Process Mapping & Gap Analysis
-
Upload
ignatius-leonard -
Category
Documents
-
view
48 -
download
2
description
Transcript of Process Mapping & Gap Analysis

CRC at CTSI
Process Mapping & Gap Analysis
Prepared by:Hilary ButlerDan Chen
Anand KulkarniGaurav Srivastava
Ingrid Warrner

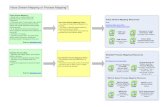
Process overview
Stage 1 Stage 2 Stage 3
Protocol approval Protocol planning Patient visits
Submission Recruitment Scheduling
Budgeting Informed consent Implementation
Feasibility review Organizational meetings Compliance documentation
Advisory Committee review Screening Data Entry
Modifications Processing instructions Sample Processing
IRB and CRC approval
2

3
Issues/Recommendations• Protocol Approval/ Stage 1
– Inefficient tracking mechanism for pre-approval of studies/ Implement “live” online tracking system which feasibility team could input feedback .
• Planning/Stage 2– SC and nursing staff fielding calls from
potential study recruits/ Create a centralized hub to pre-qualify/screen subjects and direct to proper information source

4
Issues/Recommendations• Planning/ Stage 2
– Duplicate of demographic information entry/ Develop a “one-stop” database that can be accessed by PI teams and CRC personnel
• Patient Visits/ Stage 3– Operations Mgr overloaded with
Webcamp/offload data entry of demographic info to admin asst.
– Room scheduling/ Use Outlook type calendar system that would allow the SC or nurse to book the appt. when speaking with the pt.

5
Developed a Process Map/ SOPs• A process map was created that will be a work
in progress and we will need to drill down to specifics
• Confirmed the need for us to organize the location and specific SOPs that are pertinent to the CRC and develop ones that are specific to the activity within the process map

6
Gap analysis tool• Found a tool for us to use to ensure that our
SCs are meeting FDA GCP and ICH Guidelines • It is a starting point where we can update and
customize as we further develop our SOPs for each step of the process

7
Key takeaways• Reduce manual processes by relying on
integrated electronic systems– Fully utilize WebCamp or some other
software to better track studies• Streamline scheduling and ICD verification
processes• Implement a centralized recruiting center• Improve documentation and develop SOPs