Lipids Highly diverse structures Unifying property Hydrophobic: little to no affinity to water...
-
Upload
layne-tims -
Category
Documents
-
view
216 -
download
0
Transcript of Lipids Highly diverse structures Unifying property Hydrophobic: little to no affinity to water...
Lipids
• Highly diverse structures
• Unifying property• Hydrophobic: little to no affinity to water• Contains hydrocarbons, which form nonpolar covalent bonds
• Do not form polymers
• Biologically important lipids containing molecules:– Fats– Phospholipids– Steroids
Fats
• Structure– Composed of two different molecules bonded by ester linkage
• fatty acid & glycerol
– - Fatty acid
Hydrocarbon chain with a carboxyl group at one end
– Glycerol• Three-carbon alcohol
– Each carbon attached to hydroxyl group
Try to Draw
• Hydrophobic• H2O molecules form hydrogen bonds with each other and
EXCLUDE fatty acid chains-->Fats separate from H2O (like oil
& vinegar)
Properties of Fats
Fatty acids variable chain length (often 16-18 carbons)
Saturated fat- refers to saturated fatty acid chains
- carbons contains maximum number of hydrogens
- results in 100% single bonds (no double bonds)
- more linear, packs tightly to form solid
Tend to be from animals
Unsaturated fat-contains unsaturated fatty acids-carbon-carbon double bonds (>1)-irregular hydrocarbon conformation-poor packing-forms liquids (oils) at room temperature
Tend to be from plants and fish
LE 5-12b
Unsaturated fat and fatty acid.
Oleic acid
cis double bondcauses bending
Olive oilOther liquid fats (unsaturated)?
Phospholipids• Structure
– two fatty acids bonded to glycerol through ester linkage
– Phosphate bonded to third hydroxyl group of glycerol
• Fatty acids= Hydrophobic tail• Phosphate and other groups= hydrophilic head
Draw schematic
LE 5-13
Structural formula Space-filling model Phospholipid symbol
Hydrophilichead
Hydrophobictails
Fatty acids
Choline
Phosphate
Glycerol
Hyd
rop
ho
bic
tai
lsH
ydr o
ph
ilic
hea
d
If many phospholipids were mixed in H2Ointo what structures would they self-assemble?
1. Micelle (draw)- Detergents
2. Bilayer (draw)-Cell membranes
Steroids
• Structure- Hydrophobic molecules made of 4 fused hydrocarbon rings
• Examples and Diverse Functions– Cholesterol
• Component of animal cell membranes
• Building block for steroid sex hormones such as– Estrogen, testosterone, progesterone
• High levels--> contribute to heart disease
- Boundary between intracellular compartments, living cells, and abiotic environment
– Selectively permeable– Some molecules cross membranes more readily than others
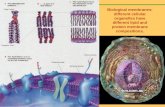
Cellular membrane-overall functions
Predominant constituent: phospholipids• Amphipathic molecules: hydrophobic AND hydrophilic
Membrane Structure
Dispersed protein components
Membrane organization and properties described by:
Fluid Mosaic Model
Singer and Nicolson 1972
LE 7-3
Hydrophilic regionof protein
Hydrophobic region of protein
Phospholipidbilayer
Mosaic: Proteins dispersed among phospholipids in membrane:
• Freeze-fracture studies of the plasma membrane
• Frozen membrane split along the middle of the phospholipid bilayer using a knife
• Imaged by EM
Supports mosaic part of model
LE 7-4
Knife
Cytoplasmic layerExtracellular layer
Cytoplasmic layer
Plasmamembrane
Extracellular layer
Proteins
The Fluidity of Membranes
• Phospholipids move laterally within the bilayer
• Some membrane proteins also drift laterally
• Rarely does a phospholipid flip-flop transversely across the membrane
LE 7-5a
Lateral movement(~107 times per second)
Flip-flop(~ once per month)
Movement of phospholipids
• Cool temp: membranes switch from fluid to more solid state
• Solidification depends on type of lipid
• What property of lipids would favor liquid versus solid state?
Effects of Temperature on membranes
LE 7-5b
ViscousFluid
Unsaturated hydrocarbontails with kinks
Membrane fluidity
Saturated hydro-carbon tails
Degree of saturation of fatty acid tails
• Tends to moderate effects of temp. on membrane state
• At warm temperatures (such as 37°C), restrains movement of phospholipids
• At cool temperatures, maintains fluidity by preventing tight packing
Steroid cholesterolalso component of membranes
Can drift within the bilayer– Proteins much larger than lipids--> move more
slowly
• Cell fusion studies support fluidity of membrane proteins
Movement of membrane proteins
Membrane Proteins and Their Functions
• Proteins determine most of the membrane’s specific functions
• Peripheral membrane proteins –not embedded–attached to extracellular or cytoplasmic
surface
• Integral membrane proteins– penetrate the hydrophobic core of bilayer– often span the membrane