isometric contractions. This result is consistentwith the linear relationship between kPi
and [Pi] in slow-twitch muscle fibers (K 5 3,100 M21zs21), although theconcentrations of Pi used in that study were not raised toan extent to test for saturation kinetics. Similar conclu-sions were reached in sinusoidal oscillation studies inskinned slow muscle fibers (481) where the [Pi] wasraised high enough (.10 mM) to observe saturation ki-netics of the process associated with the power stroke.Comparable results have been obtained in skinned car-diac muscle cells, although kPi
is somewhat greater thanin rabbit soleus fibers (8).
If the release of Pi from the cross bridge is directlyassociated with the power stroke and is controlled by thecalcium concentration, then muscle fibers activated tovarious degrees should exhibit a Pi transient rate constant
884 GORDON, HOMSHER, AND REGNIER Volume 80

that decreases as [Ca21] is reduced. This hypothesis wastested by Millar and Homsher (315) and Regnier et al.(386) using photogeneration of [Pi] from caged Pi in iso-metrically contracting muscle fibers at various [Ca21].They found no significant change in kPi
with pCa, eventhough kTR was dramatically reduced at low [Ca21]. Thusthey concluded that Ca21 must control a step that occursbefore the force-generating isomerization. Walker et al.(479), working at a higher temperature and using aslightly different caged Pi molecule, found a small butsignificant [Ca21] dependence of kPi
(a 2.5-fold change asforce was reduced to ,10% of that observed at full acti-vation). They suggested that their kPi
data could be ex-plained by a dependence of the rate constant of theforce-generating isomerization on pCa. They too foundmuch more marked effects of pCa on kTR than on kPi
.Although the model they used accounted for the effectson kPi
, it could not explain the kTR data. Additional exper-iments by Regnier et al. (386) concluded that to accountfor the variation of kTR and kPi
as force was changed bychanges in Pi, BDM, or [Ca21], the Ca21 control mustoccur on a process before the force-generating step. Thecontrolled step presumably involves Ca21 regulation of aweakly to strongly bound cross-bridge transition. Pres-sure-jump studies (120) have also found that the tran-sients thought to be associated with Pi release are inde-pendent of pCa.
Araujo and Walker (8) have also measured kPiin
cardiac muscle. They report that as [Ca21] is reduced, kPi
falls slightly (declining by 50% when the force is reducedby 80%), implying a modulation of kPi
by [Ca21] greaterthan that seen in skeletal muscle. There are at least twopossible explanations for this difference. The first is thatindeed Ca21 may modulate the kinetics of a step in thecross-bridge cycle in cardiac muscle. It would be interest-ing to compare the modulation of kPi
with the [Ca21]dependence of kTR and kCa observed in cardiac muscleunder similar conditions. The second possibility is thatthe rate-limiting process in the cardiac cross-bridge cycleis different from that of skeletal muscle.
In summary, available data from measurements of Pi
transients, pressure-jump studies, and force oscillationmeasurements suggest that Pi release from AMzADPzPi
follows the power stroke and is coupled to it. Data fromstudies of these parameters at different [Ca21] indicatethat [Ca21] exerts either a minor effect or no effect on therate of the power stroke and Pi release step per se.
5. Regulation of unloaded shortening velocity
A) STUDIES ON INTACT MUSCLE FIBERS. I) Measurement of
unloaded shortening velocity. A measure of a muscle’sability to shorten against various loads, its shorteningvelocity (v), varies inversely with the load (P). This rela-tionship is given by Hill’s classic equation (188)
~P 1 a!v 5 b~Po 2 P!
where Po is the isometric force and a and b are constantsin units of Po and muscle lengths/s, respectively. Typicalvalues for frog skeletal muscle at 0°C are 0.25 Po and 0.33lo/s (lo is usually reported as the sarcomere length of themuscle at maximal overlap or total muscle length at max-imal sarcomere overlap). When the force against whichthe muscle shortens is zero (unloaded), the equation re-duces to
vmax 5 bPo/a
and therefore
vmax 5 1.32lo/s
where vmax is the “unloaded shortening velocity.” Toavoid confusion with enzymatic rates, we designate theunloaded shortening velocity in fibers as Vu. Originally, Vu
was measured by extrapolation to zero load from a seriesof measurements of the shortening velocities obtained atvarious afterloads (188). In 1979, Edman (91) introduceda simple method for estimating Vu. In a tetanically stim-ulated muscle fiber after isometric force reached a steadystate, he rapidly released the muscle fiber by stepping oneend toward the other a distance sufficient to reduce toforce to zero (allowing the fiber to become “slack”). Dur-ing this release, force falls to zero and, when the slack istaken up by sarcomere shortening, force begins to rise atthe new shorter isometric length. The time taken to beginforce redevelopment after the release is called the “slacktime.” The greater the distance released, the greater theslack time. Plots of the distance released against the slacktime in fully activated fibers are linear, and the slope ofthis line estimates the unloaded shortening velocity (seeFig. 7). We will call this estimate of unloaded shorteningvelocity Vo to distinguish it from Vu, measured by extrap-olation. The unloaded shortening velocity values esti-mated by the two techniques are not different (91, 239).
II) The effect of filament overlap on shortening ve-
locity. The unloaded shortening velocity is much lessaffected by the extent of thin and thick filament overlapthan is the isometric force (92, 147). When measured inintact skeletal muscle fibers within the range of sarco-mere lengths from 1.7 to 2.7 mm (where significant restingforce begins to develop in single skeletal muscle fibers),Vo is independent of sarcomere length (91). At sarcomerelengths ,1.7 mm, Vo declines (91). These results comparefavorably with measurements of Vu during controlled re-leases (147). Furthermore, Vu is independent of the extentof thick and thin filament overlap from 2.2 to 3.1 mmsarcomere lengths (91, 147). In this range, the thick and
April 2000 MUSCLE REGULATION 885

thin filament overlap ranges from 0.75 mm/half-sarcomere(100% or optimal overlap) to 0.30. This important findingdemonstrates that the unloaded shortening velocity in theintact, maximally activated muscle fiber is independent ofthe number of cross bridges pulling on the thin filament(when that number is varied from 40 to 100% of thoseavailable). Unfortunately, measurements of Vu and Vo arenot available at sarcomere lengths greater than ;3.1 mm,where filament overlap is ,40% of optimal. This is be-cause passive tension (passive recoil of elastic elements)beyond 3.1 mm is large and contributes to the apparentshortening velocity, complicating the analysis. At sarco-mere lengths ,1.7 mm, Vo decreases markedly.
III) The effect of temperature on shortening velocity.Measurement of Vu in living muscle fibers over the tem-perature range of 10–25°C has shown a Q10 of 2–2.5,implying that it is limited by a process with an activationenergy of 40–60 kJ (54, 91, 376). Vo and/or Vu have beenmeasured in a variety of muscles where shortening veloc-ity varies by more than a factor of 104. In all these mus-cles, Vu is roughly proportional to the actin-activatedS1/HMM/myosin ATPase rate for myosin from that muscle(15). Thus, with the assumption that the step size ofmyosin head per ATP is the same for these various mus-cles, this implies that the rate of the power stroke (that
part of the cross-bridge cycle during which force and/ordisplacement of the thin filament occurs) is proportionalto the maximal ATPase rate, both in the unloaded condi-tion. However, this does not mean that the power strokeitself is the rate-limiting step of the cross-bridge ATPasecycle. These results suggest that maximal shortening ve-locity is dependent on the cross-bridge cycling rate and isindependent of the number of cross bridges pulling on thethin filament.
IV) What limits shortening velocity? Is the unloadedvelocity limited by 1) the intrinsic viscous drag on thefilaments, 2) the drag from attached cross bridges, 3)cytoskeletal elements that resist sliding, 4) the number ofactive cross bridges, or 5) other factors? Each of theseseparate factors is discussed below.
A) Viscous drag. During sarcomere shortening at Vo,viscous drag is produced as the thin filaments slide pastthe thick filament through the cytosol. Both theoreticaland experimental evaluations have shown that the dragoffered by such sliding is very small (61, 214, 216). Solvingthe equation for the axial viscous drag/half-thick fila-ment/two thin filaments sliding at 6 mm/s, Fd (214) gives1.4 3 10214 N/half-thick filament/2 thin filaments. If 300 S1heads are available to attach /half-thick filament (see sect.IIIC) and 40% (67, 280) attach and exert 2 pN force/crossbridge during an isometric contraction (112, 320), then theactive positive force exerted by a thick filament is 2.4 310210 N. Thus the force needed to overcome viscous dragat Vo is ,0.01% of the force the cross bridges can exert.The power required to overcome thin filament viscousdrag is the Fd 3 Vo (1.4 10214 N/thick filament/half-sarco-mere 3 6 3 1026 mzhalf-sarcomere21zs21) is 8.4 3 10220
W. This is ,0.004% of the cross-bridge power output of 2.53 10215 W produced by ATP splitting per half-thick fila-ment (assuming an ATPase rate by the cross bridges of 5cycle/s, 3 3 102 cross bridges/h; change in ATP freeenergy 5 250 kJ/mol, and 50% efficiency). Thus viscousdrag of sliding thin filaments should not limit shorteningvelocity.
B) Structural or cytoskeletal factors may limit
shortening. At sarcomere lengths ,1.7 mm, energy isdissipated in deforming both the sarcomere and thickfilament structures as well as changing filament separa-tion. For sarcomere lengths of 1.7–3.1 mm, other factorslimit Vo, and there are two major sources of energy dis-sipation. As a fiber shortens, the filament lattice expands(97), and this expansion may be resisted by cytoskeletallinks between myofibrils and attachments to the musclebasement membrane. This resistance is probably verysmall because between sarcomere lengths of 1.7 and 2.5mm in skeletal muscle there is negligible restoring forceexerted during relaxation to return the muscle to its initialsarcomere length. Alternatively, it may be that MzADPzPi
cross bridges rapidly attach and detach from the thinfilament and so constitute a resistive force (37). Careful
FIG. 7. Protocol for measurement of unloaded shortening velocityusing the slack test (91) for two pCa values, 4.5 and 6.0. Inset: protocolwith fiber length and force vs. time records at each pCa value. In eachrecord at the given pCa, the fiber was quickly released a distance Dx attime 0, and the time required for force to begin redeveloping (slacktaken up in the fiber) was measured (indicated by numbered arrows).For the pCa 6 data, records are shown at higher force sensitivity. In thegraph, the length change (Dx) is plotted against the duration of timerequired for the unloaded shortening to take up the slack, force to beginto redevelop. The numbered data points 1, 2, and 3 are for pCa 4.5 and4, 5, and 6 are for pCa 6.0. The slope of this line is the unloadedshortening velocity. Note that at shorter distances of shortening, thevelocity (slope) is independent of Ca21, but that at shortenings .10%,the unloaded shortening velocity for pCa 6.0 is substantially slower thanat pCa 4.5. [From Moss (330).]
886 GORDON, HOMSHER, AND REGNIER Volume 80

studies of the passive force exerted by such cross bridgesduring rapid stretches of relaxed muscle have shown thatsuch forces are miniscule (190). It may be that suchattachments (as well as other cytoskeletal forces) doconstitute a significant force during shortening. However,there is no compelling evidence for such forces, so thishypothesis remains to be tested.
C) Resistive drag produced by strongly attached
cross bridges. A. F. Huxley (215) suggested that the dragfrom the attached cross bridges opposes thin filamentsliding and limits Vo. He imagined that cross bridges,driven by thermal energy, oscillate axially on the thickfilament about an equilibrium position at x 5 0. As theyoscillate toward the Z line (at x . 0), they could attach ina conformation from which they would exert positiveforce acting to pull the thin filament toward the M line.With the assumption of a cross-bridge stiffness k (;5 31024 N/m) (143, 349, 354), an initial cross-bridge attach-ment to actin on the Z-line side of the cross-bridge equi-librium position would generate a force kzx. During mus-cle shortening, attached thin filaments slide toward thecenter of the sarcomere (x30), the attached cross-bridgeheads move toward their equilibrium position and at-tached cross-bridge force decreases. At its equilibriumposition, cross-bridge force equals zero, and the workdone by the cross bridge moving from its attachmentpoint, x nm from the equilibrium position, will be (kzx2)/2.As thick and thin filament sliding (shortening) continues,the attached cross-bridge is dragged passed its equilib-rium position into a negative force-bearing region whereforce is 2kzx. A. F. Huxley (215) hypothesized that theaccumulation of attached cross bridges in the negativeforce-bearing region produces a force opposing continuedsliding. When the force exerted by “negatively” strainedcross bridges is equal and opposite to the force exerted bycross bridges generating positive force, the sliding veloc-ity reaches its maximum steady-state velocity, Vu. Thusthe detachment rate of the cross bridges in the negativelystrained region (x , 0) limits unloaded shortening veloc-ity. This view is supported by the independence of Vo onthick and thin filament overlap at sarcomere lengthsgreater than 2.2 mm. Further interventions that slowcross-bridge detachment from the thin filament (e.g., in-creasing [ADP] or decreasing [ATP]) decrease Vo (62, 68,70, 108, 382). The effects of [ATP] and [ADP] are pre-dicted by several strain-dependent models (94, 351).
Josephson and Edman (236) presented evidence sup-porting the idea that the drag, imposed by negativelystrained cross bridges, limits Vo. They measured Vo earlyin a tetanus (in the first 30 ms after the beginning ofstimulation before tension had risen to 30% of maximal),during the plateau of the tetanus, and during relaxationfrom the tetanus. At first glance, A. F. Huxley’s (215) viewof Vo would seem to imply that Vo would be the same ateach point in the tetanus. However, Josephson and Ed-
man (236) found that at the start of the titanic stimulation,Vo was ;30% greater than that seen during the plateau ofthe tetanus. Furthermore, during the initial stages of re-laxation from the tetanus, Vo fell to values significantlyless than those during the tetanic plateau. Josephson andEdman (236) argued that such behavior was predicted byHuxley’s model (215). They noted that at the beginning ofa tetanus, before tension has reached the plateau, crossbridges attach and exert force. If shortening begins beforea steady state of attached positively strained cross bridgesis attained, then as cross bridges are dragged into thenegatively strained region, even more cross bridges willbe attaching in the positively strained region. Thus, atearly times during titanic contraction, the rate of attach-ment of positively strained cross bridges (from x 5 0 to x)will exceed the rate of formation of negatively strainedcross bridges and unloaded shortening velocity will behigh. If, however, shortening begins during the plateau ofthe tetanus, the negatively strained and positively strainedcross bridges will be balanced and, consequently, Vo willbe constant and independent of activation. During relax-ation, as calcium concentration drops, the rate of forma-tion of positively strained cross bridges declines, and therate of entry of cross bridges into the positively strainedstate lags behind the rate of entry of cross bridges into thenegatively strained state, resulting in a slower Vo than thatobserved during the tetanus plateau. Josephson and Ed-man (236) showed that a modification of A. F. Huxley’sequations (215) accurately accounted for their experi-mental observations.
V) Effect of cross-bridge numbers on thin filament
shortening velocity. With the assumption that during thecross-bridge throw d (of 10 nm), a cross bridge generatespositive force during the power stroke at rapid speeds fora time ts (of 5 ms), and that the maximum ATPase rate iskcat (8 s21 at 10°C) (382), the equation relating Vo to thenumber of attached cross bridges (N) (467) is
Vo 5 d/ts 3 @1 2 ~1 2 ts 3 kcat!N#
Solution of this equation for various numbers ofcross bridges attached to the thin filament shows that, toproduce shortening at 0.95 of the maximum velocity (1.9mm/s), 73 actin monomers per thin filament (27% of thosein a thin filament at optimal sarcomere overlap) must bebound to cross bridges that are pulling. Similarly, 17 crossbridges must be bound per thin filament (6% of those in athin filament at optimal sarcomere length) to generate aspeed of ;0.5Vo, and 7 cross bridges must be bound perthin filament (3% of available actin monomers) to gener-ate a speed of 0.25Vo. In section IIIC1 we estimated thatduring shortening at Vo at maximum activation, one crossbridge was attached and propelling the thin filament per11–26 actin monomers. This implies that at Vo, only three
April 2000 MUSCLE REGULATION 887

to eight cross bridges are pulling each thin filament to-ward the center of the sarcomere. If there are 1,000 thinfilaments rigidly attached to the Z line in each myofibril,3,000–8,000 cross bridges produce the Z-line movementat full activation, and the shortening velocity will be 2mmzhalf-sarcomere21zs21. Even at 10% activation, thenumber of attached cross bridges per Z line in eachmyofibril would be at least 300 cross bridges, more thanenough to reach Vo. This analysis is valid only if theattachment of thin filaments to the Z line is rigid andmoves as a single unit. In summary, the evidence in intactskeletal muscle fibers supports the conclusion that theunloaded shortening velocity is independent of the num-ber of attached cross bridges but is probably limited bythe intrinsic drag from attached cross bridges.
VI) Calcium regulation of unloaded shortening ve-
locity. If calcium regulates contraction by controlling onlythe rate of entry into the force-generating portion of thecross-bridge cycle, then steady-state Vo should be inde-pendent of the extent of muscle activation. This conclu-sion assumes that even if the cross-bridge throw in thepower stroke is only 5–10 nm, there are enough crossbridges pulling on the thin filament to ensure that it isconstantly moving (see above). Edman (91) tested theidea that Vo is independent of activation in single livingmuscle fibers by grading the intracellular [Ca21] withsodium dantrolene inhibition of Ca21 release from thesarcoplasmic reticulum. Using the slack test and releasingthe muscle by ,9% of the initial length, Edman (91) foundthat, when the isometric force is reduced to as low as 10%of maximum by exposure of the fiber to sodium dan-trolene, Vo was within 10% of its value at full activation.Although the result seems compelling, dantrolene treat-ment may not uniformly inhibit the release of calciumover the fiber cross section, i.e., it may inhibit calciumrelease more in the outer fiber annulus than the innercore. If so, force may be reduced to zero in the outerannulus but is normal in a core region experiencing max-imal Ca21 activation. In such a case, the fiber force wouldbe reduced but Vo would be unchanged. Thus experi-ments should be directed at examining the [Ca21] profilethroughout the fiber in the presence of dantrolene (474).As discussed in section IIIC9, data exist suggesting thatrapid shortening itself may deactivate the fiber (90), pos-sibly through the detachment of strongly attached crossbridges influencing either Ca21 binding to the thin fila-ment or direct activation of the thin filament. The data ofVandenboom et al. (469) suggest that at maximal calciumactivation, although the shortening may influence the rateof force development, it does not affect Vo, which in livingfibers is not greatly sensitive to the level of activation.
B) EFFECT OF [CA21] ON UNLOADED SHORTENING VELOCITY IN
SKINNED MUSCLE FIBERS. The use of skinned muscle fiberspermits variation of parameters not easily altered in theintact fiber, such as [Ca21] and the TnC isoform. More-
over, both the force and the unloaded shortening velocityexhibited by skinned muscle fibers are similar to thosemeasured in the intact fiber (238). The effect of a reduc-tion of [Ca21] on unloaded shortening in skinned fibersappears to differ from that observed with reduction ofthick and thin filament overlap in intact fibers. In the slacktest measurements of Vo at reduced or submaximal[Ca21], plots of the distance released against the slacktime interval no longer form a single straight line (see Fig.7) and are better characterized by the sum of two straightlines. For releases ,6–8% of lo (60–80 nm/h), plots of thedistance released versus slack time have a single slope,the unloaded shortening velocity of phase 1, Vo 1 which isindependent of [Ca21]. At submaximal Ca21 and releasesof .8% (phase 2), plots of distance released versus slacktime exhibit a reduced slope, Vo 2, indicative of a reducedunloaded shortening velocity compared with Vo 1. PlotsVo 1 and Vo 2 as a function of the relative isometric force,P/Po, varied by changing [Ca21], show very different be-havior. Vo 1 decreases only slightly as Ca21 is decreaseduntil P/Po has fallen to ,20% of maximal, but then fallsprecipitously as Ca21 is decreased further. Vo 2, on theother hand, decreases linearly with the decline in P/Po.Thus, at a pCa producing a P/Po of 0.5, Vo 1 is ;0.9 of themaximal Vo, whereas Vo 2 is 0.5 of maximal Vo. The be-havior of Vo 2 is different from that seen in the intact fiberwhere Vo is independent of P/Po values down to 0.4, whenforce is decreased by decreasing filament overlap. How-ever, Vo 1 (at submaximal pCa) behaves in a manner sim-ilar to Vo in the fiber whose overlap is reduced. Howclosely they correspond cannot be stated, since there isno unambiguous data at thick and thin filament overlap,40% of optimal in the fully active fiber. The similaritybetween Vo in intact fibers and Vo 1 in skinned fibers issupported also by the data discussed above of Edman(91), suggesting that Vo in intact fibers may depend littleon the level of Ca21 activation.
The behavior of Vo 2 has no analogy in the fully activefiber contracting at reduced overlap. However, Edman(91) and Vandenboom et al. (469) measurements usedslack test releases of 9% or less. This means that thereleases were not large enough to produce the Vo 2 resultseen in skinned muscle fibers. Thus there may be nodiscrepancy between intact and skinned muscle fibers visa vis the calcium dependence of Vo. As discussed insection IIID, measurement of in vitro motility slidingspeed, Vf, reveals that Vf changes little with reductions in[Ca21] until pCa is raised to 6.5. At still greater pCa, Vf
declines toward zero. Thus regulated thin filaments in thein vitro motility assay using fast muscle myosin behave ina fashion comparable to Vo 1, and not like Vo 2. Possibleexplanations for the effects of [Ca21] on Vo 1 and Vo 2
include the trivial (the skinned fiber is a poor model forthe behavior of the living fiber) and the significant: 1) areduction in [Ca21] reduces the rate of the cross-bridge
888 GORDON, HOMSHER, AND REGNIER Volume 80

power stroke, 2) a reduction in [Ca21] reduces the rate ofcross-bridge detachment from the thin filament duringshortening, or 3) a reduction in [Ca21], along with short-ening per se, reduces the rate of cross-bridge formation orthe rate of cross-bridge detachment.
These hypotheses have been tested using severalapproaches. The activation of the thin filament, indepen-dent of [Ca21], can be altered by removal of TnC (334) orreplacement of TnC with a mutant cardiac TnC, CBMII(327). This mutant TnC can be substituted for TnC in thefiber but does not bind Ca21 at the NH2-terminal Ca21
binding trigger site and thus is not activated by Ca21.Variation of relative isometric force, P/Po, at saturating[Ca21] indicates that P/Po is inversely proportional to theamount of TnC removed or replacement by the CBMII(328). The behavior of Vo after TnC removal or replace-ment by CBMII is similar to that seen when [Ca21] isvaried in the control skinned muscle fiber. As the isomet-ric force declines, Vo 1 is little affected until force isreduced to ,20% of maximal while Vo 2 declines in directproportion to the reduction in relative isometric force.These latter results suggest that the calcium-dependentchanges in Vo 2 are a consequence of regulatory proteininteraction with the structural arrangement of the con-tractile proteins in the muscle fiber. The constancy of Vo 1,even when force decreases, significantly suggests that therate of cross-bridge attachment and detachment arelargely unaffected by the extent of thin filament activa-tion. For displacements of ,80 nm/h, the rate of cross-bridge attachment, detachment, and the size of the powerstroke are unaffected by thin filament activation. Thus thefirst phase of unloaded shortening velocity (Vo 1) is largelyindependent of the effects of [Ca21], thin filament activa-tion, or the number of thick and thin filament interactions.The behavior of Vo 1 is similar to the behavior of theregulated thin filament sliding speed as pCa is variedand/or CBMII content of the thin filament is varied. Thebehavior of the in vitro unloaded, regulated thin filamentsliding speed in the in vitro motility assay, Vf, underreduced [Ca21] or increased thin filament CBMII can beaccounted for by changes in the number of cross bridgespulling on the thin filament (148, 206). The similarity ofVo 1 and the in vitro motility behavior thus invite similarexplanations.
The behavior of Vo 2 is, however, different from whatis seen for Vf in the in vitro motility assay and mustinvolve a different explanation. In the above experiments,pCa was constant and saturating. Because Vo 2 decreasesas the proportion of CBMII bound to the thin filamentincreases (i.e., as the extent of thin filament activationwas reduced) at saturating pCa, changes in Vo 2 cannot bethe result of a Ca21-mediated change in binding to Tn ora regulatory light chain. These changes are more relatedto the number of cross bridges bound to the thin filamentand remain to be explained.
Recently, similar experiments have been done in fastrabbit psoas fibers reconstituted with mixtures of skeletalTnC and the skeletal non-Ca21-binding TnC mutant[sTnC(D27A, D63)] (Regnier, unpublished observations).The results of these experiments suggest that, as thenumber of activatable Tn units is decreased, a reductionin unloaded shortening velocity can occur during maxi-mal Ca21 activation (pCa 4) even at shorter length steps(i.e., Vo 1), with no additional slowing (Vo 2) at longerlength steps. The discrepancy between these results andthose with CBMII may result from differences betweencardiac and skeletal forms of TnC. Further study isneeded to determine if this is so.
Moss (330) has suggested that cross bridges attachedto thin filaments can remain attached and be dragged longdistances (60–80 nm) past their equilibrium position be-fore they are forcibly detached, and thus produce a dragthat slows the muscle shortening velocity. This was sug-gested to occur particularly as attached cross bridges,sliding along the partially activated thin filament, found aregion of the filament that was in the “off” state and got“stuck.” This hypothesized effect would be exacerbatedby the reduction of the extent of thin filament activation.However, if Vo is 5 mmzh21zs21, this hypothesis requiresthat the cross bridge remains attached for up to 16 ms (80nmz5 mm21zh21zs21) as opposed to the 1–3 ms usuallysupposed in the Huxley model (215). Furthermore, thishypothesis requires that, over short ranges of cross-bridgetravel (22 to 15 nm), the cross-bridge stiffness would be5 3 1024 N/m; however, for deflections from the equilib-rium position between 22 and 280 nm, the stiffnesswould have to be .100 times less to account for Vo 1. Thestiffness would then need to increase again markedly atdistances .80 nm. These requirements seem unrealistic.
Another possibility is that during rapid shorteningthe thin filament is deactivated by the rapid detachment ofcross bridges. Iwamoto (230) found that at submaximalactivation, during a repetitive series of rapid shorteningsat velocities near Vo followed by quick restretches to theinitial muscle length, there is a progressive slowing ofshortening velocity. In other words, the Vo 1 phase ofshortening is lost and the Vo 2 phase predominates. Thedata also showed that a 300-ms period of isometric con-traction must be interposed between releases to reversethis inhibition. During rapid shortening at saturating pCa,the stiffness of the muscle fiber decreases by nearly 60%,indicating that the number of attached cross bridges ismarkedly reduced. At saturating Ca21, enough crossbridges are attached to forestall significant deactivationof the thin filament. However, during shortening near Vo
and submaximal [Ca21], the number of attached crossbridges (which promote thin filament activation) may fallbelow a level required for full activation of the thin fila-ment. A thin filament deactivation could manifest itself intwo ways: 1) an increase in the rate of cross-bridge de-
April 2000 MUSCLE REGULATION 889

tachment, or 2) a decrease in the rate of cross-bridgeattachment. Either effect would result in a reduced num-ber of cross bridges attached and interacting with the thinfilament. If the rate of cross-bridge detachment increased,the unloaded shortening velocity would increase (be-cause the number of negative force-generating crossbridges would decrease). On the other hand, if the rate ofcross-bridge attachment decreased, the unloaded short-ening velocity would decrease in a fashion similar to thatdescribed by Josephson and Edman (236) during relax-ation from a tetanus.
A paradoxical aspect of the reduction in Vo 2 at low[Ca21] is the effect of added Pi. Metzger (308) found thatat submaximal levels of [Ca21] there is an inhibition ofVo 2, but the addition of 10–30 mM Pi returns Vo 2 towardcontrol values of Vo. This behavior seems inconsistentwith the changes in Vo 2 arising from structural con-straints on the shortening velocity (an imposed load otherthan that arising from the cross bridges themselves) be-cause it implies that such a restraint is Pi dependent. Oneexplanation for this behavior is that an elevated [Pi] re-duces the fraction of AMzADP cross bridges by increasingthe fraction of cross bridges in the AMzADPzPi, a weaklybound cross bridge. If such a Pi binding is stronger tonegatively strained cross bridges than positively strainedcross bridges, then the drag force will be reduced andvelocity of shortening would increase. If true, at saturat-ing calcium, addition of Pi would increase Vo beyond thatof control. The data (308, 353) on this point are equivocal.In summary, the unloaded shortening velocity of musclecan be altered by many variables; the size of the cross-bridge throw, the duration of the cross-bridge duty cycle(the time the cross bridges stay attached to the thinfilament per ATP hydrolysis cycle), the number of crossbridges interacting with the thin filament, the tempera-ture, and both the rate of cross-bridge attachment (atleast transiently) and detachment. The effects of Ca21 onthe Vo seem explicable by changes in the rate of cross-bridge attachment, which in turn are set by the extent ofthe access of the cross bridge to the thin filament.
6. Activation by strong cross-bridge attachment
As was discussed in section IIIC1, strongly attachedcross bridges are involved in activating the thin filamentduring muscle contraction. There is also significant exper-imental evidence that strongly attached myosin crossbridges can activate contraction in skinned muscle fibersin the absence of Ca21. Reuben et al. (393) first showedthat decreasing the concentration of MgATP in the ab-sence of Ca21 could produce force in skinned crayfishmuscle fibers. Further decreases in [MgATP] resulted in adecrease in force so that the MgATP-force relationshipwas biphasic. This was the skinned fiber equivalent of theincreased ATPase activity observed in myofibril prepara-
tions by Bremel and Weber (32) and the biphasic MgATP-ATPase relationship seen under similar conditions. Theinterpretation of these results was that in the absence ofCa21 and presence of low MgATP, rigor (AM) crossbridges and possibly AMzADP cross bridges (453) (seenext page) bound to and activated the thin filaments in acooperative manner. The binding of a strongly boundrigor cross bridge presumably displaced Tm and allowedthe binding of S1zADPzPi cross bridges to actin, activatingthe ATPase and generating force. At very low MgATPlevels, there were insufficient numbers of S1zADPzPi crossbridges to produce force or ATPase activity. These dataindicate that the same mechanism exists for the control offorce in skinned fibers and for the control of ATPaseactivity in simpler myofibril preparations. In skinned rab-bit psoas muscle fibers, Brandt et al. (28) demonstratedthat the relationship between force and decreased MgATP{increased pMgATP (log10[MgATP])} was very steep andcould be fitted by a Hill equation (equation in sect. IIIC1)with a nH of ;5 and pMgATP1/2 (pMgATP at which theforce had increased to one-half of the maximum value) of;4.6 (2.5 3 1025 M). The maximum force was only ;70%of the maximal Ca21-activated force so that under theseconditions activation by decreased MgATP was not max-imal. It was previously shown that partial activation withCa21 decreased the nH observed with force obtained bydecreasing [MgATP] (27), implying some interaction be-tween Ca21 and rigor cross bridges in thin filament acti-vation. Furthermore, Brandt et al. (28) showed that atleast part of the activation by rigor cross bridges was dueto a spread of activation along the thin filament betweenA7TmTn regulatory units (Tm movement), since extrac-tion of most of the TnC decreased nH to ;2. The assump-tion was that extraction of TnC disrupts the coupling ofTm movement between regulatory units. This is expectedsince in the absence of TnC, TnI remains bound to actin,thus effectively anchoring the regulatory complex to thethin filament every seven actins (297). TnC extraction alsodecreases the slope of the force-pCa relationship inskinned skeletal muscle fibers (26, 334), presumably forthe same reason. Complete extraction of TnC decreasesthe maximum force at low MgATP (“rigor force”) in theabsence of Ca21 by only ;25%, implying that the maxi-mum rigor force depends less on coupling between unitsand more on activation within one unit (28).
In later comparative studies, Metzger (307) con-firmed these results for fast and slow skeletal musclefibers but showed that the nH was slightly larger for slowfibers. Additionally, he found that the nH was muchgreater for cardiac myocytes, ;15 rather than ;5, imply-ing a highly cooperative activation by cross bridges. Fur-thermore, he showed [as did Brandt et al. (28)] thatextraction of TnC (to ;10% of the normal TnC estimatedby the decline of maximal Ca21-activated force to 10% ofcontrol) decreased the nH with decreasing MgATP in the
890 GORDON, HOMSHER, AND REGNIER Volume 80

absence of Ca21 for all muscle types, reducing all tonearly the same value, ;4. [Although this number ishigher than that measured by Brandt et al. (28), variationsbetween labs may not be highly significant because deter-mining the exact nH is very sensitive to the techniqueused.] These results imply that, in the absence of Ca21,rigor cross bridges in one regulatory unit can displace theTm of neighboring units more easily in cardiac musclethan in skeletal muscle. There are several possible expla-nations for this. 1) In cardiac muscle, Tm may be less wellanchored to the thin filament through TnI, TnC, and TnTin the absence of Ca21. Cardiac Tm is less well anchoredto actin in the absence of Tn (59). 2) Cardiac Tm is lessflexible so that neighboring Tm are more strongly cou-pled. The evidence suggests the opposite (59). 3) Rigorbinding could be stronger in cardiac muscle. The lastexplanation is not likely as evidenced by Metzger’s data(307) showing that the nH from each fiber type becomessimilar after extraction of TnC. The simplest interpreta-tion is that the extraction of TnC reduces the ability ofcross bridges to activate more than just one A7TnTm unit,resulting in no coupling between neighboring units alongthe thin filament. In other words, full TnC extractionmight show the relationship for rigor activation within asingle regulatory unit (although one cannot rule out inter-actions across the thin filament, as there are Tm on bothsides).
The above discussion has assumed that it is the rigor(AM) cross bridge that provides activation in the absenceof Ca21. There is excellent evidence that a strong bindingAMzADP cross bridge can also activate. Increased MgADPincreases the skinned fiber Ca21 sensitivity (128, 195) andincreased MgADP increases tension in the absence ofCa21 (128). As discussed in section IIIC, MgADP increasesS1 binding to regulated actin filaments in a cooperativemanner (160), and discussed later in this section, MgADPincreases S1 binding to thin filaments in myofibrils in theabsence of Ca21 (516). In fact, much of the force inskinned fibers at low MgATP in the absence of Ca21 maybe attributed to the activation by AMzADP cross bridges(453). The relative population of the various cross-bridgestates is strain sensitive so that the proportion of AM andAMzADP cross bridges will vary as discussed in sectionIID. The data clearly support that the rigor binding of S1 inthe total absence of ATP can shift the Tm to an apparentactivating state, as discussed in section IIIA, which isconsistent with activation by rigor S1. Thus one mustconsider both AM and AMzADP cross bridges in activationby strongly attached cross bridges. We do not try todistinguish the relative contribution in this section andgenerally imply both when we refer to activation by rigorcross bridges.
Under these conditions of maximal TnC extraction,the nH and relative force provide information about thenumber of force-generating cross bridges activated by
each rigor cross bridge or how many rigor cross bridgesare need to activate one force-generating cross bridge.Comparing these results in different fiber types withand without TnC extraction could also provide infor-mation about the number of rigor units per A7TmTnneeded to activate that unit and the extent of the co-operative spread of activation along the thin filament.However, the exact relationship depends on the modelused. Complications in the modeling arise because[MgATP] may affect both the cross-bridge attachmenttime and the force/cross bridge, and the interpretationalso depends critically on the specific model of activa-tion (29, 31). Brandt et al. (28) developed a concerted-transition [Monod-Changeaux-Wyman (321)] model todescribe these data, but their model assumed thatthe thin filament normally activates as a unit, andthis has been disputed (50, 332) (see sect. IIIC1). Inaddition, their model predicts that if cycling crossbridges can activate the thin filament, the muscle willnot relax (29). These intriguing data await more com-plete modeling.
In summary, the results of decreasing [MgATP] stud-ies clearly show that rigor cross bridges can activate thethin filament and that the activation within one regulatoryunit can spread along the thin filament. Furthermore, itappears that spread along the thin filament in the absenceof Ca21 occurs more readily in cardiac muscle than inskeletal muscle, presumably because of a greater ease ofTm movement. Finally, it should be pointed out that thisactivation by rigorlike cross bridges is due to activationby long-lived cross bridges, whereas under more physio-logical MgATP concentrations, cycling cross bridges aremuch more short-lived.
Further evidence for activation by strongly boundcross bridges comes from the activation produced by astrongly bound myosin analog, NEM-S1 (429). As dis-cussed in sections IIIB and IIIC1, NEM-S1 was used exten-sively in biochemical experiments because it boundstrongly to and dissociated slowly from actin and maxi-mally Ca21-activated regulated thin filaments (500).Swartz and Moss (429) diffused NEM-modified S1 intoskinned muscle fibers and found that high concentrationsdecreased the maximum Ca21-activated force (presum-ably because NEM-S1 can displace endogenous crossbridges), whereas lower concentrations of NEM-S1 in-creased force at low levels of Ca21 and decreased the nH
of the force-pCa relationship without decreasing maxi-mum force. Although there is some question about thespeed of NEM-S1 diffusion into skinned muscle fibers(253), Swartz and Moss (429) observed clear effects ofNEM-S1, implying that it was not a limitation in theirexperiments. Furthermore, at low Ca21, NEM-S1 in-creased kTR to virtually its maximum value, indicating thatthese strongly attached cross bridges enhanced the ef-fects of Ca21 in activating muscle fibers. In doing so, they
April 2000 MUSCLE REGULATION 891

decreased the cooperative activation of force by cyclingcross bridges, since the steepness of the force-pCa rela-tionship was decreased. Surprisingly, NEM-S1 did notactivate skeletal muscle or cardiac muscle in the absenceof Ca21 (115), as it does the acto-S1 ATPase of S1 withregulated actin filaments (161). Thus NEM-S1 providesadditional evidence that strongly bound cross bridges canassist Ca21 activation of skinned muscle fibers. The effectof NEM-S1 to decrease the nH of the force-pCa relation-ship can be understood if it partially moved Tm, but notenough to allow attachment of cycling cross bridges in anA7TmTn unit. In this partially shifted state, Tm could bemoved sufficiently by Ca21 binding to TnC to bring aboutfull activation of that regulatory unit. Thus the increase inforce after Ca21 binding is not highly cooperative in thepresence of NEM-S1.
The studies described above used force as the mea-sure of the level of activation. Another measure of acti-vation is the amount of myosin bound to the thin filamentin the sarcomere. This was used to great advantage withisolated filaments by Greene and Eisenberg (160) and hasnow been done using fluorescently labeled S1 in myofi-brils with intact sarcomeric structure by Swartz and col-leagues (430, 516, 517). In these studies, the binding offluorescently labeled S1 to the thin filament was com-pared in the region of cross-bridge overlap and nonover-lap with the thick filament. This was done as a function ofCa21 and [S1] under rigor (430) or ADP conditions (516,517), enabling them to assess the effect on thin filamentactivation. In both rigor and S1zADP conditions, and athigh Ca21 and very low added S1 (,1 nM), S1 boundabout four times more to the overlap region than thenonoverlap region. As the added [S1] was increased inboth the presence and absence of Ca21, the binding of S1to the nonoverlap region increased in a highly cooperativemanner. This occurred particularly in the absence ofCa21, saturating at a value consistent with binding to allavailable sites. As expected, S1zADP bound somewhatmore weakly than S1 in rigor, but not by as much aswould be predicted from the relative affinities of thestrong binding complex estimated by McKillop andGeeves (303). Thus, in the nonoverlap region, where therewas no competition from endogenous cross bridges, thebinding of added S1 or S1zADP to the thin filament wasnearly four times less than in the overlap region, even inthe presence of high [Ca21]. Importantly, maximum acti-vation as measured by S1 binding did not occur with highCa21 alone. At high Ca21, endogenous rigor or ADP crossbridges in the overlap region increased the binding ofadded S1 or S1zADP to the thin filaments in that regionover that seen in the nonoverlap region. This supports theconclusion that endogenous strongly attached crossbridges in the sarcomeric structure can provide additionalactivation (cross-bridge binding) of the thin filamentabove that provided by Ca21 alone.
The increased S1 rigor or S1zADP binding in theoverlap region with high Ca21 and low added S1 can beunderstood well from the model of thin filament activa-tion from McKillop and Geeves (303), derived from theirstudies on isolated filaments. In their model, as discussedin section IIIA2, there would be a distribution of blocked,closed, and open actins on the thin filament that is depen-dent on Ca21 and strong S1 binding. In the presence oflow added S1 and high Ca21, the McKillop and Geevesmodel (303) predicts that 20–25% of the actins in thenonoverlap region would be in the open state comparedwith 100% of the actins in the overlap region. This isbecause of the presence or absence of the endogenouscross bridges in the rigor or ADP nucleotide state. Theratio of open actins in the overlap/nonoverlap regionswould be ;4, which was similar to the ratio of S1 orS1zADP binding between the two regions that they ob-served at low added S1. Thus the data of Swartz andco-workers (430, 516, 517) are supportive of the Geevesmodel at low [S1], but the data need to be fit over thewhole range of added S1 or S1zADP, as well as 6Ca21, toconclude that the data are well described by the model.
The above data appear to make less likely a model ofactivation where high [Ca21] or strong cross bridges shiftTm such that all actins in a regulatory unit have the samemyosin affinity. With this model in the presence of lowadded S1 and high Ca21, the ratio of S1 binding in theoverlap/nonoverlap regions would be equal to the in-creased cross-bridge affinity from Tm movement resultingfrom endogenous cross-bridge binding in the overlap re-gion. This increase in affinity should depend on the nu-cleotide state of the S1 (rigor or ADP). Although thisappears less likely because of the studies of Swartz andco-workers (430, 516, 517), the available data cannot en-tirely eliminate this possibility. In conclusion, these stud-ies in myofibrils with intact sarcomere structure supportthe idea that there is a distribution in states in the thinfilament with weaker and stronger S1 binding. Addition-ally, the data support the idea that binding of endogenousrigor or ADP cross bridges in the intact sarcomere canshift the cross-bridge distribution to stronger binding.Finally, the above data support the conclusion that Ca21
alone can stimulate strong binding of S1 to the thin fila-ment but that additional binding can occur in the pres-ence of endogenous rigor or ADP cross bridges.
7. Ca21 binding and TnC structural changes in fibers
Because Ca21 activates contraction in skinned mus-cle preparations, a quantitative assessment in this prepa-ration of Ca21 binding and the conformational changes inTnC and other regulatory proteins leading to activationare crucial. There have been numerous direct studies ofCa21 binding including 45Ca binding studies and electron-probe X-ray microanalysis of Ca21 binding in the sarco-
892 GORDON, HOMSHER, AND REGNIER Volume 80

mere under a number of different conditions. There havealso been indirect studies of Ca21 binding using fluores-cent or EPR probes on TnC to sense local structuralchanges in TnC again under various cross-bridge andactivation conditions. These studies show a strong cou-pling between rigor cross-bridge attachment, enhancedCa21 binding, and TnC structural changes in all musclesas in myofibrils (32). However, the coupling betweencycling cross bridges and Ca21 binding/TnC structuralchanges appears to differ between muscle types. Studiesindicate a strong coupling in cardiac muscle (168, 197),whereas similar but less decisive changes are observed infast and slow skeletal muscles (124, 292, 483). These dataare reviewed next.
Direct measures of Ca21 binding (45Ca) by Fuchs andco-workers (122–124, 196) in various muscle types, andPan and Solaro (346) in cardiac muscle, have shownclearly that Ca21 binding at the NH2-terminal Ca21 bind-ing sites is associated with activation in these muscles.Fuchs and co-workers have shown that rigor crossbridges enhance Ca21 binding in both skeletal (122) andcardiac (196) muscles. In contrast, inhibition of cyclingcross bridges by Vi or extensive muscle shortening (de-creased sarcomere lengths) decreases 45Ca21 binding incardiac muscle preparations (197), but not in either fast(124) or slow (483) mammalian skeletal muscle prepara-tions. This latter point is particularly significant becausecardiac and slow skeletal muscle share the same TnC andmyosin isoforms (231, 413, 498, 510) and both show de-creasing Ca21 sensitivity with decreasing sarcomerelengths. In further support that differences exist betweenskeletal and cardiac muscle, Wang and Fuchs (484) ob-served that osmotic compression of the fiber lattice en-hances Ca21 sensitivity (pCa1/2) of force in both fastskeletal and cardiac muscle but enhances 45Ca21 binding(at a given pCa) only in cardiac muscle. The lack of asarcomere length effect on Ca21 binding to TnC in fastskeletal muscle was verified by Patel et al. (357) using avery different technique. Cantino et al. (50) confirmed theenhanced Ca21 binding to TnC with rigor cross bridges infast skeletal muscle and demonstrated this enhancedbinding was in the region of overlap in the sarcomere,with little extension along the nonoverlapped portion ofthe thin filaments. Cantino et al. (51) also showed thatwhen fibers were stretched to beyond overlap of thick andthin filaments, Ca21 binding along skeletal muscle thinfilaments was uniform and increased with increasingCa21. Thus direct measures of Ca21 binding show thatrigor cross bridges enhance Ca21 binding, but the effectsof cycling cross bridges are less certain and appear todepend on the muscle type.
Studies of the relationship between Ca21 binding andTnC structural changes, using fluorescent or EPR probesbound to TnC, have produced similar results in somelaboratories but differing results in others. In every study,
rigor cross bridges have caused structural changes in TnC(4, 153, 163, 168, 275, 292, 374) and produce activation inevery muscle tested. This is in excellent agreement withthe 45Ca studies. However, the change in TnC structurewith cycling cross bridges in skinned cardiac musclepreparations depends to some extent on the location ofthe probe (293, 374). The results for cycling cross bridgesin skeletal muscle also depend somewhat on the labora-tory and technique used. Results of early studies (4, 163,323) suggest that cycling cross bridges in skeletal fibersproduced the largest structural change in TnC when TnCwas labeled with 5-dimethylamino-1-napthalenyl sulfonyl-aziridine (DANZ) at Met-25. This result came from com-parisons of fluorescence in the presence of Ca21 and ATPat maximum filament overlap versus the fluorescence inthe absence of overlap (sarcomere length .4.0 mm). Theincreased fluorescence at full overlap was interpreted asan effect of the cycling cross bridges. All these studiesfound little effect of added Pi on the fluorescence, despitesubstantial inhibition of force. In subsequent studies us-ing fluorescent probes sensitive to either TnC orientationor local environment, inhibition of force by either stretch-ing the fiber to 3.6 mm or addition of BDM or AlF4
2,demonstrated little influence of force or strong cross-bridge attachment on TnC structure at maximal Ca21
(292). The differences between this latter study and theothers include the use of techniques to minimize sarco-mere shortening, the use of both environmentally sensi-tive and orientation-sensitive probes, and the use of dex-tran to minimize changes in lattice spacing duringactivation. In another study using EPR, Li and Fajer (275)demonstrated only a small change in TnC structure withforce inhibition by AlF4
2. Thus the effect of cycling crossbridges on the skeletal TnC structure may be small. Thiscould be because cycling cross bridges have, in fact, littleeffect on TnC structure in skeletal muscle or because thefraction of attached cross bridges is much smaller duringcycling than in rigor.
A more indirect measure of Ca21 binding has beenthe increase in sarcoplasmic free [Ca21] seen with a stepdecrease in length of isometrically contracting cardiacmuscle (3) and barnacle muscle (149, 394) during thedeclining phase of the Ca21 transient during a twitch.These investigators interpreted the increased [Ca21] asbeing released from TnC subsequent to a decreased Ca21
affinity resulting from the detachment of cross bridgesaccompanying the shortening step. This implied that ac-tive cross bridges enhanced Ca21 binding to TnC in car-diac and barnacle muscle. Support for this hypothesiscame from the studies of Gordon and Ridgway (150, 151),Kurihara et al. (257), and Allen and Kentish (2) and themodeling studies of Gordon and Ridgway (152). In thelatter studies (measuring [Ca21] using aequorin), thechange in Ca21 affinity of TnC for a given force level wasestimated. This was done by assuming the increased
April 2000 MUSCLE REGULATION 893

[Ca21] measured with the shortening step was propor-tional to the TnC-bound Ca21, and calculating the relativeaffinity from the observed increased [Ca21] and the mea-sured free [Ca21]. These data resulted in a calculated TnCCa21 affinity that increased steeply as a function of theforce (152). It also demonstrated that, during transientstimulation, the time course of Ca21 bound to TnC boundwas intermediate between free [Ca21] and force. Duringthe rise in free [Ca21] and force, the increase in boundCa21 lagged the rise in Ca21 and led to the rise in force.During relaxation, the decrease in bound Ca21 occurredafter the decline in free [Ca21] but before the decline inforce. The calculated Ca21 affinity as a function of forcecould then be used to calculate the force resulting fromthe bound Ca21 using a simple activation model andassuming that the effect of force/cross-bridge attachmenton the TnC Ca21 affinity was through a change in thedissociation rate of Ca21 from TnC (152). These resultsagain suggest that cycling cross-bridge attachment in car-diac and barnacle muscle increase Ca21 binding to TnC.The result in cardiac muscle is similar to the 45Ca21
results discussed above (197) and the fluorescent proberesults (168).
In contrast to the direct, steady-state measure ofCa21 binding using 45Ca21 in skeletal muscle showinglittle effect of cycling cross bridges (124), there are indi-rect measures of Ca21 binding using length changes thatdemonstrate some effect of cycling cross bridges to en-hance Ca21 binding to TnC. Cannell (49) and later Caputoet al. (52) and Vandenboom et al. (469) showed there wasa transient increase in free Ca21 during nonuniform re-laxation from tetanic stimulation in skeletal muscle. Atthis time during relaxation, many sarcomeres are short-ening rapidly while others are lengthening (65, 218). Therapid shortening is accompanied by cross-bridge detach-ment and extra Ca21, presumably dissociated from TnCbecause of decreased Ca21 affinity. In addition, Caputo etal. (52) showed increased Ca21 accompanying a shorten-ing ramp during the initial phase of relaxation from te-tanic stimulation, but not during the force plateau. Later,Vandenboom et al. (469) showed that if the shorteningwere rapid enough and large enough (close to Vu and.10% lo, respectively) during the plateau, they could ob-serve extra Ca21 during shortening and decreased Ca21
during the subsequent force redevelopment. This impliesthere is some effect of cycling cross bridges on Ca21
binding to TnC in skeletal muscle, but the effect may besmaller than in mammalian cardiac muscle or barnaclemuscle. These indirect techniques suffer from uncertain-ties in calibrating the [Ca21] signal, relating free [Ca21] toTnC-bound Ca21, and calculating the effects of the otherCa21 buffers, so it is difficult to compare results betweentissues and calculate actual changes in TnC Ca21 affinity.It could be that Ca21 binding to TnC site II is moreaffected by strong cross-bridge attachment than binding
to site I (cardiac TnC and barnacle TnC have only site IICa21 binding, skeletal TnC has both site I and site II Ca21
binding) (325). If this were the case, Fuchs and Wang(124) might have had a more difficult time observingchanges in total Ca21 binding. This cannot completelyaccount for the difference between the effect of cyclingcross bridges on 45Ca21 binding to skeletal and cardiacmuscle TnC, since force-dependent Ca21 binding is notobserved in slow skeletal muscle fibers, even though itcontains the cardiac TnC isoform with Ca21 binding onlyat site II. However, there may be other variations in theregulatory protein interactions that enhance the effect incardiac muscle. Finally, another contrast between the45Ca21 binding measurements and the indirect measuresusing changes in free [Ca21] is that the former are steady-state measurements and the latter are transient measure-ments. If the change in TnC Ca21 affinity were only tran-sient, it would only be detected by latter techniques. Inany case, the data suggest the differences may be quanti-tative, rather than qualitative, and that Ca21 binding toTnC serves to initiate contraction but is not the only stepin regulation.
To more fully understand regulation, one must followstructural changes in the other components of the regu-latory system occurring during activation. This can bedone using skinned fibers where the native proteins canbe exchanged for fluorescent or EPR-labeled proteins.With the available techniques for exchanging variouscomponents of the regulatory system, additional studiesare clearly possible (TnT exchange) (173, 174). The pro-tein that has been the most difficult to exchange is Tm.Studies where Tm has been exchanged involve digestionand repolymerization of actin, followed by incorporationof Tn and labeled Tm (127, 129). These new techniquesare required to provide this important information.
In summary, studies of Ca21 binding and TnC struc-tural changes indicate a strong coupling of these changeswith attachment of cycling cross bridges in cardiac mus-cle, but less coupling in skeletal muscle. The possible roleof these differences in Ca21 activation is discussed insection V.
8. Control of relaxation
The kinetics of relaxation from force can also pro-vide information about the mechanism of activation. Themost well-defined condition for studying relaxation inintact fibers is relaxation from an isometric tetanic con-traction. Under this condition, the kinetics of cross-bridgedetachment and cross bridge-induced activation by coop-erative mechanisms are complicated by contemporane-ous processes. These other processes involve changes inintracellular [Ca21] via uptake by the sarcoplasmic retic-ulum, binding to high affinity Ca21 binding proteins suchas parvalbumin, and dissociation from Tn changing Ca21
894 GORDON, HOMSHER, AND REGNIER Volume 80

activation as well as by changes in cross bridges throughreattachment. There have been a number of studies of therelaxation process, but most have investigated the de-crease in [Ca21], whereas few have studied the mecha-nism of thin filament relaxation. A major experimentalproblem in studying relaxation is that large sarcomerenonuniformity can develop that contributes to the ob-served mechanical relaxation. Over 25 years ago,Cleworth and Edman (65) and Huxley and Simmons (217,218) showed that during relaxation there were largechanges in sarcomere spacing within fibers even when thetotal muscle length was held constant. The end sarco-meres relaxed first and were stretched as the centralsarcomeres shortened. These processes were exacer-bated after force had declined linearly by ;20–30%, pro-ducing a shoulder in the force curve as force in theshortening sarcomeres declined rapidly. This means thatin the first phase of relaxation sarcomeres are undernearly isometric conditions, whereas in the second phasethey are clearly not. Huxley and Simmons (218) showedthat when the length of the central region of a singlemuscle fiber is controlled, the initial linear phase of re-laxation and the shoulder are prolonged. This phase ismore characteristic of isometric relaxation. The rate ofdecline in force during this phase is so slow (,1 s21 at4°C, Ref. 218) that there may not be simply cross-bridgedetachment, there may be some reattachment as well.This complexity in analyzing relaxation due to sarcomerenonuniformity occurs in both intact and skinned fibers.
As outlined in sections IIA and IIIA, the Tm position isa major factor determining the attachment of crossbridges. Thus it is important to know Tm position duringrelaxation. A comparison of the time course of forcerelaxation and Tm movement in intact fibers was made byKress et al. (254) using X-ray diffraction (discussed insect. IIIA1). This study showed that Tm movement duringrelaxation, as measured by the change in intensity of thesecond actin layer line, declined faster than force. How-ever, if the amplitude rather than the intensity of thesecond actin layer line were plotted (which would be abetter measure of Tm movement if thin filaments were notactivated as units, but in segments), the amplitude woulddecline at the same rate as force. This would be consis-tent with Tm moving back toward the relaxed position asforce declined and with the rate-limiting step in relaxationbeing the net dissociation of cross bridges. Their data alsosuggested that some thin filament activation was beingmaintained by cross-bridge reattachment because the in-tensity of the second actin layer line (possible Tm move-ment) declined earlier if relaxation occurred at a longsarcomere length where there was little thick and thinfilament overlap. Furthermore, the change in intensity ofthe second actin layer line during stimulation was notsignificantly different at this longer sarcomere length withno filament overlap than the intensity at sarcomere
lengths with full overlap, implying the change in Tm po-sition during activation was similar with and withoutcross bridges. These data are consistent with cyclingcross bridges holding Tm in an activating position orattached cross bridges enhancing Ca21 binding, whiledelaying the dissociation of Ca21 and return of Tm fromthe Ca21-activated position during relaxation. There issupport for both hypotheses and reason to suggest theymay be coupled (see sects. IIIA2, IIIB, IIIC1, IIIC7, and IV).Another possibility is that the return of Tm to its restingposition can in some way accelerate cross-bridge disso-ciation. However, there is little support for such a mech-anism at this time.
If strongly attached cross bridges activate in a posi-tive-feedback manner, then how does the muscle everrelax? Of course, this will depend on the extent of thefeedback, but in any case, relaxation is accelerated by thenonuniformity in sarcomere length. These nonuniformi-ties bring about a rapid relaxation when still active sar-comeres can shorten rapidly because sarcomeres in serieslose activation and can be readily stretched. These non-uniformities are less important in cardiac muscle becauseof the larger internal stiffness (256).
Skinned fibers offer some advantages in studying themechanisms of thin filament relaxation. The [Ca21] can bedecreased rapidly using caged Ca21 chelators such asdiazo-2, and the sarcomere length can be controlled tominimize nonuniformities along the fiber. Furthermore,the steady-state force level before relaxation can be var-ied to test for the contribution of attached cross bridgeson the relaxation rate. If attached cross bridges sustainactivation, one would expect that at higher initial forcelevels there would be slower relaxation, if either cross-bridge reattachment or a reduced tendency for Tm to“push” the cross bridges off occurs.
There have been several studies using the diazo-2technique to rapidly chelate Ca21 and initiate relaxationin skinned skeletal and cardiac muscle preparations. Inskeletal muscle, the time course of relaxation of force issimilar to intact preparations, a linear phase to a shoulderand a rapid phase that can be characterized with twoexponential functions (355, 477). Because of the nonuni-formity issue discussed above, only the initial linear phasegives reliable information about relaxation in skeletalmuscle. Additionally, the Ca21 affinity of diazo-2 does notincrease enough with photolysis to bring about completerelaxation of a fiber from maximal Ca21 activation, butthe level of the initial force can be varied. In skeletalmuscle, the rate of this initial phase of relaxation isslower the higher the Ca21 level and force when relax-ation is initiated (355, 477). This effect also occurs whentechniques (38) are used that partially stabilize the sarco-meres (477). Even with this technique, the slower phaseof relaxation was always more abbreviated in the skinnedfiber preparation than in intact fibers, indicating more
April 2000 MUSCLE REGULATION 895

nonuniformity of sarcomeres during relaxation in theskinned preparation (477). This makes one cautious ininterpreting the results from skinned fiber studies, noneof which uses sarcomere length control. Slowing of relax-ation also occurs with added NEM-S1 (355) or added ADP(9), indicating that strong cross bridges influence therelaxation rate. Finally, in a very small, myofibrillar prep-aration where myofibrillar [Ca21] was decreased veryrapidly, the rate of relaxation during the linear, more“isometric,” phase of relaxation decreased with the initiallevel of force (339). Taken together with the results ofKress et al. (254), all these observations are evidence thatcycling or strongly attached cross bridges prolong activa-tion (by their positive feedback to activate the thin fila-ment) and thus allow reattachment of cross bridges. Thedata support a direct role of cross bridges in feedbackactivation in skeletal muscle, since Ca21 bound to Tndeclines more slowly than does free Ca21 during relax-ation but faster than force (152). Additionally, in skeletalmuscle, Ca21 binding to TnC does not show a strongdependence on cross-bridge attachment (124) (see sect.IIIC7), although a dependence may exist (as discussed insect. IIIC7; Refs. 49, 52, 469).
In cardiac muscle, studies of relaxation using diazo-2also have not used sarcomere control, but this may be lessimportant since sarcomere nonuniformities are reduceddue to the increased sarcomeric stiffness from passiveelements. In three studies (343, 344, 518), the rate con-stants for the decline of force did not depend on the initialforce level. This implies there is either no effect of crossbridges to slow relaxation in cardiac muscle (as thereappears to be in skeletal muscle) or that the effect ofcross bridges saturates at very low levels of attachment.This would be consistent with Tm moving more readily incardiac muscle (see sect. IIIC6). However, because strongcross-bridge attachment promotes Ca21 binding to TnC incardiac muscle (see sect. IIIC7), one might expect higherforces to promote Ca21 binding, continued thin filamentactivation, and slower relaxation if substantial reattach-ment of cross bridges occurred during relaxation. Thatthis does not occur suggests that relaxation is determinedmore by the kinetics of cross-bridge detachment than byreattachment. This was most clearly demonstrated in thestudy of Palmer and Kentish (344). They found that therate of relaxation was nearly equal to the respective max-imum kTR and kCa for rat and guinea pig cardiac muscle,whereas the maximum ATPase of the two animals dif-fered due to their respective cardiac myosin isoforms.The kinetics of relaxation in skeletal and cardiac musclepoint out major differences in activation between thesetwo tissues, with cooperative activation by cross bridgesplaying a more direct role at higher force levels in skeletalmuscle, but perhaps a less direct role in cardiac muscle(440). In cardiac muscle, strongly attached cross bridgesenhance Ca21 binding to TnC and thereby promote con-
tinued activation (discussed in sect. IIIC7). As Ca21 acti-vation falls during relaxation in cardiac muscle, both theCa21 activation and strong cross-bridge attachmentwould decline with a rate equal to the sum of the apparentrate constants for strong cross-bridge attachment anddetachment, fapp and gapp. This sum would be approxi-mately equal to the maximum kTR and kCa (see discussionof model in sects. IIIC2 and IV). Thus, in cardiac muscle,strong cross-bridge binding, Ca21 binding, and activationare more tightly coupled than in skeletal muscle.
9. Shortening-induced deactivation
An important aspect in the study of contractile reg-ulation is the question of whether the mechanical pertur-bations themselves affect regulation. For some time it hasbeen recognized that, in both skeletal and cardiac muscle,shortening and/or stretch alter contractions [see Edman(89) for a review]. In a definitive study, Edman (90) de-scribed the effect of a shortening step to decrease subse-quent force development either in a twitch or series oftwitches, a phenomenon he called “shortening-induceddeactivation.” In subsequent studies, this shortening-in-duced deactivation has been demonstrated for steady-state force and the rate of force redevelopment and, morerecently, for shortening velocity.
The main effect of shortening initially described (90)was that the force developed subsequent to a shorteningstep was less than the isometric force developed at theshorter length. Because of the effect of sarcomere lengthon isometric force, Edman (90) was careful to operate atsarcomere lengths where the maximum isometric forcevaried little with length. Edman (90) demonstrated thatthe deficit in force due to shortening increases with thedistance shortened, is present even if the shortening oc-curs after activation but before force develops, disap-pears with time (about one second) as stimulation con-tinues, and is independent of the load during shortening(loads from 0 to 1/3Po). On the other hand, the amount offorce deficit and shortening-induced deactivation de-pends on the level of Ca21 activation, decreasing as Ca21
activation approaches maximal (88). It also depends onsarcomere length but with the same dependence as themaximum force (88).
Shortening-induced deactivation was also shown todecrease the rate of force redevelopment subsequent to ashortening step (88, 469). As was the case for the forcedeficit described above, the deficit in the rate of forceredevelopment after shortening depends on the level ofCa21 activation, decreasing as the Ca21 activation ap-proaches maximum (95). Furthermore, it depends on thelevel of force at the time of shortening, decreasing thesmaller the initial force (469).
In contrast, there appears to be little effect of short-ening-induced deactivation on the unloaded shortening
896 GORDON, HOMSHER, AND REGNIER Volume 80

velocity, at least in the intact fiber (88, 469). In skinnedfibers, it has been postulated to be responsible for theslowing of shortening at submaximal Ca21 during longerlength steps (230) (see sect. IIIC5 on control of the short-ening velocity).
There are several possible mechanisms for this deac-tivation as pointed out by Ridgway and Gordon (394). Thefirst is that shortening enhances relaxation because cross-bridge detachment rates are faster when the fiber is short-ening than when it is held isometric (217, 218). The sec-ond is that shortening-induced detachment of crossbridges releases Ca21 bound to TnC by changing the Ca21
affinity of TnC (32, 394). This feedback effect of stronglyattached cross bridges to enhance Ca21 binding is dis-cussed in section IIIC7. The third mechanism is that short-ening-induced detachment of strongly attached crossbridges removes the cooperative activation or mainte-nance of activation of the thin filament by strongly at-tached cross bridges (32, 394). As suggested by Edman(90), there may be other explanations involving long-lasting structural changes in the myofilaments.
The question is which of these mechanisms is rele-vant. The first only applies to relaxation and will not beconsidered further. The effects of strongly attached crossbridges to enhance both Ca21 binding and thin filamentactivation directly (through stabilizing Tm in the activat-ing position) may be important in mediating shortening-induced deactivation. There is strong evidence that de-creases in Ca21 affinity of TnC occur with detachment ofcross bridges accompanying muscle shortening as dis-cussed in section IIIC7. Ekelund and Edman (95) sug-gested this mechanism and it was supported by the ob-servations of Ridgway and Gordon (394) and Gordon andRidgway (150), who measured the increase in intracellu-lar Ca21 accompanying shortening steps in activated,aequorin-injected barnacle single muscle fibers. Recentstudies by Vandenboom et al. (469) verified the extraintracellular Ca21 on shortening in frog single musclefibers and the correlation of the changes with deactiva-tion. The problem with these studies is the inability todetermine whether the decrease in bound Ca21 is thecause of the shortening-induced deactivation or the resultof the decrease in thin filament activation by stronglyattached cross bridges. In other words, is the decline inTnC-bound Ca21 (signaled by the rise in free Ca21) di-rectly responsible for the decrease in thin filament acti-vation and force seen with continued stimulation or is itonly secondary to changes in activation by strongly at-tached cross bridges? The only quantitative measurementof changes in bound Ca21 in skeletal muscle indicates thatbound Ca21 does not change significantly with shortening(124). This would imply the extra Ca21 seen with short-ening (by isotopic or aequorin techniques) is probably aresult of, not the cause of, the change in thin filamentactivation.
The data support the hypothesis that shortening-in-duced deactivation of the thin filament results from areduction of strongly attached cross bridges. This is con-sistent with the deactivation dependence on extent ofshortening (89) and the level of force before the shorten-ing. The smaller deactivation at higher levels of Ca21 isconsistent with either explanation. Decreases in Ca21
affinity upon shortening would change Ca21 binding littleat saturating Ca21. In like manner, decreases in stronglyattached cross bridges upon shortening would have lessof an effect at maximal activation when cross-bridge at-tachment is at a maximum. In addition, release and re-stretch experiments in barnacle muscle (Ridgway andGordon, unpublished data) indicate that substantial extraCa21 can be observed with no change in force. Recentobservations on the effect of shortening on thin filamentactivation using a fluorescent probe on TnI show thatthere is a small decrease in activation accompanying ashortening step from which the thin filament recoversquickly (41). It is not clear that this change is sufficient toexplain the deactivation seen in all cases. Finally, asdiscussed in section IIIC5, the slowing of the unloadedshortening velocity with extended shortening at submaxi-mal Ca21 activation is also most consistent with deacti-vation via a decrease in the number of strongly attachedcross bridges, resulting in a reduction of thin filamentactivation [see discussion of Iwamoto’s results (230) insect. IIIC5]. The slowing of unloaded shortening velocitywith extended shortening at submaximal activation isalso seen when using a non-Ca21-requiring activator,aTnC (see sect. IIIC2). This is consistent with the deacti-vation not being tied to Ca21 binding but to decreasedstrong cross-bridge attachment. More experiments withCa21-insensitive activation with conditions like aTnC anddirect measures of thin filament activation are needed todetermine definitively the cause of shortening-induceddeactivation.
There are significant physiological implications ofshortening-induced deactivation that must be considered.In the mechanical measurements discussed in this review,the two that are most likely to be affected are measure-ments of kTR and shortening velocity. In the kTR measure-ments, a large shortening step is followed sometime laterby a restretch to the original length. This should put themuscle back to initial conditions where activation is de-termined by Ca21 binding and the initial steps in activa-tion before strong cross-bridge attachment. Thus the ef-fect of shortening-induced deactivation on the normal kTR
measurement is to make the result less dependent on theconditions at the time of the length changes and morecharacteristic of the initial activation process. However,very short periods of shortening or not restretching thefiber to detach cross bridges could yield variable condi-tions of strong cross-bridge activation due to shortening-induced deactivation and make the results more difficult
April 2000 MUSCLE REGULATION 897

to interpret. Second, as discussed above, the shorteningvelocity in fibers and the early phases of shortening inskinned fibers are little affected by shortening-induceddeactivation, but the later phases at submaximal Ca21
activation may be. If Iwamoto’s hypothesis is correct(230), this could account for the slowing seen during thelater phases of shortening. Thus the effect of shortening-induced deactivation must be considered in understand-ing the mechanical performance of skeletal and cardiacmuscle following muscle shortening. Furthermore, theexistence of this deactivation supports the role of stronglyattached cross bridges in sustaining activation at highlevels of force in skeletal muscle.
D. Regulation of Reconstituted Thin Filaments
in In Vitro Motility Assays
The in vitro motility assay is a powerful approach forexamination of the mechanical sequelae of structural al-teration of the regulatory proteins [see Sugi (427) for arecent review]. Currently, myosin, HMM, or S1 are boundto a surface coated with nitrocellulose or dichlorometh-ylsilane. Thin filaments, usually labeled with rhodaminephalloidin (RhPh), are then applied to the surface wherethey bind to the motor proteins. On addition of MgATP,the filaments slide over the surface at speeds (Vf) compa-rable, but not identical to, speeds at which thin filamentsslide past thick filaments in the intact or skinned musclefiber (,0.75Vu). It is generally assumed that the motion ofthe thin filament is unrestrained by drag produced by thesolution (see sect. IIIC5) or interaction of the filament withthe surface. The power of this assay stems from thespecificity with which chemical and genetically modifiedproteins can be substituted in the assay and correlatedwith effects on mechanical behavior. Recently, motilityassays have been used to measure isometric force pro-duced when thin filaments, attached to flexible mi-croneedles (249, 468) or to glass beads held in opticaltraps (112, 485), are brought into contact with the surface.These approaches have made examination of mechanicalbehavior of single or small arrays of contractile proteins areality. Studies of myocytes transfected with mutant pro-teins (400, 433, 488, 494) or muscles from transgenicanimals (299, 314) are superior in that the filament geo-metric lattice is retained and the protein is in an environ-ment more like that in the living animal. However, in vitromotility assays offer experimental simplicity and speedfor evaluation of mechanistic changes consequent to pro-tein structural changes and are likely to be a useful ap-proach for screening mutations for years to come. Re-cently, Ishiwata and colleagues (127, 129) have developedan approach in which thin filaments in skinned musclefibers are enzymatically digested (by gelsolin) and thenreconstituted using actin and regulatory proteins. Al-
though this approach has exciting potential, it has notbeen successfully applied to skeletal muscle, and theformation of the thin filaments and the length and unifor-mity of the reconstituted thin have yet to be successfullycontrolled.
1. Limitations of the in vitro motility assay
Several limitations of in vitro motility system shouldbe considered when interpreting motility data. The mostserious problem is one of geometry. Myosin heads boundto the motility surface are randomly oriented. Thus themeasurements cannot be used to directly estimate theforce exerted or the cross-bridge throw size because theorientation of the motor(s) with respect to the thin fila-ment orientation is indeterminate. To alleviate this prob-lem, Sellers and Kachar (409) and Yamada et al. (508)measured thin filament sliding along isolated molluscanthick filaments. They found that thin filaments slide alongthe thick filaments at a speed comparable to those of theintact muscle. The movement of the thin filaments towardthe center of the thick filament is ;10-fold faster than thataway from the center of the thick filament. The use ofclam thick filaments would preclude the ability to usemyosin mutations and restricts the calcium concentrationused in thin filament regulation studies, since the activa-tion of the molluscan thick filaments is [Ca21] dependent.Attempts to create oriented filaments from isolated myo-sin have been problematic. Toyoshima et al. (461) boundmyosin to thin filaments in the absence of ATP and ap-plied the decorated thin filaments to the motility surface,supposing that the actin filament would orient myosinbinding to the surface. Their studies, however, indicatedthat the myosin that bound was not oriented. Recently, inan effort to orient the myosin head, Yamada and Wakaba-yashi (509) made thick filaments from myosin and Ishi-jima et al. (228) made thick filament copolymers of myo-sin rods and intact myosin at different ratios. In the lattercase (228), “thick filaments” were 5–8 mm long and somewere as wide as 0.2 mm at their center. Thin filamentsliding speeds of 9–10 mm/s toward the center of thecopolymer and ;2.5 mm/s for speeds sliding away fromthe center of the copolymer were observed, similar tothose observed by Sellers and Kachar (409) and Yamadaet al. (508). However, structural evidence showing thatcopolymers were structurally polarized at the cross-bridge level was not presented. Furthermore, measure-ment of force exerted on thin filaments attached to mi-croneedles by these thick filaments fell by only 50% for a20-fold reduction in myosin head content per unit lengthof the copolymer. Additional work should be directedtoward production of oriented myosin heads for motilityassays.
The Vf observed in the motility assays of unregulatedthin filaments is less (by 30–40%) than that seen in muscle
898 GORDON, HOMSHER, AND REGNIER Volume 80

fibers at temperatures at which the two can be compared(10–20°C) (6, 211). In addition to the myosin head orien-tation problem, this behavior could stem from the pres-ence of inactive or enzymatically damaged myosin S1heads (255), differences in ionic strength (211), structuraldifferences between the fiber or motility assay, or the lackof regulatory proteins on the thin filament. The addition ofregulatory proteins produces significant increases in thinfilament sliding speed. Sellers and co-workers (48) havereported a 30% increase in sliding speed of small smoothmuscle myosin-coated beads sliding over Nitella actincables exposed to Tm and a 10-fold increase in speed ofthin filaments with Tm bound propelled over surfacescoated by Limulus myosin. Gordon et al. (148) find thatthe unloaded unregulated thin filament sliding speed in-creases from 5 to 6.5–8 mm/s (30–60%) when skeletalTm/Tn are bound to thin filaments at pCa 5, while Marstonand co-workers (17) also found that skeletal Tn enhancedthe speed of actin-skeletal Tm filaments by 21%. Others(208) find that cardiac regulatory proteins produce a 30%increase in thin filament sliding speed at pCa 5. Marstonand co-workers (18) reported no change in thin filamentsliding speed when skeletal Tm was bound to actin fila-ments, but an ;40% increase in thin filament sliding speedwhen smooth muscle Tm binds to actin filaments. Thesmall effects of Tm alone are not dependent on the pres-ence of Ca21 (145). Thus regulatory proteins in the pres-ence of Ca21 potentiate thin filament Vf. It could beargued that binding of regulatory proteins stiffen the thinfilaments (compared with unregulated F-actin) and thusincrease Vf. However, the difference in thin filament per-sistence length (a measure of filament stiffness) in thepresence and absence of regulatory proteins is too smallto make a significant change in Vf (224). These resultsimply that regulatory proteins allosterically modify theactin structure and its interaction with myosin. In addi-tion, when myosin is oriented and thin filaments containTm/Tn, Vf may approach Vu.
Significant changes in the thin filament sliding speedare produced by isoforms of myosin (408) and differenttypes of enzymatic preparations of myosin (HMM and S1)(460). Rabbit skeletal HMM is often used because myosinproduces fragmentation and erratic movement of thinfilaments. Other groups find that rabbit myosin propelsthin filaments at high speeds [e.g., 8 mm/s at 22°C (169)and 8.6 mm/s at 27°C (229)]. S1 can propel thin filamentsbut does so at a speed 50% of that seen using HMM (460).The reason for this decrease is probably an ineffectivebinding of the S1 to the motility surface. It has beenshown that attachment of the tail of the myosin to thesurface by antibodies directed to the S2 or S2-LMM regioncan significantly increase Vf (502).
Another limiting condition for motility studies is thefact that the actin, Tm, and Tn concentrations must bedilute. The actin concentration in the motility assay is
usually ;8–50 nM. Higher concentrations reduce the con-trast between the filament and background. AlthoughRhPh stabilizes thin filament structure, actin mutants mayalter actin structure so that binding of RhPh is not strongenough to stabilize the actin filaments at very dilute actinconcentrations. The Tm and Tn concentration must besufficient to ensure saturation of the reconstituted fila-ments, but not so great that filaments bundle.
Finally, too often the methods of data selection andanalysis of thin filament movement are not described. Forall analyses, selection criteria, definition of what consti-tutes a filament, how the frame to frame speeds aredetermined, the length of time filament movement is mon-itored, how frequently the movement is sampled, and howsliding speeds are calculated should be described. This isneeded because the recorded speeds depend on themethod of speed measurement. Sampling at too low a ratetruncates the filament path and underestimates Vf. If onesamples at too great a rate, sampling error of the speedincreases and can render mean speeds meaningless. Thepresence of noise (arc wander, vibration of the micro-scope stage with respect to the image plane, photobleach-ing, and weak binding to the motility surface) contributesignificantly to movement noise and make slowly or non-moving objects appear to move, when automated dataanalysis systems are used. Finally, motility fields can varyfrom one part of the coverslip to the next. Consequently,it is wise to sample from multiple fields to produce esti-mates representing the population as a whole. Althoughthere are some limitations to the in vitro measurements,with proper attention to detail, reliable results can beobtained.
2. Characteristics of thin filament movement over
motility surfaces
Thin filaments move over a motility surface at similarspeeds over a wide range of HMM densities, but Vf isproportional to the HMM ATPase for a variety of myosinisoforms (408). Many reports of thin filament motility usesurfaces equilibrated with myosin solutions containing100–500 mg/ml yielding a surface density of 1,500–2,500HMM molecules/mm2 (17, 18, 20, 121, 148, 164, 169, 206,211, 279, 401, 408). Reduction of the HMM concentrationto 20–30 mg/ml produces little change in unregulated thinfilament sliding speed, suggesting S1 head density is suf-ficient to ensure continual movement of the thin filament.This behavior is reminiscent of the independence of Vu onsarcomere length between sarcomere lengths of 1.7 and3.1 mm. When the surface is equilibrated with ,20 mg/mlHMM, Vf is reduced, suggesting that there are an insuffi-cient number of heads available to ensure continualmovement. Another well-documented behavior is the in-dependence of thin filament sliding speed for thin filamentlengths above ;1 mm (467). This result suggests that
April 2000 MUSCLE REGULATION 899

beyond this filament length, enough heads are attached atany time to give continuous movement. In addition, itcould mean that a non-cross-bridge viscous drag forceproportional to thin filament length exists or that both thedrag force and propelling force acting on the thin filamentare proportional to thin filament length. Because calcula-tions of solution viscosity render the former possibilityunlikely, the latter possibility is more probable. Thiswould be the case if the drag force were dependent on thenumber of cross bridges interacting with the S1 heads, asin the Huxley view of unloaded sliding speed (215).
When the [ATP] is reduced or the [ADP] is increased,the rate of cross-bridge dissociation from the thin filamentis reduced and Vf falls in a manner reminiscent of thebehavior of Vu in skinned muscle fibers (211, 486). Alsogreatly elevated nonionic solute concentrations decreaseVf in a manner similar to decreases in Vu in skinnedmuscle fibers (61). Finally, Bing et al. (17) have suggestedthat the nitrocellulose surface (as compared with a sur-face of dichloromethylsilane) produces a drag on the thinfilament that slows the thin filament movement. This sug-gestion is inconsistent with the data obtained from nitro-cellulose surfaces described above. It is also inconsistentwith the fact that when regulated thin filaments attachedto microneedles are dragged over an HMM covered sur-face at pCa 9, the displacement of the microneedle isimperceptible (208). Thus the drag force is ,2% of theforce exerted on thin filaments by HMM in isometricconditions at pCa 5. In conclusion, factors that control thesliding speed in the in vitro motility assay appear to besimilar to those in skinned muscle fibers (see sect. IIIC5).
3. Early studies of regulation of thin filaments sliding
in the in vitro motility assay
Early reports of the sliding speed of regulated thinfilaments indicated that regulation occurred in an on oroff (step function) fashion seemingly predicted by Hux-ley’s view of Vo (215). At pCa .5.7 (212) or .6.1 (169),thin filaments bound to a motility surface but did notmove. However, when pCa was lowered further to 5.6 or6.0, respectively, regulated thin filaments began to move amaximum speed. A recent study by Sata et al. (401)confirmed these observations using rabbit skeletal actinand cardiac regulatory proteins. Unfortunately, thesestudies did not define the methods used to quantitatefilament movement or selection and did not specify theprotein constituency of filaments (actin:Tm:Tn). Fraserand Marston (121) successfully addressed some of theseissues by varying the amount of regulatory proteins at-tached to the thin filaments and by randomly selectingfilaments for analysis before observing their movementand quantitating the number of filaments moving and theiraverage speed. They also concluded that calcium regula-tion was an “all-or-none” process. However, their data
showed that at as [Ca21] fell from 3 3 1025 to 1 3 1029 M,sliding speed fell progressively from 5.2 6 1.0 to 3.3 6 0.8mm/s. Furthermore, at 1 3 1029 M, a [Ca21] at whichisometric force is zero, and the muscle does not activelyshorten, 20% of the thin filaments were still moving at aspeed equivalent to that of naked actin. In their experi-ments, Fraser and Marston (121) used motility solutionscontaining ,100 nM [Tn] and no added Tm. This is prob-ably too little free Tm/Tn to produce complete regulationof the thin filaments. Given the binding of Tm and Tn tothin filaments (455), ;100 nM of each will be required tosaturate the thin filament in the motility assay.
4. Effects of pCa on regulated thin filament sliding
speed and force development
Recent experiments using fully regulated thin fila-ments in the in vitro motility assay utilized cardiac orskeletal regulatory proteins (148, 206). These two studiestook care to ensure that the thin filaments were com-pletely regulated by including 80–100 nM Tm and 100 nMTn in the motility and wash solutions. Furthermore, thetwo studies used an automated motion analysis systemallowing mapping and analysis of the movement of largenumbers of thin filaments. In these studies, the movementof each filament’s centroid was followed for 2 s or more,and the number of moving and nonmoving filaments wasdetermined. The results of these two sets of experimentsare similar both in the conclusions and the absolute val-ues. The thin filament movement over motility surfaceswas measured at various pCa values ranging from 4.5 to 9.Each group measured the fraction of thin filaments mov-ing and the average speed of the moving filaments at eachionic strength. The two groups studied motility at differ-ent pH values [7.4 and 7.0 for Homsher et al. (206) andGordon et al. (148), respectively] so one expects a shift inthe Ca21 sensitivity (;0.8 pCa units), but they also useddifferent regulatory proteins, cardiac and skeletal, whichshould also affect the shift. Gordon et al. (148) also mea-sured isometric force in skinned muscle fibers in parallelexperiments. Table 3 summarizes the results of eachgroup. The primary conclusions are as following.
1) At pCa 9, few, if any, filaments move, whereas atpCa 4.5–5, 80–90% of the thin filaments move at maximalspeed. Thus complete regulation of thin filament move-ment is obtained.
2) Both the speed of moving filaments and the frac-tion of filaments moving can be fit by an equation of theform
y 5 ymax/@1 1 10n~pCa2pCa50!#
where y represents either the filament speed or fraction ofmoving filaments; ymax is the maximum sliding speed, Vf,or maximum fraction of moving filaments, fmax; n is the
900 GORDON, HOMSHER, AND REGNIER Volume 80

Hill coefficient; and pCa50 is the value of pCa at which thevalue of y is half-maximal.
3) The sliding speed is not very cooperative (n , 2.2),while the number of sliding filaments generally exhibitedgreater cooperativity.
4) When comparable plots of the force-pCa curvewere made using either skinned muscle fibers contractingunder the same conditions as the motility assay (148) orusing microneedles with single regulated thin filamentsattached in motility assays (208), both groups found thepCa50 for the force-pCa curves to be 0.4–0.8 pCa unitssmaller than the speed-pCa curves. These data suggestthat as the calcium concentration is reduced the forcedeclines before any change in speed is observed.
Table 3 shows that although the absolute valuesobtained using cardiac or skeletal regulatory proteinsare not identical (different pH values and regulatoryproteins were used), the behavior is qualitatively simi-lar and very different from the earlier studies (169, 212,401). Both groups examined the relationship betweensliding speed of thin filaments with and without regu-latory proteins. Gordon et al. (148) found that the un-loaded sliding speed of thin filaments with regulatoryproteins was 30 –50% greater than for F-actin alone atthe same ionic strength. Homsher et al. (206) found thatcardiac regulatory proteins had little effect on the max-imal sliding speeds, although in later studies they (208,279) observed a 10 –30% increase. Both groups con-cluded that that relatively few cross bridges are neededto make the filaments move but that significant num-bers are needed to propel the filaments at maximalspeed. Because force decreases significantly at pCavalues at which speed is unaffected, control of contrac-tion is primarily exerted by controlling the numbers ofattached and cycling cross bridges rather than control-ling the rate or size of the cross-bridge throw in the
cross-bridge cycle. The modified steric blocking hy-pothesis of Vibert et al. (475) and McKillop and Geeves(303) is consistent with this conclusion. In the absenceof Ca21, the Tm is in a position (blocked) that allowsweak cross-bridge attachment but not strong cross-bridge attachment. In the presence of Ca21, Tm oscil-lates between a position that allows weak attachmentand access to some of the strong binding positions(closed) and a position that allows access to all of theweak and strong binding sites (open). As Ca21 is re-duced, the fraction of actin sites occupied by crossbridges will decrease, and with it force, but enoughcross bridges can attach to ensure that the filamentslides at maximal speed. Model calculations suggestthat if .20% of the cross bridges are attaching andcycling, then the thin filament will move at near (.95%)maximal speed (206). However, as the percentage falls,the sliding speed should fall in a fashion that dependson the assumed duty cycle time and strong binding timefor myosin (467) (see discussion of shortening velocityin sect. IIIC5).
LaMadrid et al. (260) have, however, reported thatthe speed-pCa curve for moving filaments depends littleon the filament length and thus may be relatively inde-pendent of the total number of S1 heads interactingwith a thin filament. One interpretation of this obser-vation is that [Ca21] may modulate the cross-bridgepower stroke or dissociation rates as well as the con-trolling strong cross-bridge attachment. A possibleproblem with this interpretation is that the motion ofthe filaments may be erratic. As filaments slide, mostundergo periods of time when the leading edge movesmore slowly than the trailing length so that the filamenttends to fold. A few hundred milliseconds later, theleading edge accelerates and straightens the foldedsections of the thin filament. Monitoring the movement
TABLE 3. Effects of pCa on regulated thin filament sliding speed and force development
G/2, mM
Skeletal Cardiac
85 115 140 50 100
SpeedpCa50 6.1 6 0.2 5.9 6 0.1 5.8 6 0.1 7.0 6 0.2 6.8 6 0.2n 1.0 6 0.3 1.7 6 0.3 2.2 6 0.5 0.7 6 0.4 1.2 6 0.4Vfmax
, mm/s 7.1 6 1.1 7.4 6 0.4 6.2 6 0.6 6.3 6 0.8 7.4 6 0.7Fraction moving
pCa50 6.0 6 0.1 5.6 6 0.2 5.6 6 0.1 6.9 6 0.1 7.1 6 0.1n 1.4 6 0.5 0.9 6 0.2 4.3 6 1.7 2.6 6 1.4 4.1 6 0.6fmax 0.8 6 0.1 0.8 6 0.2 0.5 6 0.1 1.0 6 0.1 0.9 6 0.1
Normalized forcepK 5.4 6 0.1* 6.2 6 0.3†
n 1.9 6 0.2* 1.7 6 0.1†
fmax 1 1pH 7.0 7.0 7.0 7.4 7.4
fmax, Maximum fraction of moving filaments; n, Hill coefficient; pCa50, value of pCa at which value of filament speed is half-maximal; Vfmax,
velocity of fmax; G/2, ionic strength of solutions used. * Data from skinned skeletal muscle fiber. † Data from microneedle force measurements.
April 2000 MUSCLE REGULATION 901

of the filament centroid smooths such deceleration andacceleration changes in speed of segments of the fila-ments. Thus centroid monitoring blurs such changesand produces a slowed Vf. This analysis-dependentblurring of filament movement is similar to the reduc-tion in filament sliding speed seen when the HMMdensity on the surface of the motility assay system isreduced. An important test of this interpretation ismeasurement of the persistence length of thin filamentsmoving over various motor densities. The hypothesispredicts that the lower the motor density, the smallerthe persistence length.
5. Activation of thin filaments by attached
cross bridges
Under some conditions, the thin filament slidingspeed does depend on the number of cross bridges pro-ductively interacting with the thin filament, demonstrat-ing that strongly bound cross bridges can activate the thinfilament. Bobkov et al. (20) examined the effect of mod-ification of the SH1 by NEM or N-(1-oxyl-2,2,6,6-tetra-methl-4-piperidinyl)iodacetamide (IASL) on the regulatedthin filament sliding speed at pCa 5. They found thatSH1-modified HMM bound to the motility surface withoutunmodified HMM could not translocate thin filaments.NEM-S1 binds to actin very strongly and IASL-S1 binds toactin ;10 times more strongly than S1 in the presence ofATP. When the density of HMM on the motility surfacewas reduced (reducing the [HMM] in the labeling solutionfrom 400 to 60 mg/ml to 40 mg/ml), the regulated thinfilament sliding speed fell from 5.3 mm/s (87%) to 4.9 mm/s(64%), to 2 mm/s (9%), respectively (values in parenthesesindicate the percentage of smoothly moving filaments).However, if 1 mM IASL-S1 was added to the motilitysolution (to bind to the regulated thin filaments, but not tothe motility surface), the filament sliding speeds de-creased little with declining [HMM] being 4.8 mm/s (95%),4.9 mm/s (85%), and 4.0 mm/s (75%) at the corresponding[HMM] densities. Similar results were obtained on addingNEM-S1 to the motility solution. Thus cross bridges(IASL-S1) strongly bound to regulated F-actin (and not tothe motility surface) can activate the thin filament, per-haps stabilizing the Tm in the open position and cooper-atively exposing actin binding sites to surface-boundHMM on either side of the IASL-S1-bound head. Thisresult agrees with the work of Williams et al. (500) andSchwartz and Moss (429) on the thin filament activationeffects of SH1 modified S1.
6. Genetic modification of TnC and its effects on thin
filament sliding speed and force
Thin filaments can be reconstituted with native Tmand Tn whose TnC subunit has been modified so that it isunable to bind Ca21. Cardiac TnC has only one regulatory
calcium-binding site, and mutation of a coordinating car-boxyl group (D65A) renders TnC unable to bind Ca21
(375). The cTn containing D65A is designated CBMII-Tn(221, 375). Skeletal TnC has two calcium NH2-terminal orregulatory calcium binding sites that can be modified bypoint mutations (D27A and D63A) so that neither of itsregulatory Ca21 binding sites can be activated (444). Skel-etal Tn containing sTnC with these mutations is calledxxsTn (387) (see sect. IIIC1). The effects of varying theratio of cTn to CMBII cTn or sTn to xxsTn on motilityassays of regulated thin filament Vf and skinned musclefiber Vu have been studied by Morris and co-workers (208,210, 327) and by Chase and co-workers (277, 387), respec-tively. Their strategy was to examine how thin filamentinactivation at constant saturating pCa affects the un-loaded sliding speed, force, and kTR. The results of bothgroups are similar. First, as the fraction of CBMIITn orxxsTn bound to the thin filament increases, the thin fila-ment sliding speed in the in vitro motility assay does notchange significantly until the fraction is greater than ;0.7.This result implies that the unloaded sliding speed islargely independent of the number of cross bridges inter-acting with the thin filaments. This contrasts with theeffects of these mutants on force and kTR discussed insections IIIC1 and IIIC2, respectively. Isometric force ex-erted by single muscle fibers in which the mutant Tn hasreplaced the native Tn decreases nearly in direct propor-tion to the extent of native Tn replacement while kTR islargely independent of the amount of mutant Tn incorpo-rated. As noted in section IIIC2, these data suggest that themaximum kTR is not strongly influenced by “near neigh-bor” cooperativity between neighboring A7TmTn units.
7. Modification of actin, Tm, and Tn residues and
their effect on regulation
Recent studies of the sequelae of modification ofamino acid residues of actin, Tm, and TnT have providedadditional insights into the mechanism of regulation.These mutations produce a variety of effects on the ac-to-S1 ATPase, force, Vf, and calcium sensitivity in bothmotility assays and muscle fibers (see Table 4). Studies inthis area have been stimulated by the fact that specificdominant negative mutations in thick (myosin) and thin(actin, Tm, and TnT) filament proteins are associated withthe expression of FHC. This disease manifests itself by ahypertrophy of the myocardium in the absence of in-creased load and is followed by sudden cardiac arrest.The disease is frequently undetected until the patient hasa heart attack. Thick filament FHC mutations produce asignificant and uniform hypertrophy of the myocardiumwith few clinical symptoms and a variable frequency ofsudden death. The FHC TnT mutations usually exhibit amild hypertrophy but a high incidence of sudden death atan early age (447). The implication of many studies of the
902 GORDON, HOMSHER, AND REGNIER Volume 80

thin filament FHC mutant proteins is that point mutationsand/or deletions of single amino acid residues markedlyalter the nature of the interaction between actin andmyosin. Thus Tm and TnT mutations appear to affectsteps within the cross-bridge power stroke.
Marston and co-workers (18) expressed an actin mu-tation (E93K) and examined its effect on in vitro motility.They hypothesized that the negatively charged actin res-idue E93 repels negatively charged portions of Tm andthus holds Tm in the on state. If true, conversion of thenegatively charged residue to a positively charged residue(K93) would attract Tm to K93 and hold Tm in the off orblocked position. Their tests of this hypothesis showedthat the E93K mutant actin had no significant effect on thethin filament sliding speed in the absence of Tm butreduced sliding speed by ;35% and the number of slidingfilaments by ;60% in the presence of skeletal Tm. Thiseffect could be reversed by addition of NEM-S1. Theysuggest that electrostatic charge on the actin surface ofdomain 2 plays a role in controlling the access of the crossbridge to strong binding sites on the actin surface. Bind-ing of smooth muscle Tm to E93K actin reduced Vf by;40%, whereas smooth muscle Tm addition to wild-typeactin increased Vf by ;35%. This result is consistent withthe effects of smooth muscle Tm on the ATPase of F-actin-Tm (268). These results supported conclusionsdrawn using sTm, but also imply that smooth Tm may also
modify the rate of detachment of the cross bridge fromthe thin filament.
Lorenz et al. (281) have identified six putative siteson the actin surface (primarily in actin domains 3 and 4)whose electrostatic interaction with Tm may control itsposition on, and the activation of, the thin filament bycalcium. One of these sites, the 309–320 loop in actindomain 3, contains four charged residues. Two actin mu-tants E311A/R312A (139) and K315A/R312A (252) withinthis region have been examined. Mutations at 311/312produce no changes in regulated actomyosin ATPase,binding of the regulatory proteins to the thin filament, ormaximal Vf of regulated thin filaments, but it did reducethe [Ca21] at which Vf was half-maximal. Mutations at315/316 markedly reduced the regulated actomyosin ATP-ase and weakened the binding of Ca21 to the regulatoryproteins. These data suggest that either the modificationof the Tm position or an allosteric effect on the actinstructure alters the interaction of S1 with the thin filamentand supports the view that these sites are important in theregulation of contraction.
Three mutations of the Tm molecule, V95A, D175N,and E180G, are associated with the presence of FHC (345,456). Each produces significant changes in Vf in motilityassays (see Table 4). Studies of skinned slow musclefibers of patients containing D175N Tm revealed no sig-nificant changes in maximal isometric force or Vu, but
TABLE 4. Effect of mutant thin filament proteins on actomyosin interaction
ProteinsActomyosin
ATPase
Thin Filament Sliding Speed
Isometric Force
Ca21 Sensitivity [Ca21]50
Motility Fiber ATPase Fiber
Troponin T
Ile79Asn 1 (279, 511) 1.2–1.5 (208, 399) 1.5 (434) 0.7 (434), 0.8* (208) 0.5–0.7 (511) 1† (399), 1.3 (434)1 (326, 336)
Arg92Glu 1 (511) ? 1.6 (434) 1 (326, 434, 511) 0.7 (511) 1.6 (434), 0.7 (326)Phe110Ile 1.6 (511) ? ? ? 1 (511) ?DGlu-160 1 (456) 0.9 (456) 1 (434) 0.5 (434) ? 0.9† (434)Glu244Asp 1.6 (511), 1 (456) 1.05 (456) ? ? 1 (456, 511) 1‡ (Homsher and
Pavlov,unpublished data)
DExon 15, 16 0.3 (456) 1.1 (456) ? 0.3 (488), 0.9 (336) ? ?Tropomyosin
Asp175Asn ? 1.2 (19) 1 (22) 1 (22) ? 0.8 (22)Gln180Gly ? 1.2 (19) ? ? ? ?Val95Ala 0.8 (458) 0.9 (458) ? ? 0.6 (458) ?D23 1 (262) 0.6 (262) ? ? ? ?D234 0 (263) 0 (263) ? ? ? ?D34 0 (262) 0 (262) ? ? ? ?D345 0 (262) 0 (262) ? ? ? ?D456 0 (262) 0 (262) ? ? ? ?
Actin
Glu93Lys ? 1 (18) ? ? ? ?E311A/R312A 1 (139), 0.5 (252) 1 (139) ? ? 1 (139) 0.3 (139)K315A/E316A 0.5 (252) ? ? ? 0.25 (252) ?
All values are referenced to values obtained in the presence of native or wild-type proteins. Reference numbers are given in parentheses.ATPase was measured in acto-S1 or myofibril preparations in presence of regulatory proteins. Fiber values were measured in skinned muscle fibers,motility assays, or fibers from transgenic animals. D symbol refers to internal deletions of pseudo-repeat units in the tropomyosin molecule, e.g.,D23 corresponds to deletion of amino acid residues Q47-S123. See text for details. * Measured in motility assay system using microneedles.† Measured in studies of thin filament sliding speed at various pCa values. ‡ Sensitivity measured in in vitro motility assays of Vf.
April 2000 MUSCLE REGULATION 903

there was a significant reduction in the [Ca21] at whichthe isometric force was half-maximal (22). The D175N Tmmutation is close to a putative TnT-1 binding region, andit may be that its interaction with TnT in the regulatedsystem is responsible for the change in calcium sensitiv-ity. Like the smooth muscle Tm data above, the altered Vf
seen in these mutant Tm suggests that Tm modifies a stepin the cross-bridge power stroke perhaps an increasedrate of cross-bridge release from the thin filament.
Striated muscle Tm spans seven actin monomers andcontains a corresponding number of quasi-repeat se-quences, each having a putative actin-binding motif.These quasi-repeat sequences are hypothesized to be im-portant in the positioning of Tm over the actin surface andin the regulation of cross-bridge interaction (281). Tobac-man and co-workers (262, 263) have explored the impor-tance of these quasi-repeat sequences in the regulation ofcross-bridge function by expressing a series of mutant Tmhaving two or more internal quasi-repeat sequences de-leted from their structure (262, 263). In each case, repeatsequences 1 and 7 were retained because their overlapallows formation of a continuous Tm strand on the thinfilament. Incorporation of deletion mutants, D234, D34,D345, and D456 (corresponding to deletion of amino acidresidues, Q47-A166, N89-A166, N89-E208, D124-E234, re-spectively) and Tn onto thin filaments completely inhib-ited Ca21-activated ATPase and Vf. Incorporation of dele-tion mutant D23 (deletion of Q47-E124) had no effect onCa21 activation of acto-S1 ATPase but did produce asignificant inhibition (to 60%) of Vf. None of these dele-tions had a significant effect on Tm binding to the thinfilament in the absence or presence of Tn or in the pres-ence or absence of [Ca21]. However, the presence ofmyosin increases the binding of Tm to actin by ;1,000-fold. Deletion mutants D234, D34, D345, and D456 reducethis binding by .100-fold. These results suggest that my-osin binding induces a conformational change in actinwhich determines the azimuthal positioning and bindingof Tm to the actin filament. Deletions of quasi-repeatingsequences 4, 5, and 6 are important in promoting thisbinding and the associated allosteric changes in actinstructure greatly alter processes in the power stroke(262).
The effects of TnT mutations associated with thepresence of FHC (345, 487) have been studied with re-spect to Ca21 binding to Tn, binding of Tn to the thinfilament, acto-S1 ATPase activity, in vitro sliding speedand isometric force exerted on regulated thin filamentsand in muscle fibers. The effects of these mutations aresummarized in Table 4.
In general, the results observed using biochemicaland in vitro motility studies are consistent with the be-havior of skinned muscle fibers from transgenic animalsexpressing the mutant TnT. These studies showed thatthe effects of the mutant proteins on contractile function
do not correspond to any single stereotypic etiology. Fourof the mutant FHC proteins increase Vu or Vf, and threeinhibit maximal isometric force. Two exhibit an increasedCa21-activated regulated acto-S1 ATPase and one mark-edly inhibits Ca21-activated regulated acto-S1 ATPase.Three of the FHC mutant TnT alter calcium sensitivity.One (I79N) expressing the latter change is particularlyinteresting. At a pH .7.1, there is no change in the cal-cium sensitivity, but at pH values ,7.1 or less, there is areduction in the [Ca21] at which the filament is half-maximally activated. Changes in regulated acto-S1 AT-Pase, unloaded thin filament sliding speed, or isometricforce, caused by TnT mutations, imply that TnT directlyaffects a transition between strongly attached cross-bridge states and argue for allosteric effects on the thinfilament. Changes in Ca21 sensitivity could be accom-plished by changes in accessibility of the cross bridge toactin’s interacting surface.
Sweeney et al. (434) transfected quail myotubes withDNA coding for TnT mutations associated with FHC(I79N, R92E, and DE160). The myotubes overexpress theprotein and incorporate it into the regulatory proteins.There is no evidence of a significant histological alter-ations or defects in the myotubes (433). Sweeney et al.(434) measured the force-pCa curve and unloaded short-ening velocity of skinned myotubes containing one ofthree FHC mutant TnT. The results are summarized inTable 4 along with comparable data where available fromin vitro motility assays and acto-S1 ATPase Vmax measure-ments. The results are in good agreement with the datafrom in vitro motility assays and support the idea thatregulatory proteins can modulate the cross-bridge kinet-ics of the strongly bound state in a fashion separate fromtheir ability to control the attachment of cross bridges tothe thin filament. Thus the in vitro motility studies sup-port the conclusion that [Ca21] regulates strong cross-bridge attachment to the thin filament and modulates akinetic step in the cross-bridge cycle.
IV. MODELS OF THIN FILAMENT REGULATION
Models of Ca21 regulation of contraction have beencategorized on the basis of the method used to formulatethe model, from structural to biochemical to physiologi-cal. We are nearing a time when these approaches can beintegrated. In section IIIA, we discussed the thin filamentstructural changes following Ca21 binding, leading first tothe steric blocking model and more recently to a revisedmodel with at least three positions for Tm on actin(blocked, closed, and open). These positions correspondto 1) blocking of all but weak, electrostatic binding sitesfor myosin on actin; 2) blocking of only some hydropho-bic binding sites; and 3) exposing of virtually all myosinbinding sites on actin, respectively (475, 507).
904 GORDON, HOMSHER, AND REGNIER Volume 80

Biochemical models of regulation in the 1970s fo-cused on the effects of Ca21 binding to Tn and the regu-lation of the actin-Tm interaction by Ca21 binding andstrong myosin binding [see Tobacman (455) for a detaileddiscussion of this work]. These studies culminated in thedetailed descriptions of the interactions between the reg-ulatory proteins discussed in section IIA. They provided amore complete understanding of the factors that mayaffect Tm position on the thin filament and changes in theactin filament during activation. The models of this acti-vation evolved from studies over the last two decades(discussed in sect. IIIB) on the cooperative binding ofmyosin to the regulated filament. These models have beenmainly allosteric models of regulation [see Tobacman(455) for a detailed discussion]. By this it is meant thatCa21 binding or strong myosin binding changes the struc-ture of the thin filament so that myosin binding at anothersite is enhanced. In these models, the thin filament isswitched between a weak and strong myosin binding statewith the equilibrium determined by strongly attachedcross bridges and Ca21 binding. This model assumes thatthe two states correspond to the thin filament being ineither an off or on conformation. The first formulation ofthis model was by Hill et al. (191) and was based on thestudies of the cooperative binding of myosin to the regu-lated filament (160, 465, 499). The results were interpretedin terms of myosin binding to a A7TmTn-regulated unitwith interaction between neighboring units. The assump-tion was that the entire regulated unit was switched froma weak to strong binding state. The equilibrium betweenthe on and off forms was determined by strong cross-bridge and Ca21 binding to that unit and neighboringunits. The model was simple, mathematically precise, andfit the data. Studies of the regulation of actomyosin ATP-ase (161) ruled out a more complex model with Tmoccupying more states (192) on the basis of their bindingdata. Their studies indicated that the binding of Ca21
activated the ATPase activity to only 10–20% of maximaland that full activation required strong cross-bridge at-tachment. Lehrer and Morris (270) reached a similar con-clusion. Furthermore, Williams et al. (500) found that atlow [S1], rigorlike cross bridges (NEM-modified S1) couldincrease the ATPase activity of S1 with regulated fila-ments by increasing the Vmax over that observed withCa21 alone, but the Vmax in the presence of NEM-S1 wasnot different from that observed using unregulated F-actin filaments. They also found that the actin concentra-tion required to activate the ATPase was much less thanthat for binding, implying a different rate-limiting step forthe ATPase and binding. However, this model of Hill et al.(191) was not consistent with the structural studies of thethin filament (see sect. IIIA) which found that Ca21 causeda major movement of Tm (475). Finally, there were dis-crepancies between the experimental data and the pre-dictions for the kinetics of S1 binding to the regulated thin
filament in the presence and absence of Ca21 (465; see themodel of Geeves and co-workers, next paragraph).
More recently, Geeves and co-workers (135, 179, 302,303) proposed a detailed model with three states for thethin filament and two-step myosin binding to actin. Thetwo steps of myosin binding to actin were applicable toany nucleotide state of myosin and involved first a weakerbinding followed by an isomerization to stronger binding.The three states of the thin filament they termed blocked,closed, and open (analogous to terms describing the En-glish shop of blocked with gate locked for overnightsecurity, closed with door shut as at tea time or duringlunch break, or open for business). In the blocked state,there is no myosin binding, not even weak binding. In theclosed state, there is only weak myosin binding. In theopen state, myosin could bind weakly in any nucleotidestate and then isomerize to the strong-binding, force-producing configuration. The equilibrium constants forthe two myosin binding states depend on the nucleotidestate. The equilibrium constants for the transitions of thethin filament from blocked to closed and closed to openare a function of Ca21 with the blocked to closed transi-tion having a Hill coefficient of 1.8 (179) using fast skel-etal muscle regulatory proteins and 1.36 using cardiacregulatory proteins (390). The blocked to closed transi-tion loses its Ca21 sensitivity at ionic strengths below ;50mM at 2 mM [Mg21] but retains it at 5 mM [Mg21] (297).There was cooperativity within the A7TmTn regulatoryunit but no cooperativity between neighboring units as inthe Hill et al. (191) model, because it was not required toexplain the original data. The authors adduced other datathat the cooperative unit is larger than the seven actinunits (136) and have now expanded the model to includecooperativity between neighboring units (297). In theirnew data, the cooperative unit is not an integral multipleof 7 actins, being greater than 7 but less than 14. Theimplication is that where neighboring Tm overlap, the Tmare coupled and move together and that in between over-lap regions the Tm would be flexible so that the differentactins spanned by one Tm would not all be equivalent.This is an important point to which we will return.
The Geeves model accounts for the highly coopera-tive binding of myosin nucleotide to regulated actin. In-troducing the blocked state circumvents the major prob-lem of the Hill et al. (191) model: the discrepancy betweenthe kinetic studies of S1 binding to regulated filaments inthe presence and absence of Ca21 (465). In those studies,in the presence of Ca21, S1 bound to the regulated fila-ment with the same time course as binding to actin in theabsence of regulatory proteins. In the absence of Ca21,there was a lag in binding unless the regulated filamentwas incubated with 3 S1/A7TmTn unit. This result sug-gested that in the presence of Ca21, .90% of the regulatedfilaments were in the on state, although the equilibriumbinding data had suggested that only 20% were in the on
April 2000 MUSCLE REGULATION 905

state. Introducing a blocked state alleviated this problemand gave good fits to both the equilibrium and kineticbinding data.
This model conforms to structural studies showingthree thin filament states (366, 475) (see sect. IIIA). It alsocorresponds to the conclusion from structural studiesthat, although Ca21 binding shifted the Tm to exposemost of the myosin binding residues on actin, Tm move-ment to expose all the myosin binding sites on actinrequired strongly attached cross bridges. Thus Ca21 bind-ing by itself could not fully activate the thin filament. TheGeeves model has received broad acceptance for its sim-plicity, agreement with the structural and biochemicaldata, and quantitative development.
Despite this broad acceptance, there are some prob-lems with this model. First, there may be more than twosteps in myosin binding to actin. Geeves and Lehrer (136)could detect only total binding (through light scattering)or strong binding (through pyrene fluorescence) (136).The interaction surfaces shown in Figure 4 raise the pos-sibility that there are potentially three different strengthsof binding, if in fact they represent actual equilibriumpositions of Tm. If Tm is quite flexible (as discussedabove and also below in the third alternative), then theremay be a much broader range of binding affinities avail-able, particularly considering other nucleotide states ofthe myosin. Second, the strong binding studies only in-cluded more strongly bound nucleotide forms and did notinclude those most likely involved in the initial myosinbinding, S1zADPzPi. This may be why the blocked statewas considered truly blocked, even to weak binding.Lehrer (266) has suggested that blocked state is producedby TnI binding to actin in the absence of Ca21 to block S1attachment as an alternative to a Tm-blocked state. Be-cause TnI does not span seven actins, this is a less likelyalternative. Third, the three structural states discussed insection IIIA and the blocked, closed, and open states donot match up exactly. For example, in the presence ofCa21, McKillop and Geeves (303) found that 20% of theregulatory units were in the open state, yet the structuralstudies show no open actins with Tm still covering someof the myosin binding sites on all actins (Fig. 4). A numberof interpretations are possible. 1) The Tm position is anaverage one so that at any time, 20% of the A7TmTnregulatory units are actually in the fully open state with allmyosin binding sites on actin available while the otherregulatory units are in a more closed state. This does notseem likely, since the position of the Tm is well defined(but see Squire and Morris, Ref. 421). 2) If Tm is veryflexible, then within one regulatory unit such flexibilitycould remove much of the discrepancy with the structuralstudies. Within one unit A7TmTn, a flexible Tm in thepresence of Ca21 might allow one to two actins in theopen configuration and five or six in the closed configu-ration (or 3 actins in a A14Tm2Tn2 “unit”) and be consis-
tent with the structural, biochemical, and physiologicaldata for skeletal muscle. As discussed above, Tm may beso flexible that it could move about its equilibrium posi-tion to open up all myosin-binding sites on 20% of theactins at any one time. If this occurs within one unit, thestate of each actin must be defined. It may be that strongattachment of S1 stabilizes Tm in the fully deflected po-sition with all actin sites open. 3) Myosin binding to theactin sites available in the presence of Ca21 provides theinitial binding from which the isomerization of myosinaids in “pushing” the Tm into the fully deflected position,the fully open state. The data of Swartz and co-workers(430, 517) on S1 binding to myofibrils argues against thislatter interpretation because their data would require thatboth the closed and open states of actin would have thesame relative affinity for rigor S1 and S1zADP. ThusGeeves and co-workers (268, 297) view Tm flexibility ascentral to the understanding of regulation. Furthermore,there is evidence of preferred actins for cross-bridge at-tachment during activation (462) that also argues againsttreating all actins in a regulatory unit as equivalent. If so,a model with only three actin binding states may needrevision.
Lehrer (266) proposed a modified version of the two-state cooperative/allosteric model that differed from theGeeves model in not having a fully blocked state of thethin filament. Again, it was allosteric because strong bind-ing of myosin at one place on the thin filament coopera-tively enhanced the binding of myosin at another site.Lehrer (266) hypothesized that Ca21 does not cause thetransition from a blocked to a closed state by movementof Tm but causes TnI movement that allows weak bindingof myosin to the thin filament from which the myosin canisomerize to the strong binding form. Once in the strongbinding form, the myosin produces force and further ac-tivates the thin filament for binding of other myosinsthrough the movement of Tm. Although there is strongevidence that TnI can block myosin binding to actin on aone-to-one basis (442) (see sect. IIA) in the absence of Tm,and that TnI may bind to two actins (463), there is littleevidence that this inhibition of myosin binding extends tothe neighboring actins in the seven actin unit because ofthe presence of TnI alone and not because of the blockingposition of Tm. Again, as in the Geeves model, there areonly two proposed types of myosin binding, which reflectthe limitations of the techniques used to assess binding.Of course in the structured sarcomere, strained myosincross bridges could have a continuum of binding states. Liand Fajer (274) suggest additional thin filament states areneeded to account for the responses of EPR probes onTnC to Ca21 binding and various cross-bridge states, soone may consider more complex models.
In an alternative model of regulation, Tobacman(455) suggests that Ca21 binding activates the thin fila-ment but that strong cross-bridge binding further acti-
906 GORDON, HOMSHER, AND REGNIER Volume 80

vates the thin filament. This would give rise to the poten-tiated state described by Bremel et al. (31). As pointed outby Tobacman (455), the actin filament and myosin aloneare all that is required for movement and force in the invitro motility assay. The presence of regulatory proteinscan bring about an increase in speed in the in vitromotility assay (148) and the ATPase activity for a givenactin concentration by increasing the KATPase of regulatedactin over actin in the S1-actin ATPase assay (500). How-ever, the maximum ATPase activity given by the Vmax ofS1 and regulated thin filaments plus Ca21 in the presenceof strongly attached myosin (NEM-S1) is no different fromthat for actin alone (500). Thus this is not good evidencefor a truly potentiated state with higher Vmax. An en-hanced KATPase for actin activation of the ATPase withcardiac regulatory proteins and Ca21 is also suggested byTobacman and co-workers’ ATPase data (46) (Fig. 5B).These data show that the cardiac thin filament couldactivate the ATPase of S1 at very low [S1], but a ratio of$4 S1/7 actin is required to achieve a higher ATPaserate/S1. This could imply a cooperative activation/alloste-ric system for the thin filament, but only at S1-to-actinratios higher than those found in the sarcomere. On theother hand, during isometric contractions, the stronglybound time of cross bridges in the sarcomere is undoubt-edly much longer than in the completely unloaded condi-tion of actin-myosin in the test tube. Unloaded shorteningconditions in the fiber may be more like the conditionsseen for actin-myosin in the test tube (see the discussionin the beginning of sect. IIIC). This alternative suggestionfrom Tobacman (455) has not been developed to thequantitative model stage for testing. Furthermore, Tobac-man (455) suggested that not all actins in the regulatoryunit are necessarily equivalent (discussed above); thatmakes further experimental information necessary to for-mulate a more complete model of regulation from thebiochemical data. The model of McKillop and Geeves(303) seems like a reasonable starting point for the refine-ment, by including the possibility of different activationproperties for the various actins in the regulatory unit.
The physiological models differ from the biochemicalmodels in that the output is force and shortening (asopposed to ATPase), and the proteins are constrainedboth in concentration and arrangement within the sarco-mere. This constraint in the sarcomere results in strainedcross bridges during contractions, which modifies at leastsome of the rate constants, changing the cross-bridgekinetics and substantially altering the duration of cross-bridge attachment. These differences can have a profoundinfluence on the Ca21 regulation. The first quantitativemodel of Ca21 regulation was that of Julian (237), whohypothesized that Ca21 increased the attachment rateconstant (f) of the original Huxley (215) cross-bridgemodel. This was challenged by Podolsky and Teichholz(365) on the basis that they did not find a [Ca21] depen-
dence of maximum shortening velocity, launching a de-bate on this point (see sect. IIIC5). They hypothesized thatregulation occurred via recruitment of cross bridgesrather than regulation of cross-bridge kinetics. This de-bate on regulation by recruitment of cross bridges versusregulation of cross-bridge kinetics continues (see Bren-ner, Ref. 36) and is discussed at length in section IIIC2.
Many recent models describing Ca21 control of ten-sion generation rely on the formulation of Brenner (34),which was derived from his studies on the control of therate of tension redevelopment (kTR) in skinned musclefibers. As discussed in section IIIC2, this technique wasused to measure how Ca21 controlled the transition ofcross bridges from weak binding to strong, force-gener-ating states. He then described this transition along thelines first suggested by Kushmerick and Krasner (259).This involved using the Huxley (215) model with twoapparent rates, fapp and gapp, which lumps the rate con-stants for all steps in the actomyosin ATPase cycle relatedto cross-bridge attachment to force generation and de-tachment from force-generating states, respectively. Asdescribed in section IIIC2, he measured kTR, force, stiff-ness, and ATPase as a function of Ca21 and found that kTR
and force data could be fit if fapp is a function of [Ca21].The question in this formulation is how Ca21 regulatesfapp. It leads to two hypotheses: control of a force-gener-ating kinetic step in the actomyosin ATPase cycle orcontrol of thin filament activation. Control of thin filamentactivation is included in the fapp as defined by Brenner(34), but as will be shown below, for clearer understand-ing of the mechanisms involved one needs to know if thetwo possibilities can be experimentally distinguished.
Ca21 control of a kinetic step in the actomyosin cyclewas concluded from many biochemical studies [see Cha-lovich (55) discussed above] and provided an attractivehypothesis for tying together both biochemical and phys-iological data. However, measurements in skinned fibersusing caged compounds, pressure-jumps, and sinusoidallength changes have shown little if any Ca21 regulation ofany of the observable steps, such as Pi release (see sect.IIIC4). Thus the skinned fiber data do not support Ca21
regulation of a kinetic step by strongly attached crossbridges, and subsequent models have centered on Ca21
regulation of thin filament activation and strong cross-bridge attachment. We discuss two types of models of thisregulation: direct Ca21 regulation through Tn/Tm usingthe simple A7TmTn regulatory unit and cooperative acti-vation through strong cross-bridge attachment in one unitor interaction between A7TmTn units along the thin fila-ment.
An example of a simple Ca21 activation model is thatof Landesberg and Sideman (261), discussed in sectionIIIC2. In this model (diagrammed in Fig. 8A), Ca21 bindingto Tn can activate a simple regulatory unit so that weaklyattached cross bridges can transition to strong, force-
April 2000 MUSCLE REGULATION 907

generating cross bridges with attachment and detachmentrate constants of fapp and gapp, respectively. The modelallows strong cross bridges to remain attached even whenCa21 dissociates from Tn (state 4). However, from thisstate, cross bridges dissociate with a gapp
9 rate constant,and reattachment is negligible. With no Ca21 dependenceof the apparent rate constants for cross bridges, Ca21 actsonly through binding to Tn to activate the thin filament.This simple model can fit the kTR data for fast skeletalmuscle as described in section IIIC2 with reasonable val-ues for the various rate constants (383, 388) (see legend toFig. 8). This includes the effects of factors that modifycross-bridge kinetics (383) and Ca21 binding to TnC (385,388). This is illustrated in Figure 8, B and C, where ex-perimental data are compared with model calculations forthree conditions affecting thin filament activation: 1)
Ca21-independent activation (aTnC) as well as 2) de-creased and 3) increased Ca21 dissociation rate from TnC(calmidazolium and the NH2-terminal deletion mutant ofTnC), respectively. The model also provided a ready ex-planation for the variety of shapes observed for the kTR-force curves in different muscles and different tempera-tures. However, this model does not fit the force-pCarelationship, because it lacks cooperative feedback. Land-esberg and Sideman (261) added feedback from force-bearing cross bridges to Ca21 binding to TnC to obtainboth a steeper force-pCa relationship and a fit to theforce-sarcomere length relationship. There is good evi-dence that cycling cross bridges enhance Ca21 binding incardiac muscle, but there is much less evidence in skele-tal muscle (see sect. IIIC7). Thus, although this type offeedback has some relevance for cardiac muscle, it is
FIG. 8. Left: 4-state model of activation, separating thin filament Ca21 activation kinetics from cross-bridgeattachment/detachment kinetics. The 4 states are distinguished by the absence or presence of Ca21 binding to TnC toactivate a A7TmTn unit and by weak/no binding or strong, force-generating cross-bridge binding to actin. Ca21 is boundin states 2 and 3 and dissociated in states 1 and 4. Ca21 binding to state 1, with association/dissociation rate constantskon/koff, produces the activated thin filament state 2. Strong, force-generating cross-bridge attachment can occur to theactivated thin filament actins in state 2 to produce force and shortening with attachment rate constant fapp anddetachment rate constant gapp. Ca21 can dissociate (with a kon
9 /koff9 which is usually assumed to be the same as kon/koff)
from the activated thin filament with the cross bridge strongly attached (state 3) in the transition to state 4. From state
4, the cross bridge can detach to state 1 with rate constant gapp9 with negligible reattachment. [Modified from Regnier et
al. (388), Landesberg and Seidman (261), and Hancock et al. (165).] Right: comparison of predictions and experimentkTR-force data for 3 different manipulations that affect the kinetics of thin filament activation. Experimental data pointsare shown with theoretical lines from the model. a: Experimental data are for substitution of aTnC for some of theendogenous TnC (oxidized cTnC which activates in the absence of Ca21). Parameters used in the modeling are asfollows: fapp 5 17 s21; gapp 5 gapp
9 5 1.5 s21; fapp9 5 0, kon 5 kon
9 5 0–144 s21 (kon varied by changing [Ca21]); and koff
5 koff9 5 10 s21 for control fibers, 3.5 s21 for calmidazolium (CDZ), and ,0.3 s21 for aTnC. [Data from Chase et al. (63)
(n) for the addition of 10 mM CDZ (which slows the Ca21 dissociation rate from TnC); data from Regnier et al. (385) (C)and for control fibers with endogenous TnC before the addition of CDZ (●).] b: Experimental data are for substitutionin rabbit psoas skinned muscle fibers of an NH2-terminal deletion mutant chicken skeletal TnC (NHdel) in which theCa21 dissociation rate is greatly increased (ƒ). Control fibers have wild-type chicken skeletal TnC (WT) substituted forthe endogenous rabbit TnC (●). Parameters used in the modeling are as follows; fapp 5 17 s21; gapp 5 gapp
9 5 1.5 s21; f app9
5 0; kon 5 kon9 5 0–200 s21 (kon varied by changing [Ca21]); koff 5 koff
9 5 10 s21 for control fibers (wild type) and 70 s21
for fibers with NHdel TnC (NHdel). [Data from Regnier et al. (388).]
908 GORDON, HOMSHER, AND REGNIER Volume 80

probably not the mechanism that makes the force-pCarelationship cooperative in skeletal muscle (see sect.IIIC7). Additionally, the model is incomplete because itcannot describe how strongly attached, noncycling crossbridges can activate the thin filament, as discussed insections IIIC1 and IIIC6. Therefore, the model needs to beexpanded to more completely explain the activation ofskinned muscle preparations including more complex be-haviors such as the kCa (see sect. IIIC3), the kPi
(see sect.IIIC4), and the increase in kTR with increasing [Pi] (seesect. IIIC2).
To explain the steepness of the force-pCa relation-ship in skeletal muscle requires, in addition to direct Ca21
activation, cooperative activation of the thin filament bycycling cross bridges. Campbell (48) introduced a modelthat does this. In its most general form, this model ex-panded the four-state model of Landesberg and Seidman(261) to a six-state model including separate Ca21 bindingand thin filament activation steps, as well as the cross-bridge attachment/detachment steps with their apparentrate constants. Campbell (48) suggested two feedbackpathways: one from the force-generating states to theCa21 binding rate constants and a second from force-generating states to the rate constants for the transition ofthe thin filament into the activated state. Calculations,using a simplified version of the model with feedbackfrom force-producing states to thin filament activation,showed that the model could produce a typical kCa-forcerelationship. It also produced the two-phase force-pCacurve seen by Moss (331), but not the single-phase force-pCa curve seen by others (23) (see sect. IIIC1). An inter-esting feature of this feedback is a slow build-up of forceto its maximal level. This slow build-up occurs becausefeedback enhances the final force level so that the force“chases” this final level, which is constantly rising be-cause of the feedback. Thus feedback steepens the force-pCa relationship and slows kCa at lower levels of activa-tion. Although Campbell did not model the forceredevelopment rate constant, kTR, his formulation doesproduce a typical kTR-force relationship, as in Figure 6.This model also provides a ready mechanism by whichstrongly attached, noncycling cross bridges can activatethe thin filament. However, the shape of the kTR-forcecurve in Campbell’s model is dependent on cooperativeactivation. As discussed in section IIIC1, cooperative ac-tivation is required to explain the force-pCa relationshipbut may not be required to explain the kTR relationship(see sect. IIIC2). Furthermore, the Campbell (48) modelonly considers cooperativity within the single A7TmTnregulatory unit and does not consider cooperativity alongthe thin filament, which steepens the force-pCa relation-ship (387).
The model of Dobrunz et al. (80) is an example of amodel that includes cooperativity along the thin filament.This model has similarities to Campbell’s six-state model,
with a thin filament regulatory unit with or without Ca21
binding, an activated thin filament regulatory unit with orwithout Ca21 binding, and a regulatory unit with attachedforce-generating cross bridge(s). They assume rapid equilib-ria between the states and coupling between neighboringactivated regulatory units that increases the equilibrium be-tween active and inactive units depending on whether oneor both neighboring units are active. They found that theyneeded to consider interactions between only 9 units/thinfilament (rather than all 26 units) for their calculated param-eters to be independent of the number of units. Althoughthis model was designed to calculate the steady-state force-pCa relationship, it includes a formalism that could poten-tially be used for calculating transient behavior. The intro-duction of many parameters fit in the modeling means thatuniqueness of fit is a problem until more parameters can beindependently measured. The model demonstrates an ap-proach that could be used for a more complete model in-corporating the known mechanisms of activation and mea-sured parameters to explain both steady-state and transientbehavior.
In the models discussed thus far, the cross-bridgeATPase cycle (scheme 1, see sect. IIID) is described sim-ply by fapp and gapp (see discussion in sect. IIIC2 andearlier in this section). A more complete model of regu-lation should include rates for individual cross-bridgetransitions that have been measured in fibers and in so-lution (see sect. IIIC). Such a model was proposed byRegnier et al. (386), based on their observations on theeffects of Pi, BDM, and Ca21 on kTR, kPi
, force, and stiff-ness in skinned skeletal muscle fibers. They concludedthat Ca21 controls strong cross-bridge binding, specifi-cally a weak-to-strong binding step preceding force gen-eration and Pi release. They were able to obtain reason-able fits to the kTR and kPi
data using values of the rateconstants that they measured, which are similar to thoseshown in Table 1. They could account for Ca21 regulationof force through control of the weak-to-strong cross-bridge binding transition. However, because they did notinclude a cooperative activation by strongly bound crossbridges, there was no steep dependence of force on[Ca21]. Similar models relating the ATPase cycle to forcegeneration have been proposed by Kawai and Zhao (245)and Wahr et al. (476). Thus, rather than using the lumpedrate constants fapp and gapp (Fig. 8, left), a more completemodel of regulation needs to include Ca21 and strongcross-bridge control of the thin filament and a more de-tailed cross-bridge kinetic scheme.
In summary, the individual models mentioned aboveare each able to describe specific aspects of thin filamentactivation and regulation of cross-bridge binding by Ca21
for which they were intended. However, none of thesemodels has been able to provide a completely satisfyingpicture. The ideal model would incorporate a more com-plete description of the thin filament activation process
April 2000 MUSCLE REGULATION 909

and the chemomechanical states associated with cross-bridge cycling. This would include Ca21 activation of thethin filament and cooperative activation by strong crossbridges both within individual A7TmTn regulatory units,which may be more flexible in size and between unitsalong the thin filament, to describe steady-state and tran-sient behavior. The beginning of a more comprehensiveapproach to modeling including the coupling betweenneighboring units is the cellular automaton approach ofZou and Phillips (521), but this needs to be developedfurther to account for all of the biochemical, structural,and physiological data discussed here. Considering theflexibility of Tm, it may be necessary to consider thedifferent states (open, closed, blocked) and the openprobability of individual actins and to include more de-tailed descriptions of the three-dimensional nature of in-dividual thin and thick filaments. Finally, a kinetic de-scription of these processes and the interactions betweenthin filament regulatory proteins and cross bridges isessential to describe a realistic mechanism by which Ca21
controls dynamic processes like the rate of force devel-opment and shortening in muscle.
V. CONCLUSIONS AND FUTURE DIRECTIONS
A. Conclusions
Ca21 regulation of contraction in vertebrate striatedmuscle is exerted primarily through effects on the thinfilament to regulate the open probability of actin, whichregulates strong cross-bridge binding to actin and mayinfluence cross-bridge detachment kinetics. Thus the fo-cus of the review has been regulation of the thin filament.Data have been summarized from structural, biochemical,physiological, and in vitro motility studies.
As discussed in sections II and IIIA, the position of Tmon the thin filament determines the interaction of myosinwith actin, whether the actin is open to myosin binding,and whether the binding will be weak (electrostatic) orstronger (hydrophobic interactions). However, dependingon the Tm isoform and the Tn subunits that interact withTm, the Tm may be quite flexible so that, although it spansseven actins, the availability of myosin binding sites onthese (and neighboring) actins may vary. Biochemicallythese binding sites may be characterized as blocked,closed, or open depending on the strength of the myosinbinding and whether the myosin can isomerize to astrongly bound, force-generating state. In the blockedstate, TnI binding to actin may itself block one, two, ormore actins per A7TmTn regulatory unit. The position ofTm and TnI on the actin filament is determined by 1) theoccupancy of regulatory Ca21 binding sites on Tn, 2)conformational changes resulting from Ca21 binding, and3) changes in the interactions of Tn, Tm, and actin and as
well as by myosin S1 binding to actin. Ca21 binding toTnC enhances TnC-TnI interaction, pulling TnI away fromits binding sites on one to two of the actins in the basicA7TmTn structural regulatory unit. The reduced TnI affin-ity for actin allows increased Tm movement and exposesactin binding sites previously blocked by Tm. The cou-pling of adjacent Tm by an overlap region can result inmovement of the adjacent Tm and exposure of some oftheir associated actins. The movement of Tm is also af-fected by interactions with TnT and TnT’s interactionswith TnC-TnI that are modulated by Ca21. TnT can in-crease the coupling between neighboring Tm and, by itsinteraction with as much as 40% of the length of Tm, mayinfluence the flexibility of individual Tm. Because thisimplies there will be variations in the position of Tm onindividual actins, it means that variation of TnT isoformsmay give rise to differences with respect to the propertiesof fractionally activated Tn-Tm. This suggests a poten-tially high degree of variability in Ca21 activation depend-ing on the specific protein isoforms.
Ca21 activation and Tm movement exposes myosinbinding sites on some fraction of the actins so that theyare available for myosin attachment and isomerization tostrong-binding, force-producing states. Evidence fromstructural studies on skeletal muscle proteins supportsthe idea that strong binding of skeletal myosin to thesesites promotes additional Tm movement, stabilizes Tm inthe open position, and provides cooperative activation ofadditional actins in the A7TmTn structural regulatory unitand possibly of additional actins in neighboring regula-tory units.
These structural and biochemical findings supportphysiological observations of steady-state and transientmechanical behavior discussed in section III. The conclu-sions from these physiological studies and the section inwhich the data are discussed are as follows.
1) The regulated step in the actomyosin ATPase cy-cle is the strong binding of myosin to actin (see sect. III, B,C1, C2, C3, and C5). There is also some evidence from thein vitro motility studies (see sect. IIID) that there is mod-ulation of another kinetic step in the cross-bridge cycle,possibly cross-bridge detachment, but probably not the Pi
release step (see sect. IIIC4).2) Ca21 binding to Tn results in Tm movement and
uncovers sites on actin to which myosin can bind, isomer-ize to strong binding states, and produce force and muscleshortening.
3) The initial rate of force development dependsmostly on the level of activation of the thin filament byCa21, and on the myosin kinetic properties, but dependslittle on the initial force level (see sect. III, B and C1–C3).
4) A small number of strongly attached cross bridgeswithin an A7TmTn regulatory unit (number still to bedetermined) can activate the unit’s actins and perhapsthose in neighboring units so that additional myosins can
910 GORDON, HOMSHER, AND REGNIER Volume 80

bind, isomerize to strongly bound states, and produceforce (see sect. III, C1, C6, C8, and C9).
5) Cooperativity between neighboring regulatoryunits contributes to the activation by strong cross bridgesof steady-state force (see sect. III, C1 and C6) but does notaffect the rate of force development (see sect. IIIC2).
6) Strongly attached cycling cross bridges can delayrelaxation in skeletal muscle in a cooperative manner (seesect. IIIC8).
7) Strongly attached, cycling cross bridges can en-hance Ca21 binding to cardiac TnC but influence skeletalTnC to a lesser extent (see sect. IIIC7).
Differences have been noted in regulation betweenvertebrate skeletal and cardiac muscle. Compared withfast skeletal muscle, cardiac muscle exhibits the follow-ing: 1) a decreased steepness of the force-pCa curve (seesect. IIIC1); 2) less effect of the initial level of force on therelaxation rate; 3) less dependence of kTR on Ca21; 4)more cooperativity between neighboring units duringrigor cross-bridge activation in the absence of Ca21; 5)more feedback between cycling cross bridges and Ca21
binding to cTnC; and 6) unique regulatory protein iso-forms including TnC, TnI, TnT, Tm, myosin, and somestructural proteins, although there are some equivalentsbetween slow skeletal muscle and some cardiac cells inTnC and myosin.
Presumably, these properties are caused by the dif-ferences in protein isoforms in these two muscle tissues,since the sarcomere structure is so similar. The feedbackbetween cycling cross-bridge attachment and Ca21 bind-ing to cTnC (5) implies a strong coupling between S1binding to actin, Tm movement, and TnT-TnI-TnC inter-actions in cardiac muscle. Ca21 binding does not producethe large structural change in the NH2 terminal of cTnC asoccurs in skeletal TnC (84, 85, 411). The binding of cTnI tocTnC appears to promote the open cTnC structure asso-ciated with Ca21 binding, enhanced Ca21 binding, andactivation (276). Dissociation of cTnI from actin by Tm-TnT coupling and stronger binding to cTnC in the absenceof Ca21 may be facilitated in cardiac muscle as would beimplied by the greater cooperativity between neighboringregulatory units in rigor activation in the absence of Ca21
(4, 307). Thus strong cross-bridge attachment in cardiacmuscle and Ca21 binding to cTnC might be coupledthrough Tm-TnT-TnI. It is not because of the properties ofcTnC alone because slow-twitch muscle (with cTnc) doesnot show this effect of cross-bridge attachment (483). Theenhanced coupling of Tm movement to TnC might occurif cardiac Tm-Tn were less flexible than skeletal Tm withstrong cross-bridge binding (60) or because of interac-tions of Tm with cTnT. A stiffer Tm-TnT connectionwould be more likely to transmit to TnC the effect ofcross bridges binding to actin, which in turn shift orstabilize Tm. A tighter coupling between Ca21 binding toTnC and cycling cross bridges could reduce the coopera-
tive activation by strongly attached cross bridges separatefrom their effect to enhance Ca21 binding. This wouldlead to reduced cooperativity during relaxation (2) andpossibly a reduction in Ca21 dependence of kTR (3) (seesect. IIIC2).
A shallower force-pCa curve (1) for cardiac muscle isdue in part to the Tn isoform. In cardiac muscle reconsti-tuted with filaments made from skeletal or cardiac Tn andTm in various permutations, the increased steepness ofthe force-pCa curve follows the skeletal Tn isoform, andnot the skeletal Tm isoform (126). Although other Tnsubunits may contribute to this difference in cooperativeactivation, some is attributable to the properties of TnCalone since with TnC exchanges, the steeper curve fol-lows the skeletal TnC (11). The shallower force-pCa curveassociated with cTnC could result in small part fromhaving just one functional NH2-terminal Ca21 binding sitebut probably mainly from the lack of a one-to-one cou-pling between Ca21 binding and conformational change incTnC (84, 85). This makes cTnC more dependent on bind-ing to cTnI to achieve this conformational change. WithCa21 binding at just one TnC, the shift in Tm would beless likely to bring actins in the A7TmTn regulatory unitinto the open configuration for strong myosin binding andforce generation. Thus cardiac muscle would not be fullyactive at maximal Ca21 as observed (384) and would bemore dependent on activation by strongly attached crossbridges or anything that would promote the cTnC-cTnIinteraction. This would make cardiac muscle more sensi-tive to conditions that influenced strong cross-bridge at-tachment such as sarcomere length (filament lattice) (294,300), augmentation or inhibition of cycling cross-bridgeattachment (383, 384), or phosphorylation of myosin RLC(272) or C protein (501).
The above summary of possible reasons for differ-ences in properties between cardiac-skeletal is somewhatspeculative, but it suggests a number of lines of investi-gation that should be pursued.
B. Future Research Directions
As detailed in this review, an enormous amount ofinformation is now available on Ca21 activation of thethin filament in striated muscle, and the general conceptsare now clearer. A detailed mathematical model is stillneeded that quantitatively explains the physiological be-havior of skeletal and cardiac muscle. Before this occurs,additional information is required. There are a number ofareas that are clearly important directions for future in-vestigations that have been suggested by the materialreviewed here. Some of these are discussed below.
The flexibility of Tm and the possible variability ofactivation of individual actins have been suggested asimportant issues to be investigated. The role of TnT in
April 2000 MUSCLE REGULATION 911

stabilizing Tm and the role that various TnT isoforms playin determining the properties of regulation are worthconsidering. One of the consequences of flexible Tm is thepossibility that all actins are not equivalent. A test of thisidea will require better techniques for assessing changesat different actins in the A7TmTn unit, possibly throughX-ray and imaging techniques or through using mutant Tmor actins.
More information is needed on the detailed interac-tions between the regulatory proteins and the variationsproduced by different isoforms. Such studies cannot bedone just with isolated proteins but must include studiesin skinned muscle fibers and in vitro motility assays, inwhich the interaction with all the neighboring proteins inan ordered system can be assessed. This will necessitatedevelopment of additional techniques for probing interac-tions in skinned fibers. Our understanding of regulationhas already benefited from the ability to introduce labeledor mutant proteins into skinned fibers, cultured myocytes,transgenic animals, or filaments in the in vitro motilitysystem. This should allow both probing of protein inter-action and better testing of models of regulation.
Although we conclude that the major site of Ca21
regulation is in regulating strong myosin binding to actin,there is evidence from the in vitro motility assay thatmodulation of cross-bridge kinetics, possibly of cross-bridge detachment, can occur particularly with differentTnT or Tm isoforms. It is not clear whether this modula-tion is due to differences in interaction between Tm andmyosin or due to changes in actin, which affect its inter-action with myosin. In this review we have not stressedthe role of actin, as less is known about it, but evidence isstrong (106) that actin is much more than a passive ele-ment in the contraction and regulation processes. Furtherinvestigation must be done on these issues before wehave a complete understanding of regulation.
In addition to Ca21 binding to activate the thin fila-ment, the evidence supports a role for strongly attachedcross bridges in attaining full force development, partic-ularly in cardiac muscle. A quantitative model is neededto describe in a testable manner the roles of direct Ca21
activation and cooperative activation by strongly attachedcross bridges in muscle fibers. This will require experi-mental measures of thin filament activation in fibers bymeasuring Tm position using fluorescent or spin labels orother techniques. It will also require improved techniquesto specify strong cross-bridge attachment in fibers, possi-bly by incorporating pyrene labels on the actin in additionto the presently used stiffness measurements.
In the in vitro motility system, there is a need to studyoriented cross-bridge heads, either using long native thickfilaments, polymerized thick filaments, or nano-fabrica-tion techniques. Finally, the field is ready for single cross-bridge head measurements using optical traps to studythe Ca21 dependency of strongly attached cross-bridge
transitions. Although some single molecule techniqueshave been worked out, there are problems distinguishingbetween weak and strong cross-bridge attachment.Pyrene labeling of actin may be used to assess strongattachment, or measurements of the stiffness of attach-ment may yield the required information.
This is an exciting time to be studying the problem ofregulation of contraction because of the amazing prolif-eration of new techniques and preparations. These tech-nological and scientific advances offer the hope that amore complete understanding of the crucial question ofthe mechanism of the regulation of striated muscle con-traction can be achieved in the near future.
We acknowledge the assistance of many individuals inwriting this review. Larry Smillie and Ron Milligan preparedexcellent figures for us and provided important discussions. BillLehman provided valuable advice in preparing a figure summa-rizing some of their work. Others (Rick Moss, Sam Lehrer, andLarry Tobacman) gave us permission to use figures from theirstudies. Mike Geeves shared with us an important manuscriptbefore it was published and was generous with his time explain-ing his ideas and models. Discussions with Larry Tobacmanwere particularly helpful. Lee Sweeney shared with us resultsfrom his studies before they were published. We appreciated theediting of the manuscript and useful comments by Don Martynand P. Bryant Chase and the careful reading and comments ofEmilie Warner. We are particularly appreciative of the assis-tance with the manuscript, figures, and references of MarthaMathiason who provided important computer support. DeniseNishioka also assisted greatly with the references and figurescanning. Finally, we gratefully acknowledge the patience, sup-port, encouragement, and indulgence of Della Gordon, DianneHomsher (remembering Alexandra), and Julie Regnier in thelong process of writing this review.
This work was supported in part by National Institutes ofHealth Grants HL-52558, NS-08384 (to A. M. Gordon), AR-30988(to E. Homsher), and HL-61683 (to M. Regnier).
Address for reprint requests and other correspondence:A. M. Gordon, Dept. of Physiology and Biophysics, Univ. ofWashington, Box 357290, Seattle, WA 98195-7290 (E-mail:[email protected]).
REFERENCES
1. ADAMS, S. R., J. P. Y. KAO, G. GRYNKIEWICZ, A. MINTA, AND R. Y.TSIEN. Biologically useful chelators that release Ca21 upon illumi-nation. J. Am. Chem. Soc. 110: 3212–3220, 1988.
2. ALLEN, D. G., AND J. C. KENTISH. Calcium concentration in themyoplasm of skinned ferret ventricular muscle following changesin muscle length. J. Physiol. (Lond.) 407: 489–503, 1988.
3. ALLEN, D. G., AND S. KURIHARA. The effects of muscle length onintracellular calcium transients in mammalian cardiac muscle.J. Physiol. (Lond.) 327: 79–94, 1982.
4. ALLEN, T. S., L. D. YATES, AND A. M. GORDON. Ca21-dependenceof structural changes in troponin-C in demembranated fibers ofrabbit psoas muscle. Biophys. J. 61: 399–409, 1992.
5. AMOS, L. A. Structure of muscle filaments studied by electronmicroscopy. Annu. Rev. Biophys. Biophys. Chem. 14: 291–313,1985.
6. ANSON, M., M. A. GEEVES, S. E. KURZAWA, AND D. J. MANSTEIN.
912 GORDON, HOMSHER, AND REGNIER Volume 80

Myosin motors with artificial lever arms. EMBO J. 15: 6069–6074,1996.
7. ARAUJO, A., AND J. W. WALKER. Kinetics of tension developmentin skinned cardiac myocytes measured by photorelease of Ca21.Am. J. Physiol. Heart Circ. Physiol. 267: H1643–H1653, 1994.
8. ARAUJO, A., AND J. W. WALKER. Phosphate release and forcegeneration in cardiac myocytes investigated with caged phosphateand caged calcium. Biophys. J. 70: 2316–2326, 1996.
9. ASHLEY, C. C., T. J. LEA, I. P. MULLIGAN, R. E. PALMER, AND S. J.SIMNETT. Activation and relaxation mechanisms in single musclefibres. Adv. Exp. Med. Biol. 332: 97–115, 1993.
10. ASHLEY, C. C., I. P. MULLIGAN, AND T. J. LEA. Ca21 and activationmechanisms in skeletal muscle. Q. Rev. Biophys. 24: 1–73, 1991.
11. BABU, A., S. P. SCORDILIS, E. H. SONNENBLICK, AND J. GULATI.The control of myocardial contraction with skeletal fast muscletroponin C. J. Biol. Chem. 262: 5815–5822, 1987.
12. BACCHIOCCHI, C., AND S. S. LEHRER. Fluorescence energy trans-fer studies of the tropomyosin movement in reconstituted skeletalmuscle thin filaments (Abstract). Biophys. J. 76: A154, 1999.
13. BAGSHAW, C. R. On the location of the divalent metal binding sitesand the light chain subunits of vertebrate myosin. Biochemistry 16:59–67, 1977.
14. BAGSHAW, C. R., AND D. R. TRENTHAM. The reversibility ofadenosine triphosphate cleavage by myosin. Biochem. J. 133: 323–328, 1973.
15. BARANY, M. ATPase activity of myosin correlated with speed ofmuscle shortening. J. Gen. Physiol. 50, Suppl.: 197–218, 1967.
16. BARMAN, T., M. BRUNE, C. LIONNE, N. PIRODDI, C. POGGESI, R.STEHLE, C. TESI, F. TRAVERS, AND M. R. WEBB. ATPase andshortening rates in frog fast skeletal myofibrils by time-resolvedmeasurements of protein-bound and free Pi. Biophys. J. 74: 3120–3130, 1998.
17. BING, W., I. D. FRASER, AND S. B. MARSTON. Troponin I andtroponin T interact with troponin C to produce different Ca21-dependent effects on actin-tropomyosin filament motility. Bio-
chem. J. 327: 335–340, 1997.18. BING, W., A. RAZZAQ, J. SPARROW, AND S. MARSTON. Tropomy-
osin and troponin regulation of wild type and E93K mutant actinfilaments from Drosophila flight muscle. Charge reversal on actinchanges actin-tropomyosin from on to off state. J. Biol. Chem. 273:15016–15021, 1998.
19. BING, W., C. S. REDWOOD, I. F. PURCELL, G. ESPOSITO, H.WATKINS, AND S. B. MARSTON. Effects of two hypertrophic car-diomyopathy mutations in alpha-tropomyosin, Asp175Asn andGlu180Gly, on Ca21 regulation of thin filament motility. Biochem.
Biophys. Res. Commun. 236: 760–764, 1997.20. BOBKOV, A. A., E. A. BOBKOVA, E. HOMSHER, AND E. REISLER.
Activation of regulated actin by SH1-modified myosin subfragment1. Biochemistry 36: 7733–7738, 1997.
21. BONNE, G., L. CARRIER, P. RICHARD, B. HAINQUE, AND K.SCHWARTZ. Familial hypertrophic cardiomyopathy: from muta-tions to functional defects. Circ. Res. 83: 580–593, 1998.
22. BOTTINELLI, R., D. A. COVIELLO, C. S. REDWOOD, M. A. PEL-LEGRINO, B. J. MARON, P. SPIRITO, H. WATKINS, AND C. REG-GIANI. A mutant tropomyosin that causes hypertrophic cardiomy-opathy is expressed in vivo and associated with an increasedcalcium sensitivity. Circ. Res. 82: 106–115, 1998.
23. BRANDT, P. W., F. COLOMO, N. PIRODDI, C. POGGESI, AND C.TESI. Force regulation by Ca21 in skinned single cardiac myocytesof frog. Biophys. J. 74: 1994–2004, 1998.
24. BRANDT, P. W., R. N. COX, AND M. KAWAI. Can the binding of theCa21 to two regulatory sites on troponin C determine the steeppCa/tension relationship? Proc. Natl. Acad. Sci. USA 77: 4717–4720, 1980.
25. BRANDT, P. W., M. S. DIAMOND, J. S. RUTCHIK, AND F. H. SCHA-CHAT. Co-operative interactions between troponin-tropomyosinunits extend the length of the thin filament in skeletal muscle. J.
Mol. Biol. 195: 885–896, 1987.26. BRANDT, P. W., M. S. DIAMOND, AND F. H. SCHACHAT. The thin
filament of vertebrate skeletal muscle co-operatively activates as aunit. J. Mol. Biol. 180: 379–384, 1984.
27. BRANDT, P. W., J. P. REUBEN, AND H. GRUNDFEST. Regulation of
tension in the skinned crayfish muscle fiber. II. Role of calcium.J. Gen. Physiol. 59: 305–317, 1972.
28. BRANDT, P. W., D. ROEMER, AND F. H. SCHACHAT. Co-operativeactivation of skeletal muscle thin filaments by rigor crossbridges.The effect of troponin C extraction. J. Mol. Biol. 212: 473–480,1990.
29. BRANDT, P. W., AND F. H. SCHACHAT. Troponin C modulates theactivation of thin filaments by rigor cross-bridges. Biophys. J. 72:2262–2267, 1997.
30. BREITBART, R. E., H. T. NGUYEN, R. M. MEDFORD, A. T.DESTREE, V. MAHDAVI, AND B. NADAL-GINARD. Intricate com-binatorial patterns of exon splicing generate multiple regulatedtroponin T isoforms from a single gene. Cell 41: 67–82, 1985.
31. BREMEL, R. D., J. M. MURRAY, AND A. WEBER. Manifestations ofcooperative behavior in the regulated actin filament during actin-activated ATP hydrolysis in the presence of calcium. Cold Spring
Harb. Symp. Quant. Biol. 37: 267–275, 1972.32. BREMEL, R. D., AND A. WEBER. Cooperation within actin filament
in vertebrate skeletal muscle. Nature 238: 97–101, 1972.33. BRENNER, B. Cross-bridge attachment during isotonic shortening
in single skinned rabbit psoas fibers (Abstract). Biophys. J. 41: A33,1983.
34. BRENNER, B. The cross-bridge cycle in muscle. Mechanical, bio-chemical, and structural studies on single skinned rabbit psoasfibers to characterize cross-bridge kinetics in muscle for correla-tion with the actomyosin-ATPase in solution. Basic Res. Cardiol.
81: 1–15, 1986.35. BRENNER, B. Effect of Ca21 on cross-bridge turnover kinetics in
skinned single rabbit psoas fibers: implications for regulation ofmuscle contraction. Proc. Natl. Acad. Sci. USA 85: 3265–3269, 1988.
36. BRENNER, B. Muscle mechanics and biochemical kinetics. In:Molecular Mechanisms in Muscular Contraction, edited by J. M.Squire. Boca Raton, FL: CRC, 1990, p. 77–149.
37. BRENNER, B. Rapid dissociation and reassociation of actomyosincross-bridges during force generation: a newly observed facet ofcross-bridge action in muscle. Proc. Natl. Acad. Sci. USA 88:10490–10494, 1991.
38. BRENNER, B. Technique for stabilizing the striation pattern inmaximally calcium-activated skinned rabbit psoas fibers. Biophys.
J. 41: 99–102, 1983.39. BRENNER, B., J. M. CHALOVICH, L. E. GREENE, E. EISENBERG,
AND M. SCHOENBERG. Stiffness of skinned rabbit psoas fibers inMgATP and MgPPi solution. Biophys. J. 50: 685–691, 1986.
40. BRENNER, B., AND E. EISENBERG. Rate of force generation inmuscle: correlation with actomyosin ATPase activity in solution.Proc. Natl. Acad. Sci. USA 83: 3542–3546, 1986.
41. BRENNER, B., T. KRAFT, AND J. M. CHALOVICH. Fluorescence ofNBD-labelled troponin-I as a probe for the kinetics of thin filamentactivation in skeletal muscle fibers. Adv. Exp. Med. Biol. 453:177–184, 1998.
42. BRENNER, B., M. SCHOENBERG, J. M. CHALOVICH, L. E.GREENE, AND E. EISENBERG. Evidence for cross-bridge attach-ment in relaxed muscle at low ionic strength. Proc. Natl. Acad. Sci.
USA 79: 7288–7291, 1982.43. BRENNER, B., AND L. C. YU. Equatorial X-ray diffraction from
single skinned rabbit psoas fibers at various degrees of activation.Biophys. J. 48: 829–834, 1985.
44. BRUNE, M., J. L. HUNTER, J. E. CORRIE, AND M. R. WEBB. Direct,real-time measurement of rapid inorganic phosphate release usinga novel fluorescent probe and its application to actomyosin sub-fragment 1 ATPase. Biochemistry 33: 8262–8271, 1994.
45. BURTON, K. The magnitude of the force rise after rapid shorteningand restretch in fibres isolated from rabbit psoas muscle (Ab-stract). J. Physiol. (Lond.) 418: 66P, 1989.
46. BUTTERS, C. A., J. B. TOBACMAN, AND L. S. TOBACMAN. Coop-erative effect of calcium binding to adjacent troponin molecules onthe thin filament-myosin subfragment 1 MgATPase rate. J. Biol.
Chem. 272: 13196–13202, 1997.47. CAMPBELL, A. P., AND B. D. SYKES. Interaction of troponin I and
troponin C. Use of the two-dimensional nuclear magnetic reso-nance transferred nuclear Overhauser effect to determine thestructure of the inhibitory troponin I peptide when bound to skel-etal troponin C. J. Mol. Biol. 222: 405–421, 1991.
April 2000 MUSCLE REGULATION 913

48. CAMPBELL, K. Rate constant of muscle force redevelopment re-flects cooperative activation as well as cross-bridge kinetics. Bio-
phys. J. 72: 254–262, 1997.49. CANNELL, M. B. Effect of tetanus duration on the free calcium
during the relaxation of frog skeletal muscle fibres. J. Physiol.
(Lond.) 376: 203–218, 1986.50. CANTINO, M. E., T. S. ALLEN, AND A. M. GORDON. Subsarcomeric
distribution of calcium in demembranated fibers of rabbit psoasmuscle. Biophys. J. 64: 211–222, 1993.
51. CANTINO, M. E., J. G. EICHEN, AND S. B. DANIELS. Distributionsof calcium in A and I bands of skinned vertebrate muscle fibersstretched to beyond filament overlap. Biophys. J. 75: 948–956,1998.
52. CAPUTO, C., K. A. EDMAN, F. LOU, AND Y. B. SUN. Variation inmyoplasmic Ca21 concentration during contraction and relaxationstudied by the indicator fluo-3 in frog muscle fibres. J. Physiol.
(Lond.) 478: 137–148, 1994.53. CASSELL, M., AND L. S. TOBACMAN. Opposite effects of myosin
subfragment 1 on binding of cardiac troponin and tropomyosin tothe thin filament. J. Biol. Chem. 271: 12867–12872, 1996.
54. CECCHI, G., F. COLOMO, AND V. LOMBARDI. Force-velocity rela-tion in normal and nitrate-treated frog single muscle fibres duringrise of tension in an isometric tetanus. J. Physiol. (Lond.) 285:257–273, 1978.
55. CHALOVICH, J. M. Actin mediated regulation of muscle contrac-tion. Pharmacol. Ther. 55: 95–148, 1992.
56. CHALOVICH, J. M., P. B. CHOCK, AND E. EISENBERG. Mechanismof action of troponin-tropomyosin. Inhibition of actomyosin ATP-ase activity without inhibition of myosin binding to actin. J. Biol.
Chem. 256: 575–578, 1981.57. CHALOVICH, J. M., AND E. EISENBERG. Inhibition of actomyosin
ATPase activity by troponin-tropomyosin without blocking thebinding of myosin to actin. J. Biol. Chem. 257: 2432–2437, 1982.
58. CHANDRA, M., E. F. DA SILVA, M. M. SORENSON, J. A. FERRO,J. R. PEARLSTONE, B. E. NASH, T. BORGFORD, C. M. KAY, AND
L. B. SMILLIE. The effects of N helix deletion and mutant F29W onthe Ca21 binding and functional properties of chicken skeletalmuscle troponin. J. Biol. Chem. 269: 14988–14994, 1994.
59. CHANDY, I. K., J. C. LO, AND R. D. LUDESCHER. Differentialmobility of skeletal and cardiac tropomyosin on the surface ofF-actin. Biochemistry 38: 9286–9294, 1999.
60. CHANDY, I. K., AND R. D. LUDESCHER. Cardiac Tm dynamics onactin in the presence of myosin heads (Abstract). Biophys. J. 76:A280, 1999.
61. CHASE, P. B., T. M. DENKINGER, AND M. J. KUSHMERICK. Effectof viscosity on mechanics of single, skinned fibers from rabbitpsoas muscle. Biophys. J. 74: 1428–1438, 1998.
62. CHASE, P. B., AND M. J. KUSHMERICK. Effect of physiological ADPconcentrations on contraction of single skinned fibers from rabbitfast and slow muscles. Am. J. Physiol. Cell Physiol. 268: C480–C489, 1995.
63. CHASE, P. B., D. A. MARTYN, AND J. D. HANNON. Isometric forceredevelopment of skinned muscle fibers from rabbit activated withand without Ca21. Biophys. J. 67: 1994–2001, 1994.
64. CHOCK, S. P., P. B. CHOCK, AND E. EISENBERG. The mechanismof the skeletal muscle myosin ATPase. II. Relationship between thefluorescence enhancement induced by ATP and the initial Pi burst.J. Biol. Chem. 254: 3236–3243, 1979.
65. CLEWORTH, D. R., AND K. A. P. EDMAN. Changes in sarcomerelength during isometric tension development in frog skeletal mus-cle. J. Physiol. (Lond.) 227: 1–17, 1972.
66. COHEN, C., AND P. J. VIBERT. Actin filaments: images and models.In: Fibrous Protein Structure, edited by J. M. Squire and P. J.Vibert. New York: Academic, 1987, p. 283–306.
67. COOKE, R. Actomyosin interaction in striated muscle. Physiol.
Rev. 77: 671–697, 1997.68. COOKE, R., AND W. BIALEK. Contraction of glycerinated muscle
fibers as a function of the ATP concentration. Biophys. J. 28:241–258, 1979.
69. COOKE, R., K. FRANKS, G. B. LUCIANI, AND E. PATE. The inhibi-tion of rabbit skeletal muscle contraction by hydrogen ions andphosphate. J. Physiol. (Lond.) 395: 77–97, 1988.
70. COOKE, R., AND E. PATE. The effects of ADP and phosphate on thecontraction of muscle fibers. Biophys. J. 48: 789–798, 1985.
71. COX, J. A., M. COMTE, AND E. A. STEIN. Calmodulin-free skeletal-muscle troponin C prepared in the absence of urea. Biochem. J.
195: 205–211, 1981.72. CRAIG, R., AND W. LEHMAN. Crossbridge and tropomyosin posi-
tions observed in native, interacting thick and thin filaments (Ab-stract). Biophys. J. 76: A274, 1999.
73. CUDA, G., L. FANANAPAZIR, W. S. ZHU, J. R. SELLERS, AND N. D.EPSTEIN. Skeletal muscle expression and abnormal function ofbeta-myosin in hypertrophic cardiomyopathy. J. Clin. Invest. 91:2861–2865, 1993.
74. DAHIYA, R., C. A. BUTTERS, AND L. S. TOBACMAN. Equilibriumlinkage analysis of cardiac thin filament assembly. Implications forthe regulation of muscle contraction. J. Biol. Chem. 269: 29457–29461, 1994.
75. DANTZIG, J. A., Y. E. GOLDMAN, N. C. MILLAR, J. LACKTIS, AND
E. HOMSHER. Reversal of the cross-bridge force-generating tran-sition by photogeneration of phosphate in rabbit psoas musclefibres. J. Physiol. (Lond.) 451: 247–278, 1992.
76. DANTZIG, J. A., M. G. HIBBERD, D. R. TRENTHAM, AND Y. E.GOLDMAN. Cross-bridge kinetics in the presence of MgADP inves-tigated by photolysis of caged ATP in rabbit psoas muscle fibres.J. Physiol. (Lond.) 432: 639–680, 1991.
77. DA SILVA, A. C., AND F. C. REINACH. Calcium binding inducesconformational changes in muscle regulatory proteins. Trends Bio-
chem. Sci. 16: 53–57, 1991.78. DAVIS, B., W. LIU, AND J. A. PUTKEY. Effect of troponin I associ-
ation on the calcium-bound conformation of cardiac troponin C(Abstract). Biophys. J. 74: A143, 1998.
79. DIFFEE, G. M., M. L. GREASER, F. C. REINACH, AND R. L. MOSS.Effects of a non-divalent cation binding mutant of myosin regula-tory light chain on tension generation in skinned skeletal musclefibers. Biophys. J. 68: 1443–1452, 1995.
80. DOBRUNZ, L. E., P. H. BACKX, AND D. T. YUE. Steady-state [Ca21]i-force relationship in intact twitching cardiac muscle: direct evi-dence for modulation by isoproterenol and EMD 53998. Biophys. J.
69: 189–201, 1995.81. DONALDSON, S. K., L. HERMANSEN, AND L. BOLLES. Differential,
direct effects of H1 on Ca21-activated force of skinned fibers fromthe soleus, cardiac and adductor magnus muscles of rabbits.Pflugers Arch. 376: 55–65, 1978.
82. DONALDSON, S. K. B., P. M. BEST, AND W. G. L. KERRICK. Char-acterization of the effects of Mg21 on Ca21- and Sr21-activatedtension generation of skinned rat cardiac fibers. J. Gen. Physiol. 71:645–655, 1978.
83. DONALDSON, S. K. B., AND W. G. L. KERRICK. Characterization ofthe effects of Mg21 on Ca21 and Sr21-activated tension generationof skinned skeletal muscle fibers. J. Gen. Physiol. 66: 427–444,1975.
84. DONG, W., S. S. ROSENFELD, C. K. WANG, A. M. GORDON, AND
H. C. CHEUNG. Kinetic studies of calcium binding to the regulatorysite of troponin C from cardiac muscle. J. Biol. Chem. 271: 688–694, 1996.
85. DONG, W. J., C. K. WANG, A. M. GORDON, S. S. ROSENFELD, AND
H. C. CHEUNG. A kinetic model for the binding of Ca21 to theregulatory site of troponin from cardiac muscle. J. Biol. Chem. 272:19229–19235, 1997.
86. EATON, B. L. Tropomyosin binding to F-actin induced by myosinheads. Science 192: 1337–1339, 1976.
87. EBASHI, S., AND M. ENDO. Calcium ion and muscle contraction. In:Progress in Biophysics and Molecular Biology, edited by J. A. V.Butler and D. Noble. Oxford, UK: Pergamon, 1968, p. 123–183.
88. EDMAN, K. A. Depression of mechanical performance by activeshortening during twitch and tetanus of vertebrate muscle fibres.Acta Physiol. Scand. 109: 15–26, 1980.
89. EDMAN, K. A. Fatigue vs. shortening-induced deactivation in stri-ated muscle. Acta Physiol. Scand. 156: 183–192, 1996.
90. EDMAN, K. A. P. Mechanical deactivation induced by active short-ening in isolated muscle fibres of the frog. J. Physiol. (Lond.) 246:255–275, 1975.
91. EDMAN, K. A. P. The velocity of unloaded shortening and its
914 GORDON, HOMSHER, AND REGNIER Volume 80

relation to sarcomere length and isometric force in vertebratemuscle fibres. J. Physiol. (Lond.) 291: 143–159, 1979.
92. EDMAN, K. A. P., AND K.-E. ANDERSSON. The variation in activetension with sarcomere length in vertebrate skeletal muscle and itsrelation to fibre width. Experientia 24: 134–136, 1968.
93. EGELMAN, E. H., AND A. ORLOVA. New insights into actin filamentdynamics. Curr. Opin. Struct. Biol. 5: 172–180, 1995.
94. EISENBERG, E., T. L. HILL, AND Y.-D. CHEN. Cross-bridge model ofmuscle contraction. Quantitative analysis. Biophys. J. 29: 195–227,1980.
95. EKELUND, M. C., AND K. A. EDMAN. Shortening induced deactiva-tion of skinned fibres of frog and mouse striated muscle. Acta
Physiol. Scand. 116: 189–199, 1982.96. ELLIOTT, G. F. Measurements of the electric charge and ion-
binding of the protein filaments in intact muscle and cornea, withimplications for filament assembly. Biophys. J. 32: 95–97, 1980.
97. ELLIOTT, G. F., J. LOWY, AND C. R. WORTHINGTON. An X-ray andlight diffraction study of the filament lattice of striated muscle inthe living state and in rigor. J. Mol. Biol. 6: 295–305, 1963.
98. ELLIS-DAVIES, G. C., AND J. H. KAPLAN. Nitrophenyl-EGTA, aphotolabile chelator that selectively binds Ca21 with high affinityand releases it rapidly upon photolysis. Proc. Natl. Acad. Sci. USA
91: 187–191, 1994.99. ELLIS-DAVIES, G. C., J. H. KAPLAN, AND R. J. BARSOTTI. Laser
photolysis of caged calcium: rates of calcium release by nitrophe-nyl-EGTA and DM-nitrophen. Biophys. J. 70: 1006–1016, 1996.
100. EL-SALEH, S. C., AND R. J. SOLARO. Troponin I enhances acidicpH-induced depression of Ca21 binding to the regulatory sites inskeletal troponin C. J. Biol. Chem. 263: 3274–3278, 1988.
101. ENDO, M. Stretch-induced increase in activation of skinned musclefibres by calcium. Nature 237: 211–213, 1972.
102. FABIATO, A., AND F. FABIATO. Effects of pH on the myofilamentsand the sarcoplasmic reticulum of skinned cells from cardiac andskeletal muscles. J. Physiol. (Lond.) 276: 233–255, 1978.
103. FABIATO, A., AND F. FABIATO. Myofilament-generated tensionoscillations during partial calcium activation and activation depen-dence of the sarcomere length-tension relation of skinned cardiaccells. J. Gen. Physiol. 72: 667–699, 1978.
104. FARAH, C. S., C. A. MIYAMOTO, C. H. RAMOS, S.-A. C. DA, R. B.QUAGGIO, K. FUJIMORI, L. B. SMILLIE, AND F. C. REINACH.Structural and regulatory functions of the NH2- and COOH-terminalregions of skeletal muscle troponin I. J. Biol. Chem. 269: 5230–5240, 1994.
105. FARAH, C. S., AND F. C. REINACH. The troponin complex andregulation of muscle contraction. FASEB J. 9: 755–767, 1995.
106. FENG, L., E. KIM, W. L. LEE, C. J. MILLER, B. KUANG, E. RE-ISLER, AND P. A. RUBENSTEIN. Fluorescence probing of yeastactin subdomain 3/4 hydrophobic loop 262–274. Actin-actin andactin-myosin interactions in actin filaments. J. Biol. Chem. 272:16829–16837, 1997.
107. FERENCZI, M. A. Phosphate burst in permeable muscle fibers ofthe rabbit. Biophys. J. 50: 471–477, 1986.
108. FERENCZI, M. A., Y. E. GOLDMAN, AND R. M. SIMMONS. Thedependence of force and shortening velocity on substrate concen-tration in skinned muscle fibres from Rana temporaria. J. Physiol.
(Lond.) 350: 519–543, 1984.109. FERENCZI, M. A., Z. H. HE, R. K. CHILLINGWORTH, M. BRUNE,
J. E. CORRIE, D. R. TRENTHAM, AND M. R. WEBB. A new methodfor the time-resolved measurement of phosphate release in perme-abilized muscle fibers. Biophys. J. 68, Suppl.: 191s–193s, 1995.
110. FERENCZI, M. A., E. HOMSHER, AND D. R. TRENTHAM. Thekinetics of magnesium adenosine triphosphate cleavage in skinnedmuscle fibres of the rabbit. J. Physiol. (Lond.) 352: 575–599, 1984.
111. FERENCZI, M. A., AND I. C. SPENCER. The elementary steps of theactomyosin ATPase in muscle fibres studied with caged-ATP. Adv.
Exp. Med. Biol. 226: 181–188, 1988.112. FINER, J. T., R. M. SIMMONS, AND J. A. SPUDICH. Single myosin
molecule mechanics: piconewton forces and nanometre steps. Na-
ture 368: 113–119, 1994.113. FINK, R. H., D. G. STEPHENSON, AND D. A. WILLIAMS. Potassium
and ionic strength effects on the isometric force of skinned twitchmuscle fibres of the rat and toad. J. Physiol. (Lond.) 370: 317–337,1986.
114. FISHER, A. J., C. A. SMITH, J. THODEN, R. SMITH, K. SUTOH,H. M. HOLDEN, AND I. RAYMENT. Structural studies of myosin:nucleotide complexes: a revised model for the molecular basis ofmuscle contraction. Biophys. J. 68: 19S–28S, 1995.
115. FITZSIMONS, D. P., AND R. L. MOSS. Strong binding of myosinmodulates length-dependent Ca21 activation of rat ventricularmyocytes. Circ. Res. 83: 602–607, 1998.
116. FLICKER, P. F., G. N. PHILLIPS, JR., AND C. COHEN. Troponin andits interactions with tropomyosin. An electron microscope study. J.
Mol. Biol. 162: 495–501, 1982.117. FORD, L. E., A. F. HUXLEY, AND R. M. SIMMONS. The relation
between stiffness and filament overlap in stimulated frog musclefibres. J. Physiol. (Lond.) 311: 219–249, 1981.
118. FORD, L. E., A. F. HUXLEY, AND R. M. SIMMONS. Tension tran-sients during steady shortening of frog muscle fibres. J. Physiol.
(Lond.) 361: 131–150, 1985.119. FORTUNE, N. S., M. A. GEEVES, AND K. W. RANATUNGA. Tension
responses to rapid pressure release in glycerinated rabbit musclefibers. Proc. Natl. Acad. Sci. USA 88: 7323–7327, 1991.
120. FORTUNE, N. S., M. A. GEEVES, AND K. W. RANATUNGA. Con-tractile activation and force generation in skinned rabbit musclefibres: effects of hydrostatic pressure. J. Physiol. (Lond.) 474:283–290, 1994.
121. FRASER, I. D., AND S. B. MARSTON. In vitro motility analysis ofactin-tropomyosin regulation by troponin and calcium. The thinfilament is switched as a single cooperative unit. J. Biol. Chem. 270:7836–7841, 1995.
122. FUCHS, F. The binding of calcium to glycerinated muscle fibers inrigor: the effect of filament overlap. Biochim. Biophys. Acta 491:523–531, 1977.
123. FUCHS, F. The binding of calcium to detergent-extracted rabbitpsoas muscle fibres during relaxation and force generation. J. Mus-
cle Res. Cell Motil. 6: 477–486, 1985.124. FUCHS, F., AND Y. P. WANG. Force, length, and Ca(21)-troponin C
affinity in skeletal muscle. Am. J. Physiol. Cell Physiol. 261: C787–C792, 1991.
125. FUJIMORI, K., M. SORENSON, O. HERZBERG, J. MOULT, AND F. C.REINACH. Probing the calcium-induced conformational transitionof troponin C with site-directed mutants. Nature 345: 182–184,1990.
126. FUJITA, H., AND S. ISHIWATA. Tropomyosin modulates pH depen-dence of isometric tension. Biophys. J. 77: 1540–1546, 1999.
127. FUJITA, H., K. YASUDA, S. NIITSU, T. FUNATSU, AND S. ISHI-WATA. Structural and functional reconstitution of thin filaments inthe contractile apparatus of cardiac muscle. Biophys. J. 71: 2307–2318, 1996.
128. FUKUDA, N., H. FUJITA, T. FUJITA, AND S. ISHIWATA. Regulatoryroles of MgADP and calcium in tension development of skinnedcardiac muscle. J. Muscle Res. Cell Motil. 19: 909–921, 1998.
129. FUNATSU, T., T. ANAZAWA, AND S. ISHIWATA. Structural andfunctional reconstitution of thin filaments in skeletal muscle.J. Muscle Res. Cell Motil. 15: 158–171, 1994.
130. GAGNE, S. M., M. X. LI, S. K. SIA, L. SPYRACOPOULOS, J. A.PUTKEY, J. R. SOLARO, L. B. SMILLIE, AND B. D. SYKES. Tropo-nin-C structures reveal mechanism of calcium-regulatory proteins(Abstract). Biophys. J. 74: A9, 1998.
131. GAO, W. D., P. H. BACKX, M. AZAN-BACKX, AND E. MARBAN.Myofilament Ca21 sensitivity in intact versus skinned rat ventricu-lar muscle. Circ. Res. 74: 408–415, 1994.
132. GASMI-SEABROOK, G. M., J. W. HOWARTH, N. FINLEY, E. ABUS-AMHADNEH, V. GAPONENKO, R. M. BRITO, R. J. SOLARO, AND
P. R. ROSEVEAR. Solution structures of the C-terminal domain ofcardiac troponin C free and bound to the N-terminal domain ofcardiac troponin I. Biochemistry 38: 8313–8322, 1999.
133. GEEVES, M. A. The dynamics of actin and myosin association andthe crossbridge model of muscle contraction. Biochem. J. 274:1–14, 1991.
134. GEEVES, M. A., AND P. B. CONIBEAR. The role of three-statedocking of myosin S1 with actin in force generation. Biophys. J. 68:194S–201S, 1995.
135. GEEVES, M. A., AND D. J. HALSALL. Two-step ligand binding andcooperativity. A model to describe the cooperative binding of
April 2000 MUSCLE REGULATION 915

myosin subfragment 1 to regulated actin. Biophys. J. 52: 215–220,1987.
136. GEEVES, M. A., AND S. S. LEHRER. Dynamics of the muscle thinfilament regulatory switch: the size of the cooperative unit. Bio-
phys. J. 67: 273–282, 1994.137. GEISTERFER-LOWRANCE, A. A., S. KASS, G. TANIGAWA, H. P.
VOSBERG, W. MCKENNA, C. E. SEIDMAN, AND J. G. SEIDMAN. Amolecular basis for familial hypertrophic cardiomyopathy: a betacardiac myosin heavy chain gene missense mutation. Cell 62: 999–1006, 1990.
138. GERGELY, J., Z. GRABAREK, P. C. LEAVIS, G. STRASBURG, T.TAO, AND C. L. WANG. Transmission of the Ca21-regulatory signalin skeletal muscle thin filaments. Adv. Exp. Med. Biol. 226: 155–164, 1988.
139. GERSON, J. H., E. BOBKOVA, E. HOMSHER, AND E. REISLER. Roleof residues 311/312 in actin-tropomyosin interaction. In vitro mo-tility study using yeast actin mutant e311a/r312a. J. Biol. Chem.
274: 17545–17550, 1999.140. GLYN, H., AND J. SLEEP. Dependence of adenosine triphosphatase
activity of rabbit psoas muscle fibres and myofibrils on substrateconcentration. J. Physiol. (Lond.) 365: 259–76, 1985.
141. GODT, R. E. Calcium-activated tension of skinned muscle fibers ofthe frog. Dependence on magnesium adenosine triphosphate con-centration. J. Gen. Physiol. 63: 722–739, 1974.
142. GOLDMAN, Y. E., M. G. HIBBERD, AND D. R. TRENTHAM. Relax-ation of rabbit psoas muscle fibres from rigor by photochemicalgeneration of adenosine-59-triphosphate. J. Physiol. (Lond.) 354:577–604, 1984.
143. GOLDMAN, Y. E., AND R. M. SIMMONS. The stiffness of frogskinned muscle fibres at altered lateral filament spacing. J. Physiol.
(Lond.) 378: 175–194, 1986.144. GONG, Z., J. XING, M. CHANDRA, W.-J. DONG, R. J. SOLARO, P. K.
UMEDA, AND H. C. CHEUNG. Comparison of the regulatory domainconformation of troponin C from cardiac and skeletal muscle (Ab-stract). Biophys. J. 74: A51, 1998.
145. GORDON, A. M., Y. CHEN, B. LIANG, M. LAMADRID, Z. LUO, AND
P. B. CHASE. Skeletal Muscle Regulatory Proteins Enhance F-
actin In Vitro Motility. New York: Plenum, 1998.146. GORDON, A. M., R. E. GODT, S. K. DONALDSON, AND C. E.
HARRIS. Tension in skinned frog muscle fibers in solutions ofvarying ionic strength and neutral salt composition. J. Gen.
Physiol. 62: 550–574, 1973.147. GORDON, A. M., A. F. HUXLEY, AND F. J. JULIAN. The variation in
isometric tension with sarcomere length in vertebrate muscle fi-bres. J. Physiol. (Lond.) 184: 170–192, 1966.
148. GORDON, A. M., M. A. LAMADRID, Y. CHEN, Z. LUO, AND P. B.CHASE. Calcium regulation of skeletal muscle thin filament motil-ity in vitro. Biophys. J. 72: 1295–1307, 1997.
149. GORDON, A. M., AND E. B. RIDGWAY. Calcium transients andrelaxation in single muscle fibers. Eur. J. Cardiol. 7, Suppl.: 27–34,1978.
150. GORDON, A. M., AND E. B. RIDGWAY. Extra calcium on shorteningin barnacle muscle: is the decrease in calcium binding related todecreased cross-bridge attachment, force, or length? J. Gen.
Physiol. 90: 321–340, 1987.151. GORDON, A. M., AND E. B. RIDGWAY. Stretch of active muscle
during the declining phase of the calcium transient produces bi-phasic changes in calcium binding to the activating sites. J. Gen.
Physiol. 96: 1013–1035, 1990.152. GORDON, A. M., AND E. B. RIDGWAY. Cross-bridges affect both
TnC structure and calcium affinity in muscle fibers. Adv. Exp. Med.
Biol. 332: 183–194, 1993.153. GORDON, A. M., E. B. RIDGWAY, L. D. YATES, AND T. ALLEN.
Muscle cross-bridge attachment: effects on calcium binding andcalcium activation. Adv. Exp. Med. Biol. 226: 89–99, 1988.
154. GORDON, A. M., AND L. D. YATES. Regulatory mechanism of con-traction in skeletal muscle. In: Advances in Comparative and
Environmental Physiology, edited by H. Sugi. Berlin: Springer-Verlag, 1992, p. 1–36.
155. GRABAREK, Z., J. GRABAREK, P. C. LEAVIS, AND J. GERGELY.Cooperative binding to the Ca21-specific sites of troponin C inregulated actin and actomyosin. J. Biol. Chem. 258: 14098–14102,1983.
156. GRABAREK, Z., R. Y. TAN, J. WANG, T. TAO, AND J. GERGELY.Inhibition of mutant troponin C activity by an intra-domain disul-phide bond. Nature 345: 132–135, 1990.
157. GREASER, M. L., AND J. GERGELY. Reconstitution of troponinactivity from three protein components. J. Biol. Chem. 246: 4226–4233, 1971.
158. GREASER, M. L., AND J. GERGELY. Purification and properties ofthe components from troponin. J. Biol. Chem. 248: 2125–2133,1973.
159. GREENE, L. The effect of nucleotide on the binding of myosinsubfragment 1 to regulated actin. J. Biol. Chem. 257: 13993–13999,1982.
160. GREENE, L. E., AND E. EISENBERG. Cooperative binding of myo-sin subfragment-1 to the actin-troponin-tropomyosin complex.Proc. Natl. Acad. Sci. USA 77: 2616–2620, 1980.
161. GREENE, L. E., D. L. WILLIAMS, JR., AND E. EISENBERG. Regula-tion of actomyosin ATPase activity by troponin-tropomyosin: effectof the binding of the myosin subfragment 1 (S-1)zATP complex.Proc. Natl. Acad. Sci. USA 84: 3102–3106, 1987.
162. GULATI, J., E. SONNENBLICK, AND A. BABU. The role of troponinC in the length dependence of Ca(21)-sensitive force of mamma-lian skeletal and cardiac muscles. J. Physiol. (Lond.) 441: 305–324,1991.
163. GUTH, K., AND J. D. POTTER. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca21 affinity ofthe Ca21-specific regulatory sites in skinned rabbit psoas fibers.J. Biol. Chem. 262: 13627–13635, 1987.
164. HAEBERLE, J. R., K. M. TRYBUS, M. E. HEMRIC, AND D. M.WARSHAW. The effects of smooth muscle caldesmon on actinfilament motility. J. Biol. Chem. 267: 23001–23006, 1992.
165. HANCOCK, W. O., L. L. HUNTSMAN, AND A. M. GORDON. Modelsof calcium activation account for differences between skeletal andcardiac force redevelopment kinetics. J. Muscle Res. Cell Motil. 18:671–681, 1997.
166. HANCOCK, W. O., D. A. MARTYN, L. L. HUNTSMAN, AND A. M.GORDON. Influence of Ca21 on force redevelopment kinetics inskinned rat myocardium. Biophys. J. 70: 2819–2829, 1996.
167. HANNON, J. D., P. B. CHASE, D. A. MARTYN, L. L. HUNTSMAN,M. J. KUSHMERICK, AND A. M. GORDON. Calcium-independentactivation of skeletal muscle fibers by a modified form of cardiactroponin C. Biophys. J. 64: 1632–1637, 1993.
168. HANNON, J. D., D. A. MARTYN, AND A. M. GORDON. Effects ofcycling and rigor crossbridges on the conformation of cardiactroponin C. Circ. Res. 71: 984–991, 1992.
169. HARADA, Y., K. SAKURADA, T. AOKI, D. D. THOMAS, AND T.YANAGIDA. Mechanochemical coupling in actomyosin energytransduction studied by in vitro movement assay. J. Mol. Biol. 216:49–68, 1990.
170. HARTZELL, H. C. Effects of phosphorylated and unphosphorylatedC-protein on cardiac actomyosin ATPase. J. Mol. Biol. 186: 185–195, 1985.
171. HARTZELL, H. C., AND L. TITUS. Effects of cholinergic and adren-ergic agonists on phosphorylation of a 165,000-dalton myofibrillarprotein in intact cardiac muscle. J. Biol. Chem. 257: 2111–2120,1982.
172. HASELGROVE, J. C. X-ray evidence for a conformational change inthe actin-containing filaments of vertebrate striated muscle. Cold
Spring Harb. Symp. Quant. Biol. 37: 341–352, 1972.173. HATAKENAKA, M., AND I. OHTSUKI. Replacement of three tropo-
nin components with cardiac troponin components within singleglycerinated skeletal muscle fibers. Biochem. Biophys. Res. Com-
mun. 181: 1022–1027, 1991.174. HATAKENAKA, M., AND I. OHTSUKI. Effect of removal and recon-
stitution of troponins C and I on the Ca(21)-activated tensiondevelopment of single glycerinated rabbit skeletal muscle fibers.Eur. J. Biochem. 205: 985–993, 1992.
175. HAZARD, A. L., S. C. KOHOUT, N. L. STRICKER, J. A. PUTKEY, AND
J. J. FALKE. The kinetic cycle of cardiac troponin C: calciumbinding and dissociation at site II trigger slow conformationalrearrangements. Protein Sci. 7: 2451–2459, 1998.
176. HE, Z. H., R. K. CHILLINGWORTH, M. BRUNE, J. E. CORRIE, D. R.TRENTHAM, M. R. WEBB, AND M. A. FERENCZI. ATPase kineticson activation of rabbit and frog permeabilized isometric muscle
916 GORDON, HOMSHER, AND REGNIER Volume 80

fibres: a real time phosphate assay. J. Physiol. (Lond.) 501: 125–148, 1997.
177. HE, Z.-H., R. K. CHILLINGWORTH, M. BRUNE, J. E. T. CORRIE,M. R. WEBB, AND M. A. FERENZI. The efficiency of contraction inrabbit skeletal muscle fibres, determined from the rate of release ofinorganic phosphate. J. Physiol. (Lond.) 517: 839–854, 1999.
178. HE, Z. H., G. J. M. STIENEN, J. P. F. BARENDS, AND M. A. FE-RENCZI. Rate of phosphate release after photoliberation of aden-osine 59-triphosphate in slow and fast skeletal muscle fibers. Bio-
phys. J. 75: 2389–2401, 1998.179. HEAD, J. G., M. D. RITCHIE, AND M. A. GEEVES. Characterization
of the equilibrium between blocked and closed states of musclethin filaments. Eur. J. Biochem. 227: 694–699, 1995.
180. HEELEY, D. H., K. GOLOSINSKA, AND L. B. SMILLIE. The effects oftroponin T fragments T1 and T2 on the binding of nonpolymeriz-able tropomyosin to F-actin in the presence and absence of tropo-nin I and troponin C. J. Biol. Chem. 262: 9971–9978, 1987.
181. HELLAM, D. C., AND R. J. PODOLSKY. Force measurements inskinned muscle fibres. J. Physiol. (Lond.) 200: 807–819, 1969.
182. HERRMANN, C., C. LIONNE, F. TRAVERS, AND T. BARMAN. Cor-relation of ActoS1, myofibrillar, and muscle fiber ATPases. Bio-
chemistry 33: 4148–4154, 1994.183. HERZBERG, O., AND M. N. JAMES. Structure of the calcium regu-
latory muscle protein troponin-C at 2.8 Å resolution. Nature 313:653–659, 1985.
184. HERZBERG, O., J. MOULT, AND M. N. JAMES. A model for theCa21-induced conformational transition of troponin C. A trigger formuscle contraction. J. Biol. Chem. 261: 2638–2644, 1986.
185. HERZBERG, O., J. MOULT, AND M. N. G. JAMES. Molecular struc-ture of troponin C and its implications for the Ca21 triggering ofmuscle contraction. Methods Enzymol. 139: 610–633, 1987.
186. HIBBERD, M. G., J. A. DANTZIG, D. R. TRENTHAM, AND Y. E.GOLDMAN. Phosphate release and force generation in skeletalmuscle fibers. Science 228: 1317–1319, 1985.
187. HIBBERD, M. G., AND D. R. TRENTHAM. Relationships betweenchemical and mechanical events during muscular contraction.Annu. Rev. Biophys. Biophys. Chem. 15: 119–161, 1986.
188. HILL, A. V. The heat of shortening and the dynamic constants ofmuscle. Proc. R. Soc. Lond. B Biol. Sci. 126: 136–195, 1938.
189. HILL, A. V. The combination of haemoglobin with oxygen and withcarbon monoxide. Biochem. J. 7: 471–480, 1913.
190. HILL, D. K. Tension due to interaction between the sliding fila-ments in resting striated muscle. The effect of stimulation.J. Physiol. (Lond.) 199: 637–684, 1968.
191. HILL, T. L., E. EISENBERG, AND L. GREENE. Theoretical model forthe cooperative equilibrium binding of myosin subfragment 1 to theactin-troponin-tropomyosin complex. Proc. Natl. Acad. Sci. USA
77: 3186–3190, 1980.192. HILL, T. L., E. EISENBERG, AND L. GREENE. Alternate model for
the cooperative equilibrium binding of myosin subfragment-1-nu-cleotide complex to actin-troponin-tropomyosin. Proc. Natl. Acad.
Sci. USA 80: 60–64, 1983.193. HITCHCOCK, D.-S. E., AND Y. AN. Integral repeats and a continuous
coiled coil are required for binding of striated muscle tropomyosinto the regulated actin filament. J. Biol. Chem. 271: 3600–3603, 1996.
194. HITCHCOCK, S. E., H. E. HUXLEY, AND G.-A. G. SZENT. Calciumsensitive binding of troponin to actin-tropomyosin: a two-sitemodel for troponin action. J. Mol. Biol. 80: 825–836, 1973.
195. HOAR, P. E., C. W. MAHONEY, AND W. G. L. KERRICK. MgADP1
increases maximum tension and Ca21 sensitivity in skinned rabbitsoleus fibers. Pflugers Arch. 410: 30–36, 1987.
196. HOFMANN, P. A., AND F. FUCHS. Effect of length and cross-bridgeattachment on Ca21 binding to cardiac troponin C. Am. J. Physiol.
Cell Physiol. 253: C90–C96, 1987.197. HOFMANN, P. A., AND F. FUCHS. Evidence for a force-dependent
component of calcium binding to cardiac troponin C. Am. J.
Physiol. Cell Physiol. 253: C541–C546, 1987.198. HOFMANN, P. A., M. L. GREASER, AND R. L. MOSS. C-protein limits
shortening velocity of rabbit skeletal muscle fibres at low levels ofCa21 activation. J. Physiol. (Lond.) 439: 701–715, 1991.
199. HOFMANN, P. A., H. C. HARTZELL, AND R. L. MOSS. Alterations inCa21 sensitive tension due to partial extraction of C-protein from
rat skinned cardiac myocytes and rabbit skeletal muscle fibers.J. Gen. Physiol. 97: 1141–1163, 1991.
200. HOFMANN, P. A., J. M. METZGER, M. L. GREASER, AND R. L.MOSS. Effects of partial extraction of light chain 2 on the Ca21
sensitivities of isometric tension, stiffness, and velocity of short-ening in skinned skeletal muscle fibers. J. Gen. Physiol. 95: 477–498, 1990.
201. HOLMES, K. C. The actomyosin interaction and its control bytropomyosin. Biophys. J. 68: 2S–7S, 1995.
202. HOLMES, K. C. Muscle proteins—their actions and interactions.Curr. Opin. Struct. Biol. 6: 781–789, 1996.
203. HOLMES, K. C., D. POPP, W. GEBHARD, AND W. KABSCH. Atomicmodel of the actin filament. Nature 347: 44–49, 1990.
204. HOLROYDE, M. J., J. D. POTTER, AND R. J. SOLARO. The calciumbinding properties of phosphorylated and unphosphorylated car-diac and skeletal myosins. J. Biol. Chem. 254: 6478–6482, 1979.
205. HOMSHER, E., M. IRVING, AND A. WALLNER. High-energy phos-phate metabolism and energy liberation associated with rapidshortening in frog skeletal muscle. J. Physiol. (Lond.) 321: 423–436, 1981.
206. HOMSHER, E., B. KIM, A. BOBKOVA, AND L. S. TOBACMAN.Calcium regulation of thin filament movement in an in vitro motilityassay. Biophys. J. 70: 1881–1892, 1996.
207. HOMSHER, E., J. LACKTIS, AND M. REGNIER. Strain-dependentmodulation of phosphate transients in rabbit skeletal muscle fibers.Biophys. J. 72: 1780–1791, 1997.
208. HOMSHER, E., D. LEE, C. MORRIS, D. PAVLOV, AND E. HOMSHER.Regulation of force and sliding speed in thin filaments: effects ofregulatory proteins and calcium. J. Physiol. (Lond.) In press.
209. HOMSHER, E., AND N. C. MILLAR. Caged compounds and striatedmuscle contraction. Annu. Rev. Physiol. 52: 8758–8796, 1990.
210. HOMSHER, E., C. MORRIS, AND L. TOBACMAN. Thin filamentregulation of in vitro motility systems (Abstract). Biophys. J. 76:A5, 1999.
211. HOMSHER, E., F. WANG, AND J. R. SELLERS. Factors affectingmovement of F-actin filaments propelled by skeletal muscle heavymeromyosin. Am. J. Physiol. Cell Physiol. 262: C714–C723, 1992.
212. HONDA, H., AND S. ASAKURA. Calcium-triggered movement ofregulated actin in vitro. A fluorescence microscopy study. J. Mol.
Biol. 205: 677–683, 1989.213. HOUDUSSE, A., M. L. LOVE, R. DOMINGUEZ, Z. GRABAREK, AND
C. COHEN. Structures of four Ca21-bound troponin C at 2.0 Åresolution: further insights into the Ca21-switch in the calmodulinsuperfamily. Structure 5: 1695–1711, 1997.
214. HUNT, A. J., F. GITTES, AND J. HOWARD. The force exerted by asingle kinesin molecule against a viscous load. Biophys. J. 67:766–781, 1994.
215. HUXLEY, A. F. Muscle structure and theories of contraction. Prog.
Biophys. Biophys. Chem. 7: 255–318, 1957.216. HUXLEY, A. F. Reflections on Muscle. Princeton, NJ: Princeton
Univ. Press, 1980.217. HUXLEY, A. F., AND R. M. SIMMONS. Rapid “give” and the tension
“shoulder” in the relaxation of frog muscle fibres. J. Physiol.
(Lond.) 210: 32P–33P, 1970.218. HUXLEY, A. F., AND R. M. SIMMONS. Mechanical transients and the
origin of muscular force. Cold Spring Harb. Symp. Quant. Biol. 37:669–680, 1973.
219. HUXLEY, H. E. Structural changes in the actin- and myosin-con-taining filaments during contraction. Cold Spring Harb. Symp.
Quant. Biol. 37: 361–376, 1972.220. HUXLEY, H. E. Time resolved X-ray diffraction studies on muscle.
In: Cross-Bridge Mechanism in Muscular Contraction, edited byH. S. A. G. H. Pollack. Tokyo: Univ. of Tokyo Press, 1978, p.391–401.
221. HUYNH, Q., C. A. BUTTERS, J. M. LEIDEN, AND L. S. TOBACMAN.Effects of cardiac thin filament Ca21: statistical mechanical analy-sis of a troponin C site II mutant. Biophys. J. 70: 1447–1455, 1996.
222. IKURA, M., G. M. CLORE, A. M. GRONENBORN, G. ZHU, C. B.KLEE, AND A. BAX. Solution structure of a calmodulin-target pep-tide complex by multidimensional NMR. Science 256: 632–638,1992.
223. INGRAHAM, R. H., AND C. A. SWENSON. Binary interactions oftroponin subunits. J. Biol. Chem. 259: 9544–9548, 1984.
April 2000 MUSCLE REGULATION 917

224. ISAMBERT, H., P. VENIER, A. C. MAGGS, A. FATTOUM, R.KASSAB, D. PANTALONI, AND M. F. CARLIER. Flexibility of actinfilaments derived from thermal fluctuations. Effect of bound nucle-otide, phalloidin, and muscle regulatory proteins. J. Biol. Chem.
270: 11437–11444, 1995.225. ISHII, Y., AND S. S. LEHRER. Fluorescence studies of the confor-
mation of pyrene-labeled tropomyosin: effects of F-actin and myo-sin subfragment 1. Biochemistry 24: 6631–6638, 1985.
226. ISHII, Y., AND S. S. LEHRER. Excimer fluorescence of pyrenyli-odoacetamide-labeled tropomyosin: a probe of the state of tropo-myosin in reconstituted muscle thin filaments. Biochemistry 29:1160–1166, 1990.
227. ISHII, Y., AND S. S. LEHRER. Kinetics of the “on-off” change inregulatory state of the muscle thin filament. Arch. Biochem. Bio-
phys. 305: 193–196, 1993.228. ISHIJIMA, A., Y. HARADA, H. KOJIMA, T. FUNATSU, H. HIGUCHI,
AND T. YANAGIDA. Single-molecule analysis of the actomyosinmotor using nano-manipulation. Biochem. Biophys. Res. Commun.
199: 1057–1063, 1994.229. ISHIJIMA, A., H. KOJIMA, H. HIGUCHI, Y. HARADA, T. FUNATSU,
AND T. YANAGIDA. Multiple- and single-molecule analysis of theactomyosin motor by nanometer-piconewton manipulation with amicroneedle: unitary steps and forces. Biophys. J. 70: 383–400,1996.
230. IWAMOTO, H. Thin filament cooperativity as a major determinantof shortening velocity in skeletal muscle fibers. Biophys. J. 74:1452–1464, 1998.
231. JANDRESKI, M. A., M. J. SOLE, AND C.-C. LIEW. Two differentforms of beta myosin heavy chain are expressed in human striatedmuscle. Hum. Genet. 77: 127–131, 1987.
232. JHA, P. K., P. C. LEAVIS, AND S. SARKAR. Interaction of deletionmutants of troponins I and T: COOH-terminal truncation of tropo-nin T abolishes troponin I binding and reduces Ca21 sensitivity ofthe reconstituted regulatory system. Biochemistry 35:16573–16580, 1996.
233. JOHNSON, J. D., S. C. CHARLTON, AND J. D. POTTER. A fluores-cence stopped flow analysis of Ca21 exchange with troponin C.J. Biol. Chem. 254: 3497–3502, 1979.
234. JOHNSON, J. D., R. J. NAKKULA, C. VASULKA, AND L. B. SMILLIE.Modulation of Ca21 exchange with the Ca21-specific regulatorysites of troponin C. J. Biol. Chem. 269: 8919–8923, 1994.
235. JOHNSON, K. A., AND E. W. TAYLOR. Intermediate states of sub-fragment 1 and actosubfragment 1 ATPase: reevaluation of themechanism. Biochemistry 17: 3432–3442, 1978.
236. JOSEPHSON, R. K., AND K. A. EDMAN. Changes in the maximumspeed of shortening of frog muscle fibres early in a tetanic contrac-tion and during relaxation. J. Physiol. (Lond.) 507: 511–525, 1998.
237. JULIAN, F. J. Activation in a skeletal muscle contraction modelwith a modification for insect fibrillar muscle. Biophys. J. 9: 547–570, 1969.
238. JULIAN, F. J. The effect of calcium on the force-velocity relation ofbriefly glycerinated frog muscle fibres. J. Physiol. (Lond.) 218:117–145, 1971.
239. JULIAN, F. J., AND D. L. MORGAN. Variation of muscle stiffnesswith tension during tension transients and constant velocity short-ening in the frog. J. Physiol. (Lond.) 319: 193–203, 1981.
240. JULIAN, F. J., AND M. R. SOLLINS. Variation of muscle stiffnesswith force at increasing speeds of shortening. J. Gen. Physiol. 66:287–302, 1975.
241. KABSCH, W., H. G. MANNHERZ, D. SUCK, E. F. PAI, AND K. C.HOLMES. Atomic structure of the actin: DNase I complex. Nature
347: 37–44, 1990.242. KAPLAN, J. H., AND G. C. ELLIS-DAVIES. Photolabile chelators for
the rapid photorelease of divalent cations. Proc. Natl. Acad. Sci.
USA 85: 6571–6575, 1988.243. KAWAI, M., K. GUTH, K. WINNIKES, C. HAIST, AND J. C. RUEGG.
The effect of inorganic phosphate on the ATP hydrolysis rate andthe tension transients in chemically skinned rabbit psoas fibers.Pflugers Arch. 408: 1–9, 1987.
244. KAWAI, M., AND H. R. HALVORSON. Two step mechanism of phos-phate release and the mechanism of force generation in chemicallyskinned fibers of rabbit psoas muscle. Biophys. J. 59: 329–342,1991.
245. KAWAI, M., AND Y. ZHAO. Cross-bridge scheme and force percross-bridge state in skinned rabbit psoas muscle fibers. Biophys.
J. 65: 638–651, 1993.246. KENTISH, J. C. Combined inhibitory actions of acidosis and phos-
phate on maximum force production in rat skinned cardiac muscle.Pflugers Arch. 419: 310–318, 1991.
247. KENTISH, J. C. The inhibitory effects of monovalent ions on forcedevelopment in detergent-skinned ventricular muscle from guinea-pig. J. Physiol. (Lond.) 352: 353–374, 1984.
248. KIMURA, A., H. HARADA, J. E. PARK, H. NISHI, M. SATOH, M.TAKAHASHI, S. HIROI, T. SASAOKA, N. OHBUCHI, T. NAKA-MURA, T. KOYANAGI, T. H. HWANG, J. A. CHOO, K. S. CHUNG, A.HASEGAWA, R. NAGAI, O. OKAZAKI, H. NAKAMURA, M. MATSU-ZAKI, T. SAKAMOTO, H. TOSHIMA, Y. KOGA, T. IMAIZUMI, AND T.SASAZUKI. Mutations in the cardiac troponin I gene associatedwith hypertrophic cardiomyopathy. Nature Genet. 16: 379–382,1997.
249. KISHINO, A., AND T. YANAGIDA. Force measurements by micro-manipulation of a single actin filament by glass needles. Nature
334: 74–76, 1988.250. KOBAYASHI, T., T. TAO, J. GERGELY, AND J. H. COLLINS. Struc-
ture of the troponin complex. Implications of photocross-linking oftroponin I to troponin C thiol mutants. J. Biol. Chem. 269: 5725–5729, 1994.
251. KOHOUT, S. C., AND J. J. FALKE. Comparison of cardiac andskeletal troponin C (Abstract). Biophys. J. 76: A158, 1999.
252. KORMAN, V., AND L. S. TOBACMAN. Mutations in actin subdomain3 that impair thin filament regulation by troponin and tropomyosin.J. Biol. Chem. 274: 22191–22196, 1999.
253. KRAFT, T., M. MESSERLI, B. ROTHEN-RUTISHAUSER, J. C. PER-RIARD, T. WALLIMANN, AND B. BRENNER. Equilibration and ex-change of fluorescently labeled molecules in skinned skeletal mus-cle fibers visualized by confocal microscopy. Biophys. J. 69: 1246–1258, 1995.
254. KRESS, M., H. E. HUXLEY, A. R. FARUQI, AND J. HENDRIX.Structural changes during activation of frog muscle studied bytime-resolved X-ray diffraction. J. Mol. Biol. 188: 325–342, 1986.
255. KRON, S. J., Y. Y. TOYOSHIMA, T. Q. UYEDA, AND J. A. SPUDICH.Assays for actin sliding movement over myosin-coated surfaces.Methods Enzymol. 196: 399–416, 1991.
256. KRUEGER, J. W., AND A. DENTON. High-resolution measurementof striation patterns and sarcomere motions in cardiac musclecells. Biophys. J. 61: 129–144, 1992.
257. KURIHARA, S., Y. SAEKI, K. HONGO, E. TANAKA, AND N. SUDO.Effects of length change on intracellular Ca21 transients in ferretventricular muscle treated with 2,3-butanedione monoxime (BDM).Jpn. J. Physiol. 40: 915–920, 1990.
258. KUSHMERICK, M. J., AND R. E. DAVIES. The chemical energetics ofmuscle contraction. II. The chemistry, efficiency and power ofmaximally working sartorius muscles. Appendix. Free energy andenthalpy of atp hydrolysis in the sarcoplasm. Proc. R. Soc. Lond. B
Biol. Sci. 174: 315–353, 1969.259. KUSHMERICK, M. J., AND B. KRASNER. Force and ATPase rate in
skinned skeletal muscle fibers. Federation Proc. 41: 2232–2237,1982.
260. LAMADRID, M. A., P. B. CHASE, AND A. M. GORDON. Motilityassays of calcium regulation of actin. In: Molecular Interactions of
Actin, edited by C. G. dos Remedios and D. D. Thomas. Heidelberg,Germany: Springer-Verlag. In press.
261. LANDESBERG, A., AND S. SIDEMAN. Coupling calcium binding totroponin C and cross-bridge cycling in skinned cardiac cells. Am. J.
Physiol. Heart Circ. Physiol. 266: H1260–H1271, 1994.262. LANDIS, C., N. BACK, E. HOMSHER, AND L. S. TOBACMAN. Effects
of tropomyosoin internal deletions on thin filament function.J. Biol. Chem. 274: 31279–31285, 1999.
263. LANDIS, C. A., A. BOBKOVA, E. HOMSHER, AND L. S. TOBACMAN.The active state of the thin filament is destabilized by an internaldeletion in tropomyosin. J. Biol. Chem. 272: 14051–14056, 1997.
264. LEHMAN, W., R. CRAIG, AND P. VIBERT. Ca21-induced tropomyo-sin movement in Limulus thin filaments revealed by three-dimen-sional reconstruction. Nature 368: 65–67, 1994.
265. LEHMAN, W., P. VIBERT, P. UMAN, AND R. CRAIG. Steric-blocking
918 GORDON, HOMSHER, AND REGNIER Volume 80

by tropomyosin visualized in relaxed vertebrate muscle thin fila-ments. J. Mol. Biol. 251: 191–196, 1995.
266. LEHRER, S. S. The regulatory switch of the muscle thin filament:Ca21 or myosin heads? J. Muscle Res. Cell Motil. 15: 232–236, 1994.
267. LEHRER, S. S., AND M. A. GEEVES. The muscle thin filament as aclassical cooperative/allosteric regulatory system. J. Mol. Biol. 277:1081–1089, 1998.
268. LEHRER, S. S., N. L. GOLITSINA, AND M. A. GEEVES. Actin-tropomyosin activation of myosin subfragment 1 ATPase and thinfilament cooperativity. The role of tropomyosin flexibility and end-to-end interactions. Biochemistry 36: 13449–13454, 1997.
269. LEHRER, S. S., AND Y. ISHII. Fluorescence properties of acrylodan-labeled tropomyosin and tropomyosin-actin: evidence for myosinsubfragment 1 induced changes in geometry between tropomyosinand actin. Biochemistry 27: 5899–5906, 1988.
270. LEHRER, S. S., AND E. P. MORRIS. Dual effects of tropomyosin andtroponin-tropomyosin on actomyosin subfragment 1 ATPase.J. Biol. Chem. 257: 8073–8080, 1982.
271. LESZYK, J., T. TAO, L. M. NUWAYSIR, AND J. GERGELY. Identifi-cation of the photocrosslinking sites in troponin-I with 4-maleim-idobenzophenone labelled mutant troponin-C having single cys-teines at positions 158 and 21. J. Muscle Res. Cell Motil. 19: 479–490, 1998.
272. LEVINE, R. J., R. W. KENSLER, Z. YANG, J. T. STULL, AND H. L.SWEENEY. Myosin light chain phosphorylation affects the struc-ture of rabbit skeletal muscle thick filaments. Biophys. J. 71:898–907, 1996.
273. LEVINE, R. J., Z. YANG, N. D. EPSTEIN, L. FANANAPAZIR, J. T.STULL, AND H. L. SWEENEY. Structural and functional responsesof mammalian thick filaments to alterations in myosin regulatorylight chains. J. Struct. Biol. 122: 149–161, 1998.
274. LI, H. C., AND P. G. FAJER. Orientational changes of troponin Cassociated with thin filament activation. Biochemistry 33: 14324–14332, 1994.
275. LI, H. C., AND P. G. FAJER. Structural coupling of troponin C andactomyosin in muscle fibers. Biochemistry 37: 6628–6635, 1998.
276. LI, M. X., L. SPYRACOPOULOS, AND B. D. SYKES. Binding ofcardiac troponin-I147–163 induces a structural opening in humancardiac troponin-C. Biochemistry 38: 8289–8298, 1999.
277. LIANG, B., Y. CHEN, C. K. WANG, M. REGNIER, P. B. CHASE, AND
A. M. GORDON. Calcium control of skeletal muscle regulated actinfilament sliding: role of myosin crossbridge number (Abstract).Biophys. J. 76: A155, 1999.
278. LIAO, R., C. K. WANG, AND H. C. CHEUNG. Time-resolved trypto-phan emission study of cardiac troponin I. Biophys. J. 63: 986–995,1992.
279. LIN, D., A. BOBKOVA, E. HOMSHER, AND L. S. TOBACMAN. Al-tered cardiac troponin T in vitro function in the presence of amutation implicated in familial hypertrophic cardiomyopathy.J. Clin. Invest. 97: 2842–2848, 1996.
280. LINARI, M., I. DOBBIE, M. RECONDITI, N. KOUBASSOVA, M.IRVING, G. PIAZZESI, AND V. LOMBARDI. The stiffness of skeletalmuscle in isometric contraction and rigor: the fraction of myosinheads bound to actin. Biophys. J. 74: 2459–2473, 1998.
281. LORENZ, M., K. J. POOLE, D. POPP, G. ROSENBAUM, AND K. C.HOLMES. An atomic model of the unregulated thin filament ob-tained by X-ray fiber diffraction on oriented actin-tropomyosin gels.J. Mol. Biol. 246: 108–119, 1995.
282. LORENZ, M., D. POPP, AND K. C. HOLMES. Refinement of theF-actin model against X-ray fiber diffraction data by the use of adirected mutation algorithm. J. Mol. Biol. 234: 826–836, 1993.
283. LU, Z., R. L. MOSS, AND J. W. WALKER. Tension transients initiatedby photogeneration of MgADP in skinned skeletal muscle fibers.J. Gen. Physiol. 101: 867–888, 1993.
284. LUO, Y., R. COOKE, AND E. PATE. A model of stress relaxation incross-bridge systems: effect of a series elastic element. Am. J.
Physiol. Cell Physiol. 265: C279–C288, 1993.285. LYMN, R. W., AND E. W. TAYLOR. Mechanism of adenosine triphos-
phate hydrolysis by actomyosin. Biochemistry 10: 4617–4624,1971.
286. MA, Y. Z., AND E. W. TAYLOR. Kinetic mechanism of myofibrilATPase. Biophys. J. 66: 1542–1553, 1994.
287. MAK, A. S., AND L. B. SMILLIE. Non-polymerizable tropomyosin:
preparation, some properties and F-actin binding. Biochem. Bio-
phys. Res. Commun. 101: 208–214, 1981.288. MALINCHIK, S., S. XU, AND L. C. YU. Temperature-induced struc-
tural changes in the myosin thick filament of skinned rabbit psoasmuscle. Biophys. J. 73: 2304–2312, 1997.
289. MALNIC, B., C. S. FARAH, AND F. C. REINACH. Regulatory prop-erties of the NHSr21 and COOH-terminal domains of troponin T.ATPase activation and binding to troponin I and troponin C. J. Biol.
Chem. 273: 10594–10601, 1998.290. MARGOSSIAN, S. S. Reversible dissociation of dog cardiac myosin
regulatory light chain 2 and its influence on ATP hydrolysis. J. Biol.
Chem. 260: 13747–13754, 1985.291. MARTINOSI, A., AND M. N. MALIK. Kinetics of formation and
dissociation of H-meromyosin-ADP complex. Cold Spring Harb.
Symp. Quant. Biol. 37: 184–185, 1973.292. MARTYN, D. A., C. J. FREITAG, P. B. CHASE, AND A. M. GORDON.
Ca21 and cross-bridge-induced changes in troponin C in skinnedskeletal muscle fibers: effects of force inhibition. Biophys. J. 76:1480–1493, 1999.
293. MARTYN, D. A., C. J. FREITAG, D. XU, AND A. M. GORDON.Structural response of monocysteine mutants of cardiac TnC tocalcium and crossbridges in skinned cardiac muscle (Abstract).Biophys. J. 76: A281, 1999.
294. MARTYN, D. A., AND A. M. GORDON. Length and myofilamentspacing-dependent changes in calcium sensitivity of skeletal fibres:effects of pH and ionic strength. J. Muscle Res. Cell Motil. 9:428–445, 1988.
295. MARTYN, D. A., AND A. M. GORDON. Force and stiffness in glyc-erinated rabbit psoas fibers. Effects of calcium and elevated phos-phate. J. Gen. Physiol. 99: 795–816, 1992.
296. MAUGHAN, D. W., AND R. E. GODT. Inhibition of force productionin compressed skinned muscle fibers of the frog. Pflugers Arch.
390: 161–163, 1981.297. MAYTUM, R., S. S. LEHRER, AND M. A. GEEVES. Cooperativity and
switching within the three-state model of muscle regulation. Bio-
chemistry 38: 1102–1110, 1999.298. MCCLELLAN, G., A. WEISBERG, AND S. WINEGRAD. cAMP can
raise or lower cardiac actomyosin ATPase activity depending ona-adrenergic activity. Am. J. Physiol. Heart Circ. Physiol. 267:H431–H442, 1994.
299. MCDONALD, K. S., L. J. FIELD, M. S. PARMACEK, M. SOONPAA,J. M. LEIDEN, AND R. L. MOSS. Length dependence of Ca21 sensi-tivity of tension in mouse cardiac myocytes expressing skeletaltroponin C. J. Physiol. (Lond.) 483: 131–139, 1995.
300. MCDONALD, K. S., AND R. L. MOSS. Osmotic compression of singlecardiac myocytes eliminates the reduction in Ca21 sensitivity oftension at short sarcomere length. Circ. Res. 77: 199–205, 1995.
301. MCDONALD, K. S., M. R. WOLFF, AND R. L. MOSS. Sarcomerelength dependence of the rate of tension redevelopment and sub-maximal tension in rat and rabbit skinned skeletal muscle fibres.J. Physiol. (Lond.) 501: 607–621, 1997.
302. MCKILLOP, D. F., AND M. A. GEEVES. Regulation of the actomyosinsubfragment 1 interaction by troponin/tropomyosin. Evidence forcontrol of a specific isomerization between two acto.myosin sub-fragment 1 states. Biochem. J. 279: 711–718, 1991.
303. MCKILLOP, D. F., AND M. A. GEEVES. Regulation of the interactionbetween actin and myosin subfragment 1: evidence for three statesof the thin filament. Biophys. J. 65: 693–701, 1993.
304. MCLACHLAN, A. D., AND M. STEWART. The 14-fold periodicity inalpha-tropomyosin and the interaction with actin. J. Mol. Biol. 103:271–298, 1976.
305. MCLAUGHLIN, P. J., J. T. GOOCH, H. G. MANNHERZ, AND A. G.WEEDS. Structure of gelsolin segment 1-actin complex and themechanism of filament severing. Nature 364: 685–692, 1993.
306. MEADOR, W. E., A. R. MEANS, AND F. A. QUIOCHO. Target enzymerecognition by calmodulin: 2.4 Å structure of a calmodulin-peptidecomplex. Science 257: 1251–1255, 1992.
307. METZGER, J. M. Myosin binding-induced cooperative activation ofthe thin filament in cardiac myocytes and skeletal muscle fibers.Biophys. J. 68: 1430–1442, 1995.
308. METZGER, J. M. Effects of phosphate and ADP on shorteningvelocity during maximal and submaximal calcium activation of the
April 2000 MUSCLE REGULATION 919

thin filament in skeletal muscle fibers. Biophys. J. 70: 409–417,1996.
309. METZGER, J. M., M. L. GREASER, AND R. L. MOSS. Variations incross-bridge attachment rate and tension with phosphorylation ofmyosin in mammalian skinned skeletal muscle fibers. Implicationsfor twitch potentiation in intact muscle. J. Gen. Physiol. 93: 855–883, 1989.
310. METZGER, J. M., AND R. L. MOSS. Calcium-sensitive cross-bridgetransitions in mammalian fast and slow skeletal muscle fibers.Science 247: 1088–1090, 1990.
311. METZGER, J. M., AND R. L. MOSS. Kinetics of a Ca21-sensitivecross-bridge state transition in skeletal muscle fibers. Effects dueto variations in thin filament activation by extraction of troponin C.J. Gen. Physiol. 98: 233–248, 1991.
312. METZGER, J. M., AND R. L. MOSS. Myosin light chain 2 modulatescalcium-sensitive cross-bridge transitions in vertebrate skeletalmuscle. Biophys. J. 63: 460–468, 1992.
313. METZGER, J. M., AND R. L. MOSS. Phosphate and the kinetics offorce generation in skinned skeletal muscle fibers (Abstract). Bio-
phys. J. 59: 418a, 1991.314. METZGER, J. M., M. S. PARMACEK, E. BARR, K. PASYK, W. I. LIN,
K. L. COCHRANE, L. J. FIELD, AND J. M. LEIDEN. Skeletal troponinC reduces contractile sensitivity to acidosis in cardiac myocytesfrom transgenic mice. Proc. Natl. Acad. Sci. USA 90: 9036–9040,1993.
315. MILLAR, N. C., AND E. HOMSHER. The effect of phosphate andcalcium on force generation in glycerinated rabbit skeletal musclefibers. J. Biol. Chem. 265: 20234–20240, 1990.
316. MILLAR, N. C., AND E. HOMSHER. Kinetics of force generation andphosphate release in skinned rabbit soleus muscle fibers. Am. J.
Physiol. Cell Physiol. 262: C1239–C1245, 1992.317. MILLIGAN, R. A. Protein-protein interactions in the rigor actomy-
osin complex. Proc. Natl. Acad. Sci. USA 93: 21–26, 1996.318. MILLIGAN, R. A., AND P. F. FLICKER. Structural relationships of
actin, myosin, and tropomyosin revealed by cryo-electron micros-copy. J. Cell Biol. 105: 29–39, 1987.
319. MILLIGAN, R. A., M. WHITTAKER, AND D. SAFER. Molecular struc-ture of F-actin and location of surface binding sites. Nature 348:217–221, 1990.
320. MOLLOY, J. E., J. E. BURNS, J.-J. KENDRICK, R. T. TREGEAR, AND
D. C. WHITE. Movement and force produced by a single myosinhead. Nature 378: 209–212, 1995.
321. MONOD, J., J. WYMAN, AND J. P. CHANGEUX. On the nature ofallosteric transitions. J. Mol. Biol. 12: 88–118, 1965.
322. MOORE, P. B., H. E. HUXLEY, AND D. J. DEROSIER. Three-dimen-sional reconstruction of F-actin, thin filaments and decorated thinfilaments. J. Mol. Biol. 50: 279–295, 1970.
323. MORANO, I., AND J. C. RUEGG. What does TnCDANZ fluorescencereveal about the thin filament state? Pflugers Arch. 418: 333–337,1991.
324. MORGAN, D. L., D. R. CLAFLIN, AND F. J. JULIAN. The relationshipbetween tension and slowly varying intracellular calcium concen-tration in intact frog skeletal muscle. J. Physiol. (Lond.) 500:177–192, 1997.
325. MORIMOTO, S., AND I. OHTSUKI. Ca21 binding to skeletal muscletroponin C in skeletal and cardiac myofibrils. J. Biochem. 105:435–439, 1989.
326. MORIMOTO, S., F. YANAGA, R. MINAKAMI, AND I. OHTSUKI.Ca21-sensitizing effects of the mutations at Ile-79 and Arg-92 oftroponin T in hypertrophic cardiomyopathy. Am. J. Physiol. Cell
Physiol. 275: C200–C207, 1998.327. MORRIS, C., A. BOBKOVA, L. TOBACMAN, AND E. HOMSHER. The
relative independence of thin filament sliding speed on the fractionof activated regulatory unit at saturating Ca21 (Abstract). Biophys.
J. 72: A173, 1997.328. MORRIS, C. A., L. S. TOBACMAN, AND E. HOMSHER. Modulation of
thin filament activation using an inactivated cardiac troponin C inskinned skeletal muscle fibers (Abstract). Biophys. J. 74: A347,1998.
329. MOSS, R. L. The effect of calcium on the maximum velocity ofshortening in skinned skeletal muscle fibres of the rabbit. J. Muscle
Res. Cell Motil. 3: 295–311, 1982.330. MOSS, R. L. Effects on shortening velocity of rabbit skeletal muscle
due to variations in the level of thin-filament activation. J. Physiol.
(Lond.) 377: 487–505, 1986.331. MOSS, R. L. Ca21 regulation of mechanical properties of striated
muscle. Mechanistic studies using extraction and replacement ofregulatory proteins. Circ. Res. 70: 865–884, 1992.
332. MOSS, R. L., J. D. ALLEN, AND M. L. GREASER. Effects of partialextraction of troponin complex upon the tension-pCa relation inrabbit skeletal muscle. Further evidence that tension developmentinvolves cooperative effects within the thin filament. J. Gen.
Physiol. 87: 761–774, 1986.333. MOSS, R. L., G. M. DIFFEE, AND M. L. GREASER. Contractile
properties of skeletal muscle fibers in relation to myofibrillar pro-tein isoforms. Rev. Physiol. Biochem. Pharmacol. 126: 1–63, 1995.
334. MOSS, R. L., G. G. GIULIAN, AND M. L. GREASER. The effects ofpartial extraction of TnC upon the tension-pCa relationship inrabbit skinned skeletal muscle fibers. J. Gen. Physiol. 86: 585–600,1985.
335. MOSS, R. L., M. R. LAUER, G. G. GIULIAN, AND M. L. GREASER.Altered Ca21 dependence of tension development in skinned skel-etal muscle fibers following modification of troponin by partialsubstitution with cardiac troponin C. J. Biol. Chem. 261: 6096–6099, 1986.
336. NAKAURA, H., S. MORIMOTO, F. YANAGA, M. NAKATA, H. NISHI,T. IMAIZUMI, AND I. OHTSUKI. Functional changes in troponin T bya splice donor site mutation that causes hypertrophic cardiomyop-athy. Am. J. Physiol. Cell Physiol. 277: C225–C232, 1999.
337. NASSAR, R., N. N. MALOUF, M. B. KELLY, A. E. OAKELEY, P. A.ANDERSON, AND L. S. TOBACMAN. Force-pCa relation and tropo-nin T isoforms of rabbit myocardium. Structure-function studies ofthe amino-terminal region of bovine cardiac troponin T. Circ. Res.
69: 1470–1475, 1991.338. NATORI, R. The property and contraction process of isolated myo-
fibrils. Jikeikai Med. J. 1: 119–126, 1954.339. NENCINI, S., N. PIRODDI, F. COLOMO, C. TESI, AND C. POGGESI.
Relaxation rate of single skeletal myofibrils following rapid solu-tion change (Abstract). Biophys. J. 76: A163, 1999.
340. OFFER, G., C. MOOS, AND R. STARR. A new protein of the thickfilaments of vertebrate skeletal myofibrils Extraction purificationand characterization. J. Mol. Biol. 74: 653–676, 1973.
341. OLAH, G. A., AND J. TREWHELLA. A model structure of the muscleprotein complex 4Ca21.troponin C.troponin I derived from small-angle scattering data: implications for regulation. Biochemistry 33:12800–12806, 1994.
342. PADRON, R., L. ALAMO, J. MURGICH, AND R. CRAIG. Towards anatomic model of the thick filaments of muscle. J. Mol. Biol. 275:35–41, 1998.
343. PALMER, S., AND J. C. KENTISH. Differential effects of the Ca21
sensitizers caffeine and CGP 48506 on the relaxation rate of ratskinned cardiac trabeculae. Circ. Res. 80: 682–687, 1997.
344. PALMER, S., AND J. C. KENTISH. Roles of Ca21 and crossbridgekinetics in determining the maximum rates of Ca21 activation andrelaxation in rat and guinea pig skinned trabeculae. Circ. Res. 83:179–186, 1998.
345. PALMITER, K. A., AND R. J. SOLARO. Molecular mechanisms reg-ulating the myofilament response to Ca21: implications of muta-tions causal for familial hypertrophic cardiomyopathy. Basic Res.
Cardiol. 92, Suppl. 1: 63–74, 1997.346. PAN, B., AND R. J. SOLARO. Calcium-binding properties of troponin
C in detergent-skinned heart muscle fibers. J. Biol. Chem. 262:7839–7849, 1987.
347. PARRY, D. A., AND J. M. SQUIRE. Structural role of tropomyosin inmuscle regulation: analysis of the X-ray diffraction patterns fromrelaxed and contracting muscles. J. Mol. Biol. 75: 33–55, 1973.
348. PARSONS, B., D. SZCZESNA, J. ZHAO, S.-G. VAN, W. G. KERRICK,J. A. PUTKEY, AND J. D. POTTER. The effect of pH on the Ca21
affinity of the Ca21 regulatory sites of skeletal and cardiac troponinC in skinned muscle fibres. J. Muscle Res. Cell Motil. 18: 599–609,1997.
349. PATE, E., AND R. COOKE. A model for the interaction of musclecross-bridges with ligands which compete with ATP. J. Theor. Biol.
118: 215–230, 1986.350. PATE, E., AND R. COOKE. Addition of phosphate to active muscle
920 GORDON, HOMSHER, AND REGNIER Volume 80

fibers probes actomyosin states within the powerstroke. Pflugers
Arch. 414: 73–81, 1989.351. PATE, E., AND R. COOKE. A model of crossbridge action: the
effects of ATP, ADP and Pi. J. Muscle Res. Cell Motil. 10: 181–196,1989.
352. PATE, E., S.-K. FRANKS, H. WHITE, AND R. COOKE. The use ofdiffering nucleotides to investigate cross-bridge kinetics. J. Biol.
Chem. 268: 10046–10053, 1993.353. PATE, E., K. L. NAKAMAYE, K. FRANKS-SKIBA, R. G. YOUNT, AND
R. COOKE. Mechanics of glycerinated muscle fibers using non-nucleoside triphosphate substrates. Biophys. J. 59: 598–605, 1991.
354. PATE, E. F., AND C. J. BROKAW. Cross-bridge behavior in rigormuscle. Biophys. Struct. Mech. 7: 51–63, 1980.
355. PATEL, J. R., G. M. DIFFEE, X. P. HUANG, AND R. L. MOSS.Phosphorylation of myosin regulatory light chain eliminates force-dependent changes in relaxation rates in skeletal muscle. Biophys.
J. 74: 360–368, 1998.356. PATEL, J. R., G. M. DIFFEE, AND R. L. MOSS. Myosin regulatory
light chain modulates the Ca21 dependence of the kinetics oftension development in skeletal muscle fibers. Biophys. J. 70:2333–2340, 1996.
357. PATEL, J. R., K. S. MCDONALD, M. R. WOLFF, AND R. L. MOSS.Ca21 binding to troponin C in skinned skeletal muscle fibers as-sessed with caged Ca21 and a Ca21 fluorophore. Invariance of Ca21
binding as a function of sarcomere length. J. Biol. Chem. 272:6018–6027, 1997.
358. PEARLSTONE, J. R., AND L. B. SMILLIE. Binding of troponin-Tfragments to several types of tropomyosin. Sensitivity to Ca21 inthe presence of troponin-C. J. Biol. Chem. 257: 10587–10592, 1982.
359. PEARLSTONE, J. R., AND L. B. SMILLIE. Effects of troponin-Iplus-C on the binding of troponin-T and its fragments to alpha-tropomyosin: Ca21 sensitivity and cooperativity. J. Biol. Chem.
258: 2534–2542, 1983.360. PEARLSTONE, J. R., AND L. B. SMILLIE. Evidence for two-site
binding of troponin I inhibitory peptides to the N and C domains oftroponin C. Biochemistry 34: 6932–6940, 1995.
361. PERRY, S. V. Troponin T: genetics, properties and function. J. Mus-
cle Res. Cell Motil. 19: 575–602, 1998.362. PERRY, S. V., H. A. COLE, J. F. HEAD, AND F. J. WILSON. Local-
ization and mode of action of the inhibitory protein component ofthe troponin complex. Cold Spring Harbor Symp. Quant. Biol. 37:251–262, 1972.
363. PHILLIPS, G. N., JR., J. P. FILLERS, AND C. COHEN. Tropomyosincrystal structure and muscle regulation. J. Mol. Biol. 192: 111–131,1986.
364. PIAZZESI, G., M. RECONDITI, I. DOBBIE, M. LINARI, P.BOESECKE, O. DIAT, M. IRVING, AND V. LOMBARDI. Changes inconformation of myosin heads during the development of isometriccontraction and rapid shortening in single frog muscle fibres.J. Physiol. (Lond.) 514: 305–312, 1999.
365. PODOLSKY, R. J., AND L. E. TEICHHOLZ. The relation betweencalcium and contraction kinetics in skinned muscle fibres.J. Physiol. (Lond.) 211: 19–35, 1970.
366. POOLE, K. J. V., G. ROSENBAUM, M. LORENZ, AND K. C. HOLMES.The effect of crossbridges on the calcium sensitivity of the struc-tural change of the regulated thin filament (Abstract). Biophys. J.
68: A365, 1995.367. POOLE, K. V., K. C. HOLMES, I. RAYMENT, AND M. LORENZ.
Control of the actomyosin interaction. Biophys. J. 68, Suppl.: 348s,1994.
368. POPP, D., AND Y. MA’EDA. Calcium ions and the structure ofmuscle actin filament. An X-ray diffraction study. J. Mol. Biol. 229:279–285, 1993.
369. POPP, D., Y. MAEDA, A. A. STEWART, AND K. C. HOLMES. X-raydiffraction studies on muscle regulation. Adv. Biophys. 27: 89–103,1991.
370. POTMA, E. J., AND G. J. STIENEN. Increase in ATP consumptionduring shortening in skinned fibres from rabbit psoas muscle:effects of inorganic phosphate. J. Physiol. (Lond.) 496: 1–12, 1996.
371. POTTER, J. D., AND J. GERGELY. Troponin, tropomyosin, and actininteractions in the Ca21 regulation of muscle contraction. Bio-
chemistry 13: 2697–2703, 1974.372. POTTER, J. D., T. PANAVELIL, G. GUZMAN, M. JONES, J. ZHAO,
AND D. SZCZESNA. The role of troponin T in the regulation ofcontraction (Abstract). Biophys. J. 74: A143, 1998.
373. POTTER, J. D., Z. SHENG, B. S. PAN, AND J. ZHAO. A directregulatory role for troponin T and a dual role for troponin C in theCa21 regulation of muscle contraction. J. Biol. Chem. 270: 2557–2562, 1995.
374. PUTKEY, J. A., W. LIU, X. LIN, S. AHMED, M. ZHANG, J. D.POTTER, AND W. G. KERRICK. Fluorescent probes attached to Cys35 or Cys 84 in cardiac troponin C are differentially sensitive toCa(21)-dependent events in vitro and in situ. Biochemistry 36:970–978, 1997.
375. PUTKEY, J. A., H. L. SWEENEY, AND S. T. CAMPBELL. Site-di-rected mutation of the trigger calcium-binding sites in cardiactroponin C. J. Biol. Chem. 264: 12370–12378, 1989.
376. RANATUNGA, K. W. Temperature-dependence of shortening veloc-ity and rate of isometric tension development in rat skeletal mus-cle. J. Physiol. (Lond.) 329: 465–483, 1982.
377. RAYMENT, I., AND H. M. HOLDEN. The three-dimensional structureof a molecular motor. Trends Biochem. Sci. 19: 129–134, 1994.
378. RAYMENT, I., H. M. HOLDEN, M. WHITTAKER, C. B. YOHN, M.LORENZ, K. C. HOLMES, AND R. A. MILLIGAN. Structure of theactin-myosin complex and its implications for muscle contraction.Science 261: 58–65, 1993.
379. RAYMENT, I., W. R. RYPNIEWSKI, B.-K. SCHMIDT, R. SMITH,D. R. TOMCHICK, M. M. BENNING, D. A. WINKELMANN, G.WESENBERG, AND H. M. HOLDEN. Three-dimensional structure ofmyosin subfragment-1: a molecular motor. Science 261: 50–58,1993.
380. RAYMENT, I., C. SMITH, AND R. G. YOUNT. The active site ofmyosin. Annu. Rev. Physiol. 58: 671–702, 1996.
381. REGNIER, M., AND E. HOMSHER. The effect of ATP analogs onposthydrolytic and force development steps in skinned skeletalmuscle fibers. Biophys. J. 74: 3059–3071, 1998.
382. REGNIER, M., D. M. LEE, AND E. HOMSHER. ATP analogs andmuscle contraction: mechanics and kinetics of nucleoside triphos-phate binding and hydrolysis. Biophys. J. 74: 3044–3058, 1998.
383. REGNIER, M., D. A. MARTYN, AND P. B. CHASE. Calcium regula-tion of tension redevelopment kinetics with 2-deoxy-ATP or low[ATP] in rabbit skeletal muscle. Biophys. J. 74: 2005–2015, 1998.
384. REGNIER, M., D. A. MARTYN, AND P. B. CHASE. The calciumsensitivity of steady-state force and force development kinetics incardiac muscle is enhanced by 2-deoxy-ATP (Abstract). Biophys. J.
78: 141A, 2000.385. REGNIER, M., D. A. MARTYN, AND P. B. CHASE. Calmidazolium
alters Ca21 regulation of tension redevelopment rate in skinnedskeletal muscle. Biophys. J. 71: 2786–2794, 1996.
386. REGNIER, M., C. MORRIS, AND E. HOMSHER. Regulation of thecross-bridge transition from a weakly to strongly bound state inskinned rabbit muscle fibers. Am. J. Physiol. Cell Physiol. 269:C1532–C1539, 1995.
387. REGNIER, M., A. J. RIVERA, M. A. BATES, AND P. B. CHASE.Calcium regulation of steady-state force and rate of tension rede-velopment in rabbit skeletal muscle (Abstract). Biophys. J. 76:A161, 1999.
388. REGNIER, M., A. J. RIVERA, P. B. CHASE, L. B. SMILLIE, AND M. M.SORENSON. Regulation of skeletal muscle tension redevelopmentby troponin C constructs with different Ca21 affinities. Biophys. J.
76: 2664–2672, 1999.389. REIFFERT, S., R. MAYTUM, M. GEEVES, K. LOHMANN, T. GREIS,
M. BLUGGEL, H. E. MEYER, L. M. HEILMEYER, AND K. JAQUET.Characterization of the cardiac holotroponin complex reconsti-tuted from native cardiac troponin T and recombinant I and C. Eur.
J. Biochem. 261: 40–47, 1999.390. REIFFERT, S. U., K. JAQUET, L. M. HEILMEYER, JR., M. D.
RITCHIE, AND M. A. GEEVES. Bisphosphorylation of cardiac tro-ponin I modulates the Ca(Sr21)-dependent binding of myosin sub-fragment S1 to reconstituted thin filaments. FEBS Lett 384: 43–47,1996.
391. REISER, P. J., M. WESTFALL, AND R. J. SOLARO. Developmentaltransition in myocardial troponin T (TnT) isoforms correlates witha change in calcium sensitivity (Abstract). Biophys. J. 57: 549A,1990.
392. RENNIE, E. R., J. D. JOHNSON, L. B. SMILLIE, AND J. A. RALL.
April 2000 MUSCLE REGULATION 921

Effects of troponin C mutants with altered Ca21 affinity on forceregulation in skeletal muscle (Abstract). Biophys. J. 72: A282, 1997.
393. REUBEN, J. P., P. W. BRANDT, M. BERMAN, AND H. GRUNDFEST.Regulation of tension in the skinned crayfish muscle fiber. I. Con-traction and relaxation in the absence of Ca (pCa is greater than 9).J. Gen. Physiol. 57: 385–407, 1971.
394. RIDGWAY, E. B., AND A. M. GORDON. Muscle calcium transient:effect of post-stimulus length changes in single muscle fibers.J. Gen. Physiol. 83: 75–103, 1984.
395. ROBERTSON, S. P., J. D. JOHNSON, M. J. HOLROYDE, E. G.KRANIAS, J. D. POTTER, AND R. J. SOLARO. The effect of troponinI phosphorylation on the Ca21-binding properties of the Ca21-regulatory site of bovine cardiac troponin. J. Biol. Chem. 257:260–263, 1982.
396. ROBERTSON, S. P., AND W. G. KERRICK. The effects of pH onCa21-activated force in frog skeletal muscle fibers. Pflugers Arch.
380: 41–45, 1979.397. ROSENFELD, S. S., AND E. W. TAYLOR. Kinetic studies of calcium
and magnesium binding to troponin C. J. Biol. Chem. 260: 242–251,1985.
398. RUEGG, J. C. Cardiac contractility: how calcium activates themyofilaments. Naturwissenschaften 85: 575–582, 1998.
399. RUST, E. M., F. P. ALBAYYA, AND J. M. METZGER. Identification ofa contractile deficit in adult cardiac myocytes expressing hypertro-phic cardiomyopathy-associated mutant troponin T proteins.J. Clin. Invest. 103: 1459–1467, 1999.
400. RUST, E. M., M. V. WESTFALL, AND J. M. METZGER. Stability of thecontractile assembly and Ca21-activated tension in adenovirus in-fected adult cardiac myocytes. Mol. Cell. Biochem. 181: 143–155,1998.
401. SATA, M., H. YAMASHITA, S. SUGIURA, H. FUJITA, S. MOMO-MURA, AND T. SERIZAWA. A new in vitro motility assay techniqueto evaluate calcium sensitivity of the cardiac contractile proteins.Pflugers Arch. 429: 443–445, 1995.
402. SCHACHAT, F. H., M. S. DIAMOND, AND P. W. BRANDT. Effect ofdifferent troponin t-tropomyosin combinations on thin filamentactivation. J. Mol. Biol. 198: 551–554, 1987.
403. SCHAERTL, S., S. S. LEHRER, AND M. A. GEEVES. Separation andcharacterization of the two functional regions of troponin involvedin muscle thin filament regulation. Biochemistry 34: 15890–15894,1995.
404. SCHOENBERG, M. Characterization of the myosin adenosinetriphosphate (M.ATP) crossbridge in rabbit and frog skeletal mus-cle fibers. Biophys. J. 54: 135–148, 1988.
405. SCHOENBERG, M. The kinetics of weakly- and strongly-bindingcrossbridges: implications for contraction and relaxation. In: Mo-
lecular Mechanism of Muscle Contraction, edited by H. Sugi andG. H. Pollack. New York: Plenum, 1988, p. 189–200.
406. SCHUTT, C. E., U. LINDBERG, J. MYSLIK, AND N. STRAUSS. Mo-lecular packing in profilin: actin crystals and its implications. J.
Mol. Biol. 209: 735–746, 1989.407. SEILER, S. H., D. A. FISCHMAN, AND L. A. LEINWAND. Modulation
of myosin filament organization by C-protein family members. Mol.
Biol. Cell 7: 113–127, 1996.408. SELLERS, J. R., G. CUDA, F. WANG, AND E. HOMSHER. Myosin-
specific adaptations of the motility assay. Methods Cell Biol. 39:23–49, 1993.
409. SELLERS, J. R., AND B. KACHAR. Polarity and velocity of slidingfilaments: control of direction by actin and of speed by myosin.Science 249: 406–408, 1990.
410. SHENG, Z., W. L. STRAUSS, J. M. FRANCOIS, AND J. D. POTTER.Evidence that both Ca(21)-specific sites of skeletal muscle TnC arerequired for full activity. J. Biol. Chem. 265: 21554–21560, 1990.
411. SIA, S. K., M. X. LI, L. SPYRACOPOULOS, S. M. GAGN’E, W. LIU,J. A. PUTKEY, AND B. D. SYKES. Structure of cardiac muscletroponin C unexpectedly reveals a closed regulatory domain.J. Biol. Chem. 272: 18216–18221, 1997.
412. SIEMANKOWSKI, R. F., AND H. D. WHITE. Kinetics of the interac-tion between actin, ADP, and cardiac myosin-S1. J. Biol. Chem.
259: 5045–5053, 1984.413. SINHA, A. M., D. J. FRIEDMAN, J. M. NIGRO, S. JAKOVCIC, M.
RABINOWITZ, AND P. K. UMEDA. Expression of rabbit ventricular
alpha-myosin heavy chain messenger RNA sequences in atrial mus-cle. J. Biol. Chem. 259: 6674–6680, 1984.
414. SLEEP, J. A., AND R. L. HUTTON. Exchange between inorganicphosphate and adenosine 59-triphosphate in the medium by acto-myosin subfragment 1. Biochemistry 19: 1276–1283, 1980.
415. SLUPSKY, C. M., AND B. D. SYKES. NMR solution structure ofcalcium-saturated skeletal muscle troponin C. Biochemistry 34:15953–15964, 1995.
416. SMILLIE, L. B., K. GOLOSINSKA, AND F. C. REINACH. Sequences ofcomplete cDNAs encoding four variants of chicken skeletal muscletroponin T. J. Biol. Chem. 263: 18816–18820, 1988.
417. SMITH, C. A., AND I. RAYMENT. X-ray structure of the magne-sium(II)-ADP-vanadate complex of the Dictyostelium discoideum
myosin motor domain to 1.9 Å resolution. Biochemistry 35: 5404–5417, 1996.
418. SORENSON, M. M., A. C. DA SILVA, C. S. GOUVEIA, V. P. SOUSA,W. OSHIMA, J. A. FERRO, AND F. C. REINACH. Concerted action ofthe high affinity calcium binding sites in skeletal muscle troponinC. J. Biol. Chem. 270: 9770–9777, 1995.
419. SPYRACOPOULOS, L., M. X. LI, S. K. SIA, S. M. GAGNE, M.CHANDRA, R. J. SOLARO, AND B. D. SYKES. Calcium-inducedstructural transition in the regulatory domain of human cardiactroponin C. Biochemistry 36: 12138–12146, 1997.
420. SQUIRE, J. M., H. A. AL-KHAYAT, AND N. YAGI. Muscle thin-filament structure and regulation. Actin subdomain movements andthe tropomyosin shift modelled from low-angle X-ray diffraction.J. Chem. Soc. Faraday Trans. 89: 2717–2726, 1993.
421. SQUIRE, J. M., AND E. P. MORRIS. A new look at thin filamentregulation in vertebrate skeletal muscle. FASEB J. 12: 761–771,1998.
422. STARR, R., R. ALMOND, AND G. OFFER. Location of C-protein,H-protein and X-protein in rabbit skeletal muscle fibre types.J. Muscle Res. Cell Motil. 6: 227–256, 1985.
423. STEFANCSIK, R., P. K. JHA, AND S. SARKAR. Identification andmutagenesis of a highly conserved domain in troponin T responsi-ble for troponin I binding: potential role for coiled coil interaction.Proc. Natl. Acad. Sci. USA 95: 957–962, 1998.
424. STEIN, L. A., R. P. SCHWARZ, JR., J. B. CHOCK, AND E. EISEN-BERG. Mechanism of actomyosin adenosine triphosphatase. Evi-dence that adenosine 59-triphosphate hydrolysis can occur withoutdissociation of the actomyosin complex. Biochemistry 18: 3895–3909, 1979.
425. STRYNADKA, N. C., M. CHERNEY, A. R. SIELECKI, M. X. LI, L. B.SMILLIE, AND M. N. JAMES. Structural details of a calcium-inducedmolecular switch: X-ray crystallographic analysis of the calcium-saturated N-terminal domain of troponin C at 1.75 Å resolution. J.
Mol. Biol. 273: 238–255, 1997.426. STUYVERS, B. D., M. MIURA, AND H. E. TER KEURS. Diastolic
viscoelastic properties of rat cardiac muscle: involvement of Ca21.Adv. Exp. Med. Biol. 430: 13–28, 1997.
427. SUGI, H. In vitro motility assay. In: Current Methods in Muscle
Physiology, edited by H. Sugi. New York: Oxford Univ. Press, 1998,p. 173–195.
428. SUNDARALINGAM, M., R. BERGSTROM, G. STRASBURG, S. T.RAO, P. ROYCHOWDHURY, M. GREASER, AND B. C. WANG. Mo-lecular structure of troponin C from chicken skeletal muscle at3-angstrom resolution. Science 227: 945–948, 1985.
429. SWARTZ, D. R., AND R. L. MOSS. Influence of a strong-bindingmyosin analogue on calcium-sensitive mechanical properties ofskinned skeletal muscle fibers. J. Biol. Chem. 267: 20497–20506,1992.
430. SWARTZ, D. R., R. L. MOSS, AND M. L. GREASER. Calcium alonedoes not fully activate the thin filament for S1 binding to rigormyofibrils. Biophys. J. 71: 1891–1904, 1996.
431. SWEENEY, H. L., B. F. BOWMAN, AND J. T. STULL. Myosin lightchain phosphorylation in vertebrate striated muscle: regulation andfunction. Am. J. Physiol. Cell Physiol. 264: C1085–C1095, 1993.
432. SWEENEY, H. L., R. M. BRITO, P. R. ROSEVEAR, AND J. A. PUT-KEY. The low-affinity Ca21-binding sites in cardiac/slow skeletalmuscle troponin C perform distinct functions: site I alone cannottrigger contraction. Proc. Natl. Acad. Sci. USA 87: 9538–9542, 1990.
433. SWEENEY, H. L., AND H. FENG. Structure-function analysis of
922 GORDON, HOMSHER, AND REGNIER Volume 80

cytoskeletal/contractile proteins in avian myotubes. Methods Cell
Biol. 52: 275–282, 1998.434. SWEENEY, H. L., H. S. FENG, Z. YANG, AND H. WATKINS. Func-
tional analyses of troponin T mutations that cause hypertrophiccardiomyopathy: insights into disease pathogenesis and troponinfunction. Proc. Natl. Acad. Sci. USA 95: 14406–14410, 1998.
435. SWEENEY, H. L., AND E. L. HOLZBAUR. Mutational analysis ofmotor proteins. Annu. Rev. Physiol. 58: 751–792, 1996.
436. SWEENEY, H. L., AND M. J. KUSHMERICK. Myosin phosphoryla-tion in permeabilized rabbit psoas fibers. Am. J. Physiol. Cell
Physiol. 249: C362–C365, 1985.437. SWEENEY, H. L., AND J. T. STULL. Alteration of cross-bridge ki-
netics by myosin light chain phosphorylation in rabbit skeletalmuscle: implications for regulation of actin-myosin interaction.Proc. Natl. Acad. Sci. USA 87: 414–418, 1990.
438. SWEENEY, H. L., Z. YANG, G. ZHI, J. T. STULL, AND K. M. TRYBUS.Charge replacement near the phosphorylatable serine of the myo-sin regulatory light chain mimics aspects of phosphorylation. Proc.
Natl. Acad. Sci. USA 91: 1490–1494, 1994.439. SWEENEY, L., A. STRACESKI, L. LEINWAND, B. TIKUNOV, AND L.
FAUST. Heterologous expression and characterization of a cardiacmyosin mutant that causes hypertrophic cardiomyopathy. J. Biol.
Chem. 269: 1603–1605, 1994.440. SWEITZER, N. K., AND R. L. MOSS. The effect of altered tempera-
ture on Ca21-sensitive force in permeabilized myocardium andskeletal muscle. Evidence for force dependence of thin filamentactivation. J. Gen. Physiol. 96: 1221–1245, 1990.
441. SWENSON, C. A., AND N. C. STELLWAGEN. Flexibility of smoothand skeletal tropomyosins. Biopolymers. 28: 955–963, 1989.
442. SYSKA, H., J. M. WILKINSON, R. J. GRAND, AND S. V. PERRY. Therelationship between biological activity and primary structure oftroponin I from white skeletal muscle of the rabbit. Biochem. J.
153: 375–387, 1976.443. SZCZESNA, D., AND P. G. FAJER. The tropomyosin domain is
flexible and disordered in reconstituted thin filaments. Biochemis-
try 34: 3614–3620, 1995.444. SZCZESNA, D., G. GUZMAN, T. MILLER, J. ZHAO, K. FAROKHI, H.
ELLEMBERGER, AND J. D. POTTER. The role of the four Ca21
binding sites of troponin C in the regulation of skeletal musclecontraction. J. Biol. Chem. 271: 8381–8386, 1996.
445. SZCZESNA, D., J. ZHAO, AND J. D. POTTER. The regulatory lightchains of myosin modulate cross-bridge cycling in skeletal muscle.J. Biol. Chem. 271: 5246–5250, 1996.
446. SZENT-GYORGYI, A. G. Calcium regulation of muscle contraction.Biophys. J. 15: 707–723, 1975.
447. TARDIFF, J. C., S. M. FACTOR, B. D. TOMPKINS, T. E. HEWETT,B. M. PALMER, R. L. MOORE, S. SCHWARTZ, J. ROBBINS, AND
L. A. LEINWAND. A truncated cardiac troponin T molecule intransgenic mice suggests multiple cellular mechanisms for familialhypertrophic cardiomyopathy. J. Clin. Invest. 101: 2800–2811,1998.
448. TASCHE, C., E. MEYHOFER, AND B. BRENNER. Calcium depen-dency of force redevelopment in rat cardiac myocytes (Abstract).Biophys. J. 74: A350, 1998.
449. TAYLOR, E. W. Mechanism of actomyosin ATPase and the problemof muscle contraction. CRC Crit. Rev. Biochem. 6: 103–164, 1979.
450. TAYLOR, E. W. Kinetic studies on the association and dissociationof myosin subfragment 1 and actin. J. Biol. Chem. 266: 294–302,1991.
451. TESI, C., K. KITAGISHI, F. TRAVERS, AND T. BARMAN. Cryoenzy-mic studies on actomyosin ATPase: kinetic evidence for commu-nication between the actin and ATP sites on myosin. Biochemistry
30: 4061–4067, 1991.452. THAMES, M. D., L. E. TEICHHOLZ, AND R. J. PODOLSKY. Ionic
strength and the contraction kinetics of skinned muscle fibers.J. Gen. Physiol. 63: 509–530, 1974.
453. THIRLWELL, H., J. E. CORRIE, G. P. REID, D. R. TRENTHAM, AND
M. A. FERENCZI. Kinetics of relaxation from rigor of permeabil-ized fast-twitch skeletal fibers from the rabbit using a novel cagedATP and apyrase. Biophys. J. 67: 2436–2447, 1994.
454. TOBACMAN, L. S. Structure-function studies of the amino-terminalregion of bovine cardiac troponin T. J. Biol. Chem. 263: 2668–2672,1988.
455. TOBACMAN, L. S. Thin filament-mediated regulation of cardiaccontraction. Annu. Rev. Physiol. 58: 447–481, 1996.
456. TOBACMAN, L. S., N. BACK, C. BUTTERS, A. KARIBE, AND J.STRAND. A novel alpha-tropomyosin mutation associated with amalignant form of hypertropic cardiomyopathy causes increasedthin filament calcium affinity and altered myosin cycling. Circula-
tion. 100: I-276, 1999.457. TOBACMAN, L. S., AND R. LEE. Isolation and functional compari-
son of bovine cardiac troponin T isoforms. J. Biol. Chem. 262:4059–4064, 1987.
458. TOBACMAN, L. S., D. LIN, C. BUTTERS, C. LANDIS, N. BACK, D.PAVLOV, AND E. HOMSHER. Functional consequences of troponinT mutations found in hypertrophic cardiomyopathy. J. Biol. Chem.
274: 28363–28370, 1999.459. TOBACMAN, L. S., AND D. SAWYER. Calcium binds cooperatively
to the regulatory sites of the cardiac thin filament. J. Biol. Chem.
265: 931–939, 1990.460. TOYOSHIMA, Y. Y., S. J. KRON, E. M. MCNALLY, K. R. NIEBLING,
C. TOYOSHIMA, AND J. A. SPUDICH. Myosin subfragment-1 issufficient to move actin filaments in vitro. Nature 328: 536–539,1987.
461. TOYOSHIMA, Y. Y., C. TOYOSHIMA, AND J. A. SPUDICH. Bidirec-tional movement of actin filaments along tracks of myosin heads.Nature 341: 154–156, 1989.
462. TREGEAR, R. T., R. J. EDWARDS, T. C. IRVING, K. J. POOLE, M. C.REEDY, H. SCHMITZ, E. TOWNS-ANDREWS, AND M. K. REEDY.X-ray diffraction indicates that active cross-bridges bind to actintarget zones in insect flight muscle. Biophys. J. 74: 1439–1451,1998.
463. TRIPET, B., E.-J. E. VAN, AND R. S. HODGES. Mapping of a secondactin-tropomyosin and a second troponin C binding site within theC terminus of troponin I, and their importance in the Ca21-depen-dent regulation of muscle contraction. J. Mol. Biol. 271: 728–750,1997.
464. TRYBUS, K. M. Role of myosin light chains. J. Muscle Res. Cell
Motil. 15: 587–594, 1994.465. TRYBUS, K. M., AND E. W. TAYLOR. Kinetic studies of the cooper-
ative binding of subfragment 1 to regulated actin. Proc. Natl. Acad.
Sci. USA 77: 7209–7213, 1980.466. UYEDA, T. Q., P. D. ABRAMSON, AND J. A. SPUDICH. The neck
region of the myosin motor domain acts as a lever arm to generatemovement. Proc. Natl. Acad. Sci. USA 93: 4459–4464, 1996.
467. UYEDA, T. Q., S. J. KRON, AND J. A. SPUDICH. Myosin step size.Estimation from slow sliding movement of actin over low densitiesof heavy meromyosin. J. Mol. Biol. 214: 699–710, 1990.
468. VANBUREN, P., G. S. WALLER, D. E. HARRIS, K. M. TRYBUS,D. M. WARSHAW, AND S. LOWEY. The essential light chain isrequired for full force production by skeletal muscle myosin. Proc.
Natl. Acad. Sci. USA 91: 12403–12407, 1994.469. VANDENBOOM, R., D. R. CLAFLIN, AND F. J. JULIAN. Effects of
rapid shortening on rate of force regeneration and myoplasmic[Ca21] in intact frog skeletal muscle fibres. J. Physiol. (Lond.) 511:171–180, 1998.
470. VAN EERD, J. P., AND K. TAKAHASHI. Determination of the com-plete amino acid sequence of bovine cardiac troponin C. Biochem-
istry 15: 1171–1180, 1976.471. VAN EYK, J. E., L. T. THOMAS, B. TRIPET, R. J. WIESNER, J. R.
PEARLSTONE, C. S. FARAH, F. C. REINACH, AND R. S. HODGES.Distinct regions of troponin I regulate Ca21-dependent activationand Ca21 sensitivity of the acto-S1-TM ATPase activity of the thinfilament. J. Biol. Chem. 272: 10529–10537, 1997.
472. VASSYLYEV, D. G., S. TAKEDA, S. WAKATSUKI, K. MAEDA, AND Y.MA’EDA. Crystal structure of troponin C in complex with troponinI fragment at 2.3-Å resolution. Proc. Natl. Acad. Sci. USA 95:4847–4852, 1998.
473. VENTURA-CLAPIER, R., H. MEKHFI, P. OLIVIERO, AND B. SWYN-GHEDAUW. Pressure overload changes cardiac skinned-fiber me-chanics in rats, not in guinea pigs. Am. J. Physiol. Heart Circ.
Physiol. 254: H517–H524, 1988.474. VERGARA, J., AND M. DIFRANCO. Imaging of calcium transients
during excitation-contraction coupling in skeletal muscle fibers.Adv. Exp. Med. Biol. 311: 227–236, 1992.
April 2000 MUSCLE REGULATION 923

475. VIBERT, P., R. CRAIG, AND W. LEHMAN. Steric-model for activa-tion of muscle thin filaments. J. Mol. Biol. 266: 8–14, 1997.
476. WAHR, P. A., H. C. CANTOR, AND J. M. METZGER. Nucleotide-dependent contractile properties of Ca(21)-activated fast and slowskeletal muscle fibers. Biophys. J. 72: 822–834, 1997.
477. WAHR, P. A., J. D. JOHNSON, AND J. A. RALL. Determinants ofrelaxation rate in skinned frog skeletal muscle fibers. Am. J.
Physiol. Cell Physiol. 274: C1608–C1615, 1998.478. WAHR, P. A., AND J. A. RALL. Role of calcium and cross bridges in
determining rate of force development in frog muscle fibers. Am. J.
Physiol. Cell Physiol. 272: C1664–C1671, 1997.479. WALKER, J. W., Z. LU, AND R. L. MOSS. Effects of Ca21 on the
kinetics of phosphate release in skeletal muscle. J. Biol. Chem. 267:2459–2466, 1992.
480. WANG, F., B. M. MARTIN, AND J. R. SELLERS. Regulation ofactomyosin interactions in Limulus muscle proteins. J. Biol.
Chem. 268: 3776–3780, 1993.481. WANG, G., AND M. KAWAI. Force generation and phosphate release
steps in skinned rabbit soleus slow-twitch muscle fibers. Biophys.
J. 73: 878–894, 1997.482. WANG, K. Titin/connectin and nebulin: giant protein rulers of
muscle structure and function. Adv. Biophys. 33: 123–134, 1996.483. WANG, Y. P., AND F. FUCHS. Length, force, and Ca21-troponin C
affinity in cardiac and slow skeletal muscle. Am. J. Physiol. Cell
Physiol. 266: C1077–C1082, 1994.484. WANG, Y. P., AND F. FUCHS. Osmotic compression of skinned
cardiac and skeletal muscle bundles: effects on force generation,Ca21 sensitivity and Ca21 binding. J. Mol. Cell. Cardiol. 27: 1235–1244, 1995.
485. WARRICK, H. M., R. M. SIMMONS, J. T. FINER, T. Q. UYEDA, S.CHU, AND J. A. SPUDICH. In vitro methods for measuring force andvelocity of the actin-myosin interaction using purified proteins.Methods Cell Biol. 39: 1–21, 1993.
486. WARSHAW, D. M., J. M. DESROSIERS, S. S. WORK, AND K. M.TRYBUS. Effects of MgATP, MgADP, and Pi on actin movement bysmooth muscle myosin. J. Biol. Chem. 266: 24339–24343, 1991.
487. WATKINS, H., W. J. MCKENNA, L. THIERFELDER, H. J. SUK, R.ANAN, A. O’DONOGHUE, P. SPIRITO, A. MATSUMORI, C. S.MORAVEC, AND J. G. SEIDMAN. Mutations in the genes for cardiactroponin T and alpha-tropomyosin in hypertrophic cardiomyopa-thy. N. Engl. J. Med. 332: 1058–1064, 1995.
488. WATKINS, H., C. E. SEIDMAN, J. G. SEIDMAN, H. S. FENG, AND
H. L. SWEENEY. Expression and functional assessment of a trun-cated cardiac troponin T that causes hypertrophic cardiomyopathy.Evidence for a dominant negative action. J. Clin. Invest. 98: 2456–2461, 1996.
489. WEBB, M. R., M. G. HIBBERD, Y. E. GOLDMAN, AND D. R.TRENTHAM. Oxygen exchange between Pi in the medium andwater during ATP hydrolysis mediated by skinned fibers fromrabbit skeletal muscle. Evidence for Pi binding to a force-generat-ing state. J. Biol. Chem. 261: 15557–15564, 1986.
490. WEGNER, A., AND T. P. WALSH. Interaction of tropomyosin-tropo-nin with actin filaments. Biochemistry 20: 5633–5642, 1981.
491. WEISBERG, A., AND S. WINEGRAD. Alteration of myosin crossbridges by phosphorylation of myosin-binding protein C in cardiacmuscle. Proc. Natl. Acad. Sci. USA 93: 8999–9003, 1996.
492. WEISBERG, A., AND S. WINEGRAD. Relation between crossbridgestructure and actomyosin ATPase activity in rat heart. Circ. Res.
83: 60–72, 1998.493. WESTERBLAD, H., AND D. G. ALLEN. The role of sarcoplasmic retic-
ulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. J. Physiol. (Lond.) 474: 291–301, 1994.
494. WESTFALL, M. V., E. M. RUST, AND J. M. METZGER. Slow skeletaltroponin I gene transfer, expression, and myofilament incorpora-tion enhances adult cardiac myocyte contractile function. Proc.
Natl. Acad. Sci. USA 94: 5444–5449, 1997.495. WHITE, H. D., B. BELKNAP, AND M. R. WEBB. Kinetics of nucleo-
side triphosphate cleavage and phosphate release steps by associ-ated rabbit skeletal actomyosin, measured using a novel fluores-cent probe for phosphate. Biochemistry 36: 11828–11836, 1997.
496. WHITE, H. D., AND E. W. TAYLOR. Energetics and mechanism ofactomyosin adenosine triphosphatase. Biochemistry 15: 5818–5826,1976.
497. WHITE, S. P., C. COHEN, AND G. N. PHILLIPS, JR. Structure of co-crystals of tropomyosin and troponin. Nature 325: 826–828, 1987.
498. WILKINSON, J. M. Troponin C from rabbit slow skeletal and car-diac muscle is the product of a single gene. Eur. J. Biochem. 103:179–188, 1980.
499. WILLIAMS, D. L., JR., AND L. E. GREENE. Comparison of the effectsof tropomyosin and troponin-tropomyosin on the binding of myo-sin subfragment 1 to actin. Biochemistry 22: 2770–2774, 1983.
500. WILLIAMS, D. L., JR., L. E. GREENE, AND E. EISENBERG. Coop-erative turning on of myosin subfragment 1 adenosinetriphos-phatase activity by the troponin-tropomyosin-actin complex. Bio-
chemistry 27: 6987–6993, 1988.501. WINEGRAD, S. Cardiac myosin binding protein C. Circ. Res. 84:
1117–1126, 1999.502. WINKELMANN, D. A., L. BOURDIEU, A. OTT, F. KINOSE, AND A.
LIBCHABER. Flexibility of myosin attachment to surfaces influ-ences F-actin motion. Biophys. J. 68: 2444–2453, 1995.
503. WOLEDGE, R. C., N. A. CURTIN, AND E. HOMSHER. Energetic as-pects of muscle contraction. Monogr. Physiol. Soc. 41: 1–357, 1985.
504. WOLFF, M. R., K. S. MCDONALD, AND R. L. MOSS. Rate of tensiondevelopment in cardiac muscle varies with level of activator cal-cium. Circ. Res. 76: 154–160, 1995.
505. WRAY, J. S. Structure of relaxed myosin filaments in relation tonucleotide state in vertebrate skeletal muscle (Abstract). J. Muscle
Res. Cell Motil. 8: 62, 1987.506. XIE, X., D. H. HARRISON, I. SCHLICHTING, R. M. SWEET, V. N.
KALABOKIS, G.-A. G. SZENT, AND C. COHEN. Structure of theregulatory domain of scallop myosin at 2.8 Å resolution. Nature
368: 306–312, 1994.507. XU, C., R. CRAIG, L. TOBACMAN, R. HOROWITZ, AND W. LEH-
MAN. Tropomyosin positions in regulated thin filaments revealedby cryoelectron microscopy. Biophys. J. 77: 985–992, 1999.
508. YAMADA, A., N. ISHII, AND K. TAKAHASHI. Direction and speed ofactin filaments moving along thick filaments isolated from mollus-can smooth muscle. J. Biochem. 108: 341–343, 1990.
509. YAMADA, A., AND T. WAKABAYASHI. Movement of actin awayfrom the center of reconstituted rabbit myosin filament is slowerthan in the opposite direction. Biophys. J. 64: 565–569, 1993.
510. YAMAUCHI-TAKIHARA, K., M. J. SOLE, J. LIEW, D. ING, AND C. C.LIEW. Characterization of human cardiac myosin heavy chaingenes. Proc. Natl. Acad. Sci. USA 86: 3504–3508, 1989.
511. YANAGA, F., S. MORIMOTO, AND I. OHTSUKI. Ca21 sensitizationand potentiation of the maximum level of myofibrillar ATPaseactivity caused by mutations of troponin T found in familial hyper-trophic cardiomyopathy. J. Biol. Chem. 274: 8806–8812, 1999.
512. YANAGIDA, T., M. TANIGUCHI, AND F. OOSAWA. Conformationalchanges of F-actin in the thin filaments of muscle induced in vivoand in vitro by calcium ions. J. Mol. Biol. 90: 509–522, 1974.
513. YATES, L. D. Calcium activation of fibers. Rec. Meat Conf. Proc. 38:9–25, 1985.
514. YOUNT, R. G., D. LAWSON, AND I. RAYMENT. Is myosin a “backdoor” enzyme? Biophys. J. 68, Suppl.: 44s–49s, 1995.
515. YUE, D. T., E. MARBAN, AND W. G. WIER. Relationship betweenforce and intracellular [Ca21] in tetanized mammalian heart mus-cle. J. Gen. Physiol. 87: 223–242, 1986.
516. ZHANG, D., K. W. YANCEY, P. SANGHANI, AND D. R. SWARTZ.Effect of ADP on the activation of the thin filament by cross-bridgesin myofibrils (Abstract). Biophys. J. 76: A155, 1999.
517. ZHANG, D., K. W. YANCEY, AND D. R. SWARTZ. Influence of ADPon myosin subfragment-1 binding to myofibrils (Abstract). Bio-
phys. J. 74: A266, 1998.518. ZHANG, R., J. ZHAO, A. MANDVENO, AND J. D. POTTER. Cardiac
troponin I phosphorylation increases the rate of cardiac musclerelaxation. Circ. Res. 76: 1028–1035, 1995.
519. ZOT, H. G., AND J. D. POTTER. A structural role for the Ca21-Mg21
sites on troponin C in the regulation of muscle contraction. J. Biol.
Chem. 257: 7678–7683, 1982.520. ZOT, H. G., AND J. D. POTTER. Calcium binding and fluorescence
measurements of dansylaziridine-labeled troponin C in reconsti-tuted thin filaments. J. Muscle Res. Cell Motil. 8: 428–436, 1987.
521. ZOU, G., AND G. N. PHILLIPS, JR. A cellular automaton model forthe regulatory behavior of muscle thin filaments. Biophys. J. 67:11–28, 1994.
924 GORDON, HOMSHER, AND REGNIER Volume 80