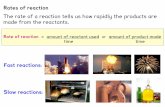
Chapter 16. Thermodynamics tells us if a reaction can occur while Kinetics tells us how quickly the...
-
Upload
malcolm-lamb -
Category
Documents
-
view
219 -
download
3
Transcript of Chapter 16. Thermodynamics tells us if a reaction can occur while Kinetics tells us how quickly the...
Kinetics
KineticsChapter 16Kinetics Thermodynamics tells us if a reaction can occur while Kinetics tells us how quickly the reaction occurs
some reactions that are thermodynamically feasible are kinetically so slow they are hardly noticeable2
23KineticsKinetics is the study of rates of chemical reactions and the mechanisms by which they occur.
Reaction rateincrease in concentration of a product per unit time ordecrease in concentration of a reactant per unit time
Reaction mechanismseries of steps by which a reaction occursthe rate of rxn is always determined by the slowest step in its mechanism (rate determining step)
Ways to measure rxn speed: for gases - pressure for concentration color intensity
3
Reaction rates ~ rates at which reactants disappear or products appear
the change in concentration of a reactant or product. = change in concentration / (number of moles x change in time) Note: reactants are negative and products are positive
Reaction Rates4Ex. 1) 4A + B 2C + 3D
Reaction MechanismReaction Mechanism: a series of elementary steps that must agree with the experimentally determined rate law and the sum of the elementary steps must give the overall balanced equation for the rxn.
the individual steps in a reaction mechanism are called elementary steps. Assume they happen as written, collision wiseYou dont want to have intermediates in a rate expression. An intermediate is a chemical that is neither a reactant nor a product. It is formed and consumed in the course of the rxn. NO + Br2 NOBr2NOBr2 + NO 2NOBrNOBr2 is the intermediate
Elementary StepMolecularity Rate LawA productsUnimolecular rxn involving one moleculeRate = k[A]A + A products (2A products)Bimolecular rxn involving collision of two speciesRate = k[A]2A + B products BimolecularRate = k[A][B]A + A + B prod. (2A + B prod.)Termolecular rxn involving collision of 3 speciesRate = k[A]2[B]A + B + C prod.TermolecularRate = k[A][B][C]Molecularity the number of species that must collide to produce the reaction indicated by that step
Examples of Elementary Steps8The Rate of ReactionRate Law Expressions must be determined experimentallycannot be determined from balanced equationsmost chemical reactions are not one-step reactions
Rate law expressions are also called:rate lawsrate equationsrate expressions8By definition, the rate law for the reaction: 2 A + 3 B 5 C is: Rate = k Ax By
k = specific rate constant at a given temp.
x and y are called orders of rxn. x + y += overall order of rxn
Note that rate laws are written using only the reactants. The orders of reaction,x & y, are from data, NOT the coefficients of the equation. In fact, the only time the orders equal the coefficients is when the reactions are elementary rxns.
10Orders of a reactionOrders of a reaction are expressed in terms of either:each reactant or overall reaction
For example:
1011
1112CatalystsA catalyst is a substance that increases the rate of the rxn, but it remains unchanged when the rxn is completeCatalysts change reaction rates by providing a faster alternative pathway where a different, lesser amount of activation energy is needed.
1213Homogeneous catalysts exist in same phase as the reactants.
Heterogeneous catalysts exist in different phases than the reactants. Often are solids
Examples of catalysts include:
13Ex. 2) Step 1: NO + Cl2 + Pt NOCl2Pt FastStep 2: NOCl2Pt + NO 2NOCl + Pt SlowA. What is the overall reaction?B. What is the catalyst?
C. What is the intermediate? (an intermediate is a substance that is produced and then used up during a reaction)
D. What is the molecularity in step 1?
E. What is the rate determining step? (For a mechanism to be consistent, the steps must add up to the overall rxn, and the rate determining step must give the derived rate law.)
F. If this rxn is 1st order [NOCl2Pt] and 1st order [NO] because they are the reactants in the rate determining step, what is the rate law for this expression?
G. What is the overall reaction order?
17Rate of a simple one-step reaction is directly proportional to the concentration of the reacting substance[X] is concentration of X in molarity or moles/L
For a simple expression like R = k[A]:doubling the initial concentration of A doubles the initial rate of reactionhalving the initial concentration of A halves the initial rate of reaction1718The Rate of ReactionLook at the following reaction and its experimentally determined rate-law expression
because it is a second order rate-law expressiondoubling the [A] increases the rate of reaction by a factor of 422 = 4 halving the [A] decreases the rate of reaction by a factor of 4(1/2)2 = 1/4
18Ex. 3) For the reaction 3A + B C, the rate law is R = k [A]2
A. What order is the reaction?
B. How will these change the rate if you triple the concentration of A
C. Double the concentration of B
The orders of the reactions are derives by the following mathematical equation: using conc. from experiments
ratios of the rates = ratios of Axratios of By
Or
rate1 = k[A]x1[B]y1[C]z1 rate2 = k[A]x2[B]y2[C]z2
(well solve some examples later)What do orders really mean?
Reactant How [M] Effect on rate Order
A Doubledno change 0A Doubleddoubles 1
A Doubledquadruples 2 A Doubledeight times 3
Order [M]order =rate 0 20 = 1
1 21 = 2
2 22 = 4
3 23 = 8
Or in other words: If concentration doubles (2) and the rate stays the same: ask yourself ~ 2to what power = 1 so 2X = 1( the power is 0 so the reaction is 0 order in A)
Conc. doubles & rate doubles: 2X = 2 ( x = 1 or first order)
Conc. doubles & rate quadruples: 2x = 4 ( x = 2 or second order)
Collision Theory of Reaction Rates
Three basic events must occur for a reaction to occur the atoms, molecules or ions must:
1. collide
2. collide with enough energy to break and form bonds
3. collide with the proper orientation
Factors that affect rate1. Temperature if the temp. increases, then the rxn tends to speed up. Generally a raise of 10oC doubles the rate.
2. Concentration the more concentrated the reactants are the quicker the rxn.
3. Nature of Reactions some chemicals react very quickly, some are very slowNa + H2O Na+ + OH- + H2 very fastAl + H2O Al3+ + OH- + H2 very slow
4. Catalyst chemical that speeds up a rxn w/o being used up.
26Temperature: The Arrhenius EquationFor reactions that have an Ea50 kJ/mol, (Ea = activation energy ) the rate approximately doubles for a 100C rise in temperature, near room temperature.
2 ICl(g) + H2(g) I2(g) + 2 HCl(g)
The rate-law expression is known to be R=k[ICl][H2]
2627Concentrations of ReactantsSimplified representation of effect of different numbers of molecules in the same volume.Increase in concentration, increases the chance of a rxn occurring. A(g) + B(g) ProductsA B
A BA B BA BA BA BA B4 different possible A-B collisions6 different possible A-B collisions9 different possible A-B collisions2728Nature of ReactantsBroad category that includes the different reacting properties of substances.
For example:~ Sodium reacts with water explosively at room temperature to liberate hydrogen and form sodium hydroxide.
~ Calcium reacts with water only slowly at room temperature to liberate hydrogen and form calcium hydroxide.
28~ The reaction of magnesium with water at room temperature is so slow that that the evolution of hydrogen is not perceptible to the human eye.
~ However, Mg reacts with steam rapidly to liberate H2 and form magnesium oxide.
Differences due to nature of the reactants
30Reaction Mechanisms & the Rate-Law ExpressionUse experimental rate-law to postulate a mechanism.
The slowest step in a reaction mechanism is the rate determining step.
Note: Experimentally determined reaction orders indicate the number of molecules involved in:
the slow step onlyorthe slow step and the equilibrium steps preceding the slow step.
3031Reaction is known to be first order in H2O2 , first order in I- , and second order overall.
Mechanism is thought to be:
Consider the iodide ion catalyzed decomposition of hydrogen peroxide to water and oxygen.3132Important notes:
one hydrogen peroxide molecule and one iodide ion are involved in the rate determining step
the iodide ion catalyst is consumed in step 1 and produced in step 2 in equal amounts
hypoiodite ion has been detected in reaction mixture as a short-lived reaction intermediate 3233Reaction Mechanisms & the Rate-Law ExpressionOzone, O3, reacts very rapidly with nitrogen oxide, NO, in a reaction that is first order in each reactant and second order overall.
A possible mechanism is:3334Reaction Mechanisms & the Rate-Law ExpressionThis mechanism is inconsistent with the rate-law expression b/c the slowest step doesnt match the rate-law found in the lab:
3435Concentration vs. Time: The Integrated Rate EquationIntegrated rate equation relates time and concentration for a chemical or nuclear reactionWhy do we need this? Problem with normal rate law is that you cant figure out the rate at some later time.
First Order Reactions1st order in reactant A & 1st order overallFor example:a A productscommon for many chemical reactions and all simple radioactive decays
3536First Order Integrated Rate Lawwhere:[A]0= mol/L of A at time t=0.[A] = mol/L of A at time t.k = specific rate constantt = time elapsed since beginning of reactiona = stoichiometric coefficient of A in balanced overall equation
36To find units for k, the specific rate constant, look at overall order
Copy Table 16-2, summary of orders of reactions, and Table16-3, graphing for orders of reactions, into your notes before starting on Kinetics Example ProblemsOverall OrderUnits0[M] time-1 1time -1 2[M]-1 time-1 3[M]-2 time-1 38Bonus QuestionThe Chernobyl nuclear reactor accident occurred in 1986. At the time that the reactor exploded some 2.4 MCi of radioactive 137Cs into the atmosphere. The half-life of 137Cs is 30.1 years. In what year will the amount of 137Cs released from Chernobyl finally decrease to 100 Ci? A Ci is a unit of radioactivity called the Curie, MCi = MegaCurie38