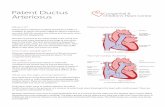
Ductus dependent circulation
-
Upload
raghu-kishore-galla -
Category
Health & Medicine
-
view
318 -
download
0
Transcript of Ductus dependent circulation
Congenital heart defects with ductus-dependentcirculation are defined as abnormalities, in which thepermeability of the ductus arteriosus is mandatory inorder to maintain systemic perfusion
Physiology of Ductus Arteriosus
• Carries 60% of combinedventricular Output
• Diverts blood from high resistancepulmonary circulation to lowresistance descending aorta andplacental circulation.
• PGE1 and PGI2 formed intramurally and in placenta maintainductal patency in fetal life
Post Natal Closure of PDA
Functional closure• In 15 hr., contraction of medial
smooth muscles due to ↑ PO2 & ↓PGE1.
Anatomical closure• In 3 wk., replacement of muscle
fibres with fibrosis creatingligamentum arteriosus & the ductloses the ability to reopen
• Cassels et al. defined the true persistence of the PDA when it persists in an infant beyond 3 months of age.
• Takizava et al concluded that endogenous NO has a major role inregulating the patency of the DA in earlier fetal stages, while dilatorprostaglandins may play a role in the near-term fetus
• The PGE1 was beneficial in opening the ductus and raising the systemicarterial oxygen saturation in neonatal patients with duct-dependentcongenital heart defects.
• According to Mc Namara, the period of 1946 to 1982 saw the revolutionin pediatric cardiac care due to the evolution of the methods to keepopen the PFO and PDA.
• Successful medical manipulation of PDA became the routine practicenow a days .
• If ductal closure causes significant decrease in systemic circulation, the conditionis called ductus dependent systemic blood flow
• If ductal closure causes significant decrease in pulmonary circulation, thecondition is called ductus dependent pulmonary blood flow
BEFORE BIRTH AFTER BIRTH
DUCTUS ARTERIOSUS DEPENDENT
PULMONARY BLOOD FLOW
• Pulmonary Atresia with or without VSD• Critical Pulmonary Stenosis• Tricuspid atresia• Tetralogy of Fallot (severe form)• Severe Ebstein’s anomaly• Severe TR
DUCTUS ARTERIOSUS DEPENDENT
SYSTEMIC BLOOD FLOW
• Critical aortic stenosis
• Coarctation of the aorta
• Interruption of aortic arch
• Hypoplastic left heart syndrome
DUCTUS ARTERIOSUS DEPENDENT
PULMONARY & SYSTEMIC CIRCULATIONS
TWO PARALLEL CIRCULATIONSMIXING IS NEEDED
Transposition of Great Arteries
Duct-dependent pulmonary Circulation
• Lungs are underperfused in these babies.
• PDA diverts partially saturated systemic blood towards the pulmonarycirculation to improve the overall saturation.
• Rarely, a widely open duct may raise the PaO2 > 49 mm Hg. Therefore, theconcentration of oxygen, to start ductal constriction, is seldom achieved byoxygen supplementation
• PaO2 remains in the range of 35 to 40 mm Hg
• least benefitted by oxygen administration and the administration of 100percent oxygen only increases the dissolved oxygen level.
OBSTRUCTION OF PULMONARY FLOW
RVH
RIGHT ATRIAL PRESSURE ↑
SYSTEMIC CYANOSIS
PERSISTENT OPENING FORAMEN OVALE
Resistance Blood Flow
Fails
Shunting of unoxygenated blood from the right atrium into the left atrium
Duct-dependent systemic Circulation
• Obstructive left-sided lesions are responsible for the decreased perfusion to thebody leading to acidosis of the vital organs including the brain and kidney.
• The aortic stenosis leads to pressure overload of the heart.
• Clinically present with hepatomegaly and right ventricular dominance.
• The duct in such cases becomes not only a decompressing channel, but alsoprovides volume and perfusion pressure for the whole body.
• Echocardiographic evaluation shows characteristic reverse filling of the arch andascending aorta.
Hypoplastic left heart syndrome (HLHS)
• Leads to complete intracardiac mixing of pulmonary andsystemic venous blood and the PA is the single outlet forthe total cardiac output.
• When PVR falls in the early postnatal period, the Qpincreases at the cost of the systemic (Qs) and coronaryblood flow.
Qp & Qs mismatch1. Excessive Qp leads to pulmonary edema and tachypneaaugments the overall metabolic rate
2. Excessive Qp results in an added volume load to thesingle ventricle resulting in ventricular dysfunction andvalvular regurgitation
3. Qs falls leading to diminished oxygen delivery ,acidosis, necrotizing enterocolitis, renal and hepaticdysfunction and other complications
Differential Diagnoses
• Pulmonary hypertension of the New born (PPHN)
• Neonatal Sepsis
• Metabolic disorders
• Primary lung pathology
• Obstructed TAPVC
• Methemoglobinemia
• Other disorders - choanal atresia, Pierre Robin sequence, vascular ring,diaphragmatic hernia, acyanotic CHDs with shunt reversal, chest infections,hypothermia and hypoglycemia.
Clinical presentation
• Usually coincide with the time of ductal constriction or closure .
• Often present in the first few days of life with incremental cyanosis.
• Neonatal cyanosis becomes more conspicuous due to their higherhemoglobin levels.
• Central cyanosis is dependent on absolute concentration of deoxygenatedHb and is more than 3 grams/L in arterial blood and more than 5 grams/Lin capillary blood.
Clinical presentation
• babies with higher fetal Hb level will have late visible cyanosis.
• Very sick babies usually have cyanotic spell or congestive heart failure andcirculatory collapse without clinical cyanosis.
• An inaudible murmur must not be criteria for exclusion of CHD, and some times,the deterioration of the clinical condition with disappearance of murmur is apointer for an urgent intervention.
• Involvement of multiple organs like kidney, brain or skeletal system, which mayadd up to the morbidity and mortality. Hence a detailed examination and parentalcounseling is required.
Confirmation of Cyanosis
• Central cyanosis must be confirmed by monitoring saturation with pulseoximetry (PO) and subsequently with ABG.
• Monitor pre- and postductal oxygen saturations.
• If there is a difference of saturation in upper and lower limb of more than3 to 7 % , the chances of having ductal flow from the pulmonary artery toaorta are high.
• In first few hours, differential saturation may be fallacious due to highpulmonary artery pressure and patent duct.
Apply hyperoxia test
• In the absence of fixed cardiac shunts, 100 percent oxygen will increase alveolarPO2, leading to an increase in pulmonary venous and systemic arterial PO2.
• In cyanotic CHDs (e.g. decreased pulmonary blood flow or TGA), little or no rise inPaO2 would be expected after breathing 100 percent O2.
• However, the same finding may occur in infants with significant pulmonaryhypertension, if significant right-to-left shunting persists through extrapulmonaryshunts (ductus arteriosus and foramen ovale).
Validity of pulse oximetry in screening for CHD
• Ten studies (44,969 newborns,71 severe defects) evaluating the usefulness ofneonatal PO screening in timely detection of CHDs showed a high specificity(99.99%) and the overall rate of detection of 15 individual defects with PO was 72% (range 46-100%), exceeding that of the clinical examination, 58 % (9-86%).
• PO should be documented at preductal and postductal sites to assess fordifferential or reverse differential cyanosis. If the preductal saturation is higher thanthe post ductal saturation (3 to 7% difference), differential cyanosis exists.
• In Sweden the use of PO and clinical examination led to an increased sensitivity of82.8 % and specificity of 100 % for the duct dependent lesions
Goals of management
• To establish the diagnosis after initial stabilization or resuscitation
• Intubation whenever indicated
• To minimize hypoxemia
• Ensure balance between Qp & Qs
• Increase the FiO2 once ductal patency is maintained.
• Do not try to achieve complete abolition of cyanosis
• A saturation of 75 to 85 % can be adequate to avoid tissue hypoxemia andeventual lactic acidosis
• PCO2 levels should be optimized between 35 to 45 mm Hg, with orwithout ventilation
• Give IV fluids, blood transfusion, address the underlying cause for acidosis
Ductal stenting - Advantages
1. Eliminating the need for neonatal palliative surgery.
2. Reducing the number of operations required.
3. Optimizing the time of definitive surgical correction.
4. High-flexibility coronary stent is an effective alternative inhigh-risk surgical candidates
Procedure of PDA stenting
• Under GA
• Antegrade through FV or retrogtade through FA
• 4F Mullin sheeth
• Coronary stent on 0.14 wire
• Ensure to cover whole length especially PA end
• After dilatation with balloon may be done
• Asprin 1-3mg/kg as long as duct patency required
Bioengineering in duct patency
• Transfection is the delivery of DNA, RNA, proteins, and macromolecules into theeukaryotic cells.
• A protein called fibronectin, the concentration of which increases in the advancedstage of gestation, is responsible for closure of the duct.
• The gene for a fibronectin decoy was introduced directly in utero in the ductaltissue to keep ductal patency in animal experiments.
• Percutaneous postnatal transfection of gene for PG in ductal tissue also ensuredprolonged patency of duct.
• These and several other projects are underway to get the safest technique to keepduct open.