The Nuclear Fuel
-
Upload
david-thomson -
Category
Documents
-
view
7 -
download
0
description
Transcript of The Nuclear Fuel

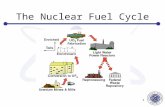
The Nuclear Fuel ‘Cycle’
Figure 1 - The Nuclear Fuel Cycle
Many processing steps are required to produce nuclear fuel and then deal with it after burn-up in a
power reactor.
Figure 1 shows the ‘cycle’ for producing 1000MW of
electricity to the grid, with the amounts of material
involved at each stage. There’s some rather serious
mistakes in the numbers – I’ll leave you to find them for
yourself.
Uranium Mining The primary nuclear fuel in use today is Uranium either
natural (0.71% ) or slightly enriched. Uranium is a
natural metal and is found in commercial quantities in
many locations in the World. Even in the best ores the
Uranium concentration is very low (0.1 to 0.2% by weight)
and so large quantities of rock need to be moved.
The standard method of extracting Uranium has been
conventional mining, either open-cast or underground. Table 1 - World Uranium Production

Once the ore has been mined the Uranium is removed in a mill, often located near the mine due to
the quantity of ore involved. In the mill, the ore is crushed and the Uranium removed, usually by
sulphuric acid. The liquid extract also contains other materials (like molybdenum, vanadium,
selenium, iron, lead and arsenic). The Uranium is precipitated from the extract and is then filtered
and dried to produce yellow cake ( .
Uranium mining produces a large residue (the tailings) which is more than 500-1000 times the mass
of the Uranium recovered. The tailings contain radium and radon and are a radioactive hazard. They
also contain a variety of heavy metals, so pose a serious chemical toxicity problem. In the past, the
record of the Uranium mining industry in dealing with these tailings has been shocking. In the US,
where many of the first mines were in Navaho reservations, the tailings were simply left large piles
contaminating the air and ground water. Similar practises were followed at many other mines and
have left a legacy that is still to be cleaned up. Modern mines claim to manage their tailings
according to strict environmental criteria, but the history means that the industry has many vocal
critics.
In- situ leaching is a more modern technique that conventional mining and is now used for all
Uranium production in the USA. The technique involves pumping acidic water into the ore-bearing
rock. The water dissolves the Uranium Oxide, which is recovered and precipitated at the surface. The
method avoids mine tailings completely, since almost all of the original rock is left in place. The
leaching water will contain contaminants, which need to be deal with properly. The disadvantages of
in-situ leaching are that it is only suitable for some sites – the rock containing the ore needs to be
porous – and there is also the possibility of ground water contamination, so great care needs to be
taken in performing a site assessment.
Table 2 shows the estimates of
recoverable Uranium (when the
price is $130/kg) present in the
World. The price for Uranium has
fluctuated widely in recent years:
in 2001 it hit $15/kg, in 2007 it
reached a near record price of
$248/kg. Current prices (July
2009) are around $97/kg. The
reason for the price variations
are the current political
intentions for a revived World
nuclear energy programme and
the relative scarcity of
economically recoverable
Uranium. Mining in 2007 only met 67% of the requirement for power production – the remainder of
the Uranium was obtained from stocks and other sources. The current World demand for Uranium
for power production is about 60,000 tonnes/year. In 2005, nuclear power provided 2.1% of the
World’s power (electricity, heating, transport, etc) and 15% of its electricity. Renewables provided
about the same fraction. If nuclear energy was to be expanded to provide 50% of the World’s power
(assuming no increase in total World energy consumption), then the World’s recoverable Uranium
Table 2 - known, recoverable, Uranium reserves 2007

Mf, xf
Mp, xp
Mt, xt
Separative
Work (∆U)
would be used up within 4 years. Obviously, as Uranium became even scarcer, the price would
increase making more marginal reserves economically recoverable, but there is a finite and close
limit on how long an expanded nuclear energy programme can be fuelled with Uranium dug out of
the ground.
Breeder reactors, which convert fertile materials like and into fissile materials, are a
necessary component in any long-term World nuclear energy strategy. The use of breeder reactors
also necessitates nuclear fuel reprocessing plants.
Uranium Enrichment Most nuclear reactors require the Uranium in their fuel to be enriched to achieve criticality. There
are two methods in common use to do this: gas centrifuges; gaseous diffusion.
Separative Work and Separation Factors
Nuclear engineers have invented new terms to describe the amount of separation
required/produced in enrichment plants. These terms are
commonly used.
The first is separative work and is a measure of the amount of
effort required to achieve a particular separation. It is used in
some countries (e.g. USA) as the purchasable unit of
enrichment. Separative work is expressed in mass units (1kg of
separative work is called a Separative Work Unit (SWU)), but
does correlate with the amount of energy required for
enrichment .
Consider the enrichment plant shown in figure 2. Mf
kilogrammes of feed are supplied to the plant with a
mole/mass fraction of of xf. The separator splits this into two streams: a product stream of
mass MP and fraction of xp; and a ‘tails’ stream of mass Mt and fraction of xt.
The following equations apply:
And the separative work is defined as:
( ) ( (
where V(x) is the value function and is
( ( (
)
Figure 2 - An enrichment

Figure 3 uses the equations above to look at the production of 1kg of 4% enriched Uranium. You can
see that by allowing the amount of in the tails to increase the separative work can be reduced,
but the amount of natural Uranium supplied to the process must increase. Since separative work
correlates with energy (and hence cost), there is an optimum residue fraction which depends on the
price of Uranium and the cost of energy.
The other term in common use in Uranium enrichment is the separation factor, α:
⁄
where = mole/weight fraction of in product
= mole/weight fraction of in tails
For isotope separation, the atomic masses are so similar than weight and mole fractions are almost
identical. For low levels of enrichment, the expression for α can be simplified:
The separation factor is a measure of the separation in a single-stage of an enrichment plant. The
actual amount of separation is dependent on the mass balance for the system, i.e.
needs to be satisfied (I’m making the low enrichment assumption in this equation). Figure 4 shows
how the production concentration from a separator (with a separation factor of 1.2) which is fed
with natural Uranium Hexafluoride ( = 0.007) at 10 kg.h-1, changes with the product flowrate. The
two limiting points are where the product flow equals the inlet flow (product concentration equals
inlet concentration) and where the product flowrate approaches zero - at this point the following
component balance applies:

Figure 4 - effect of tails residue on separation
Figure 3 - the relationship between product concentration and flowrate

This relationship is useful, as it allows the minimum number of stages required to achieve a
particular separation to be estimated.
The ratio between the product stream and the feed to an enrichment separator is called the cut.
Low values of cut give a lower number of stages to the required enrichment, but require more
separators per stage to provide the required throughput (because the product flowrate per
separator is reduced.) The optimum value of cut is usually around 0.5 – the feed rate is split evenly
between the product and the tails from a separator stage.
(
)
(
(
)
(
Example
A separator for Uranium enrichment has a separation factor equal to 1.1, what is the minimum
number of stages required to enrich natural uranium to 4%?
The maximum increase in a single stage is
so, for ‘n’ stages, the enrichment is given by,
So, the number of stages for a given enrichment can be estimated from,
Hence, for this example,

We know that for a single-stage, if we operate with a cut of 0.5,
Also, we know that when enrichments are small,
Hence,
⁄
Now, we know that the ratios
and
will be near one, with the product ratio slightly above and
tails ratio slightly below. Let’s compare how far each is away from 1:
(
)
( )
(
)
(
( ))
(
)
(
)
(
( )
Example
Using the system in the example above, how many stages are required if the cut have a value of
0.5?
This gives the enrichment per stage, so the enrichment for ‘n’ stages is
So, the number of stages required is,

This gives us the interesting result that, for a 0.5 cut, the two ratios are equally spaced on each side
of 1.
Separator Cascades
All of the currently used separators require multiple stages to achieve the enrichments required by
civil and military nuclear programmes. Also multiple stages are required to strip the tailings down to
the required level of . The stages are
arranged in a cascade. The first stage is the
stage where the fresh feed is added, the
stages above this where the ‘product’ streams
are directed are called the enrichment section
because is become enriched as the gas
moves through the stages, the stages below
the feed stage where the tailings are directed
are called the stripping section because
is being stripped from the gas at each
consecutive stage.
Now, each separation stage produces two
streams: the ‘product’ stream and the ‘tails’
stream. Consider the situation where we go for a 0.5 cut – the feed to the stage is split evenly
between the product and tails stream, and let’s look at two stages in the enrichment section of the
cascade (see figure 5).
We know that,
(
)
And also that,
(
Now, if α is close to one, then the tails from the second separator stage will have almost the same
weight fraction as the feed to the first separator stage. It makes sense, therefore, to recycle the
tails from a separator stage in the enrichment section into the feed of the separator stage
immediately prior in the chain. Figure 6 shows a possible arrangement for an enrichment section
with a cut of 0.5. Note how the ‘fresh’ flowrate (total flow minus recycle) changes as we move back
into the enrichment chain. The fresh flow into a 0.5 cut enrichment chain is equal to (
where ‘n’ is the number of stages and Mp is the final flow of product required. Note also that, at
each stage, the difference between the fresh feed to the stage and the recycle FROM the stage is
always equal to Mp – the overall mass balance needs to be maintained.
M,x
f
0.5M,xp
1
0.5M,xt
1
0.25M,xp2
0.25M,xt2
Figure 5 - two simple stages in an enrichment section

A similar argument can be used for the stripping section of a cascade – figure 7 shows and example
with three separation
stages. Note that, once
again, the total ‘fresh’
feed into the cascade is
(n+1) times the feed
leaving the cascade as
‘tails’ and that, at each
stage, the difference
between the ‘fresh’ feed
and the recycle from the
stage is each to the
amount being removed at
the end of the cascade.
Now we have the enrichment and stripping sections of the cascade, the last bit we need is the feed
stage. Figure 8 shows the feed stage of a separator cascade with connections to the enrichment and
stripping sections. Although we’ve used a fixed cut (of 0.5) on the stripping and enrichment stages,
we can’t do this on the feed stage and
maintain an overall component
balance. To keep an overall component
balance,
also, the total mass balance needs to
be maintained,
Mt
Mt
2Mt
3Mt
2Mt
3Mt
4Mt
Mp
Mp
2Mp
2Mp
3Mp
3Mp
4Mp
4Mp
5Mp
Figure 9 - a stripping section with recycle
Figure 7 - a stripping section with recycle
Fresh feed,
Mf, xf
Enrichment section
ne Mp (1+ne)Mp
Stripping section
ns Mt (1+ns)Mt
Figure 6 - an enrichment section with recycle
Figure 8 - The feed stage of a cascade

so,
( )
( )
( )
The cut on the first stage will be further modified by the number of enrichment and stripping stages
(which change the amount of recycle in the system). The cut to the product is
(
In calculating the number of stages required for enrichment or stripping above, I’ve assumed a
constant cut of 0.5 over all the stages. By changing the cut in a particular stage, the amount of
enrichment/stripping that takes place in that stage will be altered. This may make a slight difference
to the number of enrichment and stripping stages required. Since only the feed stage is affected by
this the effect shouldn’t be too large, but it is worth checking.
From the mass balances you can see that the mass flowrates passing through the separator stages
are substantially different. Rather than using different sizes of separators to deal with the different
flowrates, all significant enrichment facilities use multiple separator units, of equal capacity, for
every separation stage. The units within a stage are operated in parallel, with the feed being split
evenly amongst them. Using the same sized separator units greatly eases maintenance and reduces
the overall capital cost.
Let be the feed capacity (e.g. kg.h-1) of the separator that is being used in a cascade.
For the enrichment section, the number of separators required is given by,
∑
∑
(
(
and for the stripping section, the number is,
∑
(
Finally, the number of the feed section is,
So, the total number of separator units required is,
(
(

(
)
(
( )
( )
(
( )
(
)
(
)
( )
(
)
( )
( )
(
(
(
Example
Natural Uranium is to be enriched to 3%, and the tails from the enrichment should contain 0.3%
. Separator units with a separation factor of 1.1 and a feed capacity of 10 kg.h-1 (of Uranium
Hexafluoride) are available. How many separation stages and how many units in each stage are
required to process a feed of 20kg.h-1 of natural uranium hexafluoride?
Assuming a 0.5 cut is used, the number of enrichment stages required is
and the number of stripping stages is
If we round up the number of stages, and treat the feed stage as the first stage in both the
enrichment and stripping sections, then the total number of stages required is 49 divided into 1
feed stage, 17 stripping stages and 31 enrichment stages.
The product flow from the system is given by
and the split to the product on the feedstage is
This is quite a long way from our 0.5 split assumption for the feed stage, in favour of enrichment
(the less product drawn the higher the enrichment), so we might need one less enrichment stage
and one more stripping stage.
The number of units required per stage were calculated in Excel and the results summarised in
figure 10

Figure 10 - Solution to example
Gas centrifuges
The Uranium to be enriched is
converted to Uranium Hexafluoride
( ), which is gaseous at the
temperatures and pressures used. The
gas is then passed through a series of
gas centrifuges, where the slight mass
difference between the isotopes
allows separation.
Separation factors range from 1.02 to
1.4 depending on the centrifuge
design. A typical centrifuge is capable
of providing 4 – 40 SWUs per year. The
energy costs/SWU are around 50-
300kWh.
Gas centrifuges are the most common
modern technology used for Uranium enrichment because the energy costs per SWU are low.
Gaseous Diffusion
Once again, the uranium to be enriched is converted to gaseous uranium hexafluoride. In a gaseous
diffusion plant, the separation units are diffusion barriers (often made of sintered powders to
provide pores). The slight difference in isotope weight provides produces slightly different diffusion
coefficients, resulting in a slightly enriched gas on the other side of the barrier.
0
10
20
30
40
50
60
70
1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49
Nu
mb
er
of
un
its
Stage Number
Feed stage
Enrichment stages
Stripping stages
Figure 11 - a cascade of gas centrifuges in an enrichment plant (Wikipedia)

Gaseous diffusion separators have low separation factor of 1.003-1.0043. This means that a very
large number of stages are required to produce useful enrichments. The energy costs are also very
high at around 2400kWh/SWU. The individual separator units can have higher capacity than
centrifuges and they may be more reliable (fewer moving parts, but possible fouling of diffusion
barrier.)
Gaseous diffusion was the original technology used for enrichment and only France and the USA still
operate diffusion plants. The USA is now constructing a gas centrifuge plant and it’s likely that no
new diffusion plants will be constructed.
(
( )
( )
( ) ( (
( ( (
)
Example
An enrichment plant is required that will produce 1,000kg/annum of Uranium enriched to 5%
from a natural Uranium feedstock. The tailings from the plant should have a mass fraction of
0.3%. Compare the number of stages required and energy costs for a Gaseous Diffusion plant and
a Gas Centrifuge plant.
Assume a separation factor of 1.2 for gas centrifuge, 1.004 for gaseous diffusion and a 0.5 cut.
Gas Centrifuge Gaseous diffusion
Enrichment Stages 23 986
Stripping Stages 9 424
Total (taking into account the feed stage)
31 1,409
The overall component balance for the plant is
so
and so the mass flow of tailings is 10,750kg/annum
The separative work required
where
Energy cost for gas centrifuge is therefore 3.61x105 to 2.17x106 kWh and for the diffusion plant is
around 1.73x107 kWh.

Fuel Fabrication Once the Uranium has been suitably enriched it then needs to
be made into fuel rods to power a nuclear reactor. The earliest
rods were Uranium metal rods encased in a cladding. The
problem with these rods is that they were limited in the
temperature that they could operate at and this seriously
reduced the thermal efficiency of the nuclear reactor. All
modern reactors now use pellets of sintered Uranium Dioxide
as nuclear fuel.
Once the pellets are produced they are packed into cladding tubes usually made
of either stainless steel (AGR) or zircaloy (PWR). The cladding holds the pellets
in place and also limits the leakage of fission products into the reactor primary
coolant. The individual rods are then assembled into fuel bundles (or
assemblies) appropriate to the reactor they are destined for. The whole process
is described nicely in the Westinghouse brochure available on the LearnOnline
site.
Some countries (including the UK) produce mixed-oxide (MOX) fuel which is
made up of a mixture of enriched Uranium and Plutonium oxide. The primary reason for doing this is
to attempt to reduce Plutonium stockpiles at reprocessing plants.
At the reactor Once the fuel bundle has been assembled it’s delivered to the nuclear reactor site. Some reactors,
e.g. AGRs and CANDU were designed to be capable of refuelling while still operating, but problems
with this have led to the practice being discontinued. A nuclear reactor being refuelled is therefore
usually shutdown. The refuelling process is just mechanical handling (with the complication of
intense radioactivity). The old fuel bundles are removed and the new ones dropped (I mean
lowered) into place.
Once in the reactor the fuel bundle generates heat over its lifetime. The consumption of nuclear fuel
is usually expressed as its burnup and is measured in GW-days/Metric tonne of Uranium metal.
Figure 12 - Uranium dioxide fuel pellet
Example
A nuclear reactor core contains fuel rods with a total of 120 tonnes of Uranium Dioxide has been
generating an average thermal output of 3800MW for 100 days. What is the fuel burnup?
Assume that the power generation is equally distributed throughout the reactor.
We first need to convert the mass or Uranium Dioxide to an equivalent mass of Uranium metal:
GW-days/MTU
Figure 13 - A PWR fuel assembly

Most current reactors refuel when the burnup is 40-60 GW-days/MTU. The higher the anticipated
burnup the higher the amount of enrichment that is required. The main limitation on burnup is not
that the has all been consumed, but is instead due to neutron absorbing fission products
building up to a level where the reactivity of the reactor becomes too low. Another factor
contributing to extended burnup is the consumption of which is produce by neutron
absorption in . At low burnups Plutonium is, on balance, produced as the rate of production is
greater than the rate of fission. As the burnup and hence the amount of Plutonium increases, then
the balance changes and Plutonium starts to be consumed.
The neutron distribution and hence power generation across a reactor core is not usually uniform.
This means that some fuel assemblies will be exposed to higher burnups (usually those nearest the
centre) than others. During a refuelling shutdown it is common to replace only one quarter to one
third of the fuel assemblies and move the others into alternative positions in the reactor.
After a spent fuel assembly has been removed from the reactor core it is too radioactive (‘hot’) to be
safely transported. Instead the assembly is stored for a period (Magnox fuel needs a minimum of 90
days) while the short-lived, and so highly radioactive, fission products decay away. The fuel is stored
in large ponds filled with water, which provides radiation shielding and also the cooling which is
required (nuclear decay produces quite large quantities of heat.) In reality the storage period in
reactor ponds is much longer than the minimum required. In the USA, where no civil reprocessing
takes place, reactor ponds have been used as medium term storage and many are nearly full.
Long term storage of spent fuel In countries where civil reprocessing does not take place (e.g. the USA) and where the spent fuel
isn’t sent away for reprocessing, then the spent fuel assemblies become nuclear waste that have to
be dealt with in some way. The assemblies will remain dangerously radioactive for thousands of
years and finding a method of permanent, safe, storage is not trivial. Currently no long-term solution
for the storage of complete fuel assemblies exists, although various options are being investigated.
Example
A fuel assembly in a PWR has been exposed to a burnup of 50 GWd/MTU. If the original fuel was
enriched to 6% what is the percentage of in the fuel now (ignore plutonium
contributions to the burnup)?
The fission of 1 atom of Uranium produces approximately 200MeV (3.2x10-14kJ) of energy. The
mass of used to produce the burnup is therefore (per tonne of Uranium metal)
The original mass was, roughly, 60kg, so the mass of in the spent fuel is 6.6kg and the
percentage is approximately

Reprocessing In countries where nuclear fuel reprocessing takes place (the UK, France, Russia and soon Japan) the
cooled spent fuel is transported from the reactor ponds to the nuclear reprocessing facility. Once it
arrives at the facility it is once again stored in ponds for a time both to allow shorter lived
radioactivity to decay and also to ease production scheduling.
The object of nuclear fuel reprocessing is to remove the Uranium and Plutonium from the other
elements in the spent fuel, conserving useful material and also vastly reducing the bulk of the
radioactive waste. Figure 14 shows the breakdown on typical PWR spent fuel, with cladding
removed. The amount of the various constituents will change slightly with burnup. By the time fuel is
reprocessed the short-lived fission products have decayed away to negligible levels, leaving mainly
those with fairly long half-lives.
Although a number of process routes have been developed for reprocessing spent fuel, all of the
facilities currently operating use the PUREX process. The description below relates to the UK’s
THORP plant, but it generally describes what happens elsewhere too.
The first stage is to get the spent fuel out of the
cladding and into solution. This is done by moving the
fuel assemble into a shearing machine. The shear blade
cuts right through the assembly and chops the fuel
rods into 1-2cm long pieces. The chopped up fuel rods
then fall into a mesh basket inside a dissolver. When
the basket is loaded, 7N Nitric acid is added and this
dissolves the fuel. The acid containing most of the
dissolved contents of the spent fuel is sucked out (no
pumps are used in reprocessing plant) and the basket is washed and removed (all that is left is the
stainless steel or zircaloy cladding, although it will be slightly contaminated and needs to be treated
as nuclear waste). Finally the dissolver is washed out and readied for the next batch. The
development of the washing systems for the dissolver in THORP is my main contribution to Britian’s
nuclear future!
Figure 14 - What's in spent fuel (Dr. Terry Todd, Idaho National Laboratory 2008)
Figure 15 - Shearing a fuel assembly (Dr. Terry Todd)

The next step of the process involves multiple stages of solvent extraction and precipitation and is
shown in figure 16.
The solvent used in the PUREX process is Tri-Butyl Phosphate dissolved in Odourless Kerosene
(TBP/OK). This can hold both Uranium and Plutonium Nitrate in solution, provided they have the
correct valency (U(VI) and Pu(IV)). The valency of the Uranium and Plutonium is manipulated
throughout the process by chemical reactions to move them in and out of the solvent stream. In the
Highly Active (HA) cycle the Nitric acid from the dissolver is contacted with TBP/OK. The first step
HA/HS moves the Uranium and Plutonium into the solvent, leaving the bulk of the fission products in
the aqueous acid stream. In the 1BX/1BS step, chemicals are added which adjust the valency of the
Plutonium causing it to migrate from the solvent to the aqueous stream. The final step involves the
contacting of the Uranium laden solvent with a dilute acid stream – the low acidity causes the
Uranium to move out of the solvent and back into the aqueous phase.
The first steps in the Uranium (UP) and Plutonium (PP) purification cycles involve contacting the
solvent containing the uranium and plutonium with an aqueous scrub to reduce the fission product
impurities to very low levels. The final step in both cycles moves the Uranium or Plutonium back into
the aqueous phase and recovers the solvent for re-use.
The primary benefit of reprocessing at the moment is that the radioactive fission products are
removed from the much higher quantity of low radioactive Uranium, reducing the problems of
waste management. Now that our nuclear weapons programme has been scaled down, the
recovery of Plutonium is a bit of a nuisance, but if a breeder reactor programme is to be started,
reprocessing will be a vital element.
Figure 16 - the THORP PUREX extraction process