Building Science Champions Describe how covalent bonds form. Identify the properties of molecular...
-
Upload
franklin-watson -
Category
Documents
-
view
217 -
download
0
Transcript of Building Science Champions Describe how covalent bonds form. Identify the properties of molecular...

Building Science Champions
Covalent Bonds

Describe how covalent bonds form.Identify the properties of molecular
compounds.Distinguish between polar and nonpolar
bonds, and between polar and nonpolar compounds.
Objectives

Covalent bondDouble bondMolecular compoundPolarNonpolar
Key Terms

Co = share valent = valence electrons = shared electrons
A covalent bond is a chemical bond formed when two atoms SHARE electrons.
Form between 2 or more nonmetals. Oxygen, carbon, nitrogen and the halogen
family frequently bond with other nonmetals by sharing electrons.
Covalent Bond

F + F F F
Each fluorine has 7 valence electronsThey share 1 pairWhen you count the valence electrons, you count the
shared pair each time.By sharing they have 8 valence electrons. In a covalent bond, both atoms attract the 2
shared electrons at the same time.
Fluorine
Shared pair of electrons

There are 3 types of pairing for covalent bonds:
Single Bond – shares one pair of electronsDouble Bond – shares two pairs of electronsTriple Bond – shares three pairs of electrons
F F O O N N
Number of Bonds
Single Bond
Double Bond
Triple Bond

Properties Molecular Compounds
consist of molecules having covalently bonded atoms.
Weaker bonds than ionic bonds
Poor conductors of electricity
No charged particlesUsed for insulation: ie.
rubber and plastic
Compound Formula Melting Pt (0C)
Boiling Pt (0C)
Water H2O 0 100
Methane CH4 -182 -164
Carbon Dioxide
CO2 - -78.6*
Ammonia
NH3 -77.7 -33.6
Rubbing Alcohol
C3H7OH -89.5 82.4
Sugar C12H22O
11
185-186
decomposes
Molecular Compounds Melting and Boiling Points of
Molecular Compounds
*Carbon Dioxide changes from a solid to a gas

PolarA covalent bond
where electrons are not shared equally.
H O H
Pull is toward oxygen = Polar
A covalent bond where electrons are shared equally.
F Cl
Pull in opposite directions = nonpolar
Sharing of Electrons
Nonpolar
+ - +

Some atoms pull more strongly on the shared electrons than other atoms do. As a result, the electrons move closer to one atom, causing the atoms to have a slight electrical charge.
One electron is being pulled closer to one atom causing the atom losing an electron to become slightly positive and the one receiving an electron is slightly negative.
Unequal Sharing of Electrons

Valence electrons are shared between covalent bonds
Electrons are rapidly moving from one atom to another giving an atom a slight positive or negative charge
Review

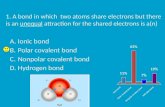
Draw dot diagrams for the following compounds and write if they are Ionic or Covalent. IF THEY ARE COVALENT WRITE IF THEY ARE polar or nonpolar and what number bond they have. N2 MgO CO2O2 NaCl CaSRbI NH3 CH4Al2O3
Higher difficulty
Your Turn

ReferencesAnderson, M. et all (2012) Physical Science.
McGraw-Hill: ColumbusFrank, D.V et al (2001). Physical Science.
Prentice Hall: New Jersey