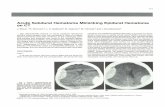
Subdural Hematoma
description
Transcript of Subdural Hematoma
Subdural HematomaLast Updated: May 11, 2006
Background: An acute subdural hematoma (SDH) is a rapidly clotting blood collection below the inner layer of the dura but external to the brain and arachnoid membrane. Two further stages, subacute and chronic, may develop with untreated acute SDH. Each type has distinctly different clinical, pathological, and imaging characteristics.
Generally, the subacute phase begins 3-7 days after acute injury. (Surgical literature favors 3 days; radiological literature favors 7).
The chronic phase begins about 2-3 weeks after acute injury.
Pathophysiology: Typically, low-pressure venous bleeding of bridging veins (between the cortex and venous sinuses) dissects the arachnoid away from the dura and layers out along the cerebral convexity. Cerebral injury results from direct pressure, increased intracranial pressure (ICP), or associated intraparenchymal insults.
In the subacute phase, the clotted blood liquifies. Occasionally, in the prone patient, the cellular elements layer can appear on CT imaging as a hematocritlike effect.
In the chronic phase, cellular elements have disintegrated, and a collection of serous fluid remains in the subdural space. In rare cases, calcification develops.
Frequency:
In the US: Frequency is related directly to the incidence of blunt head trauma. An SDH is the most common type of intracranial mass lesion, occurring in about a third of those with severe head injuries (Glasgow Coma Scale [GCS] score less than 9).
Mortality/Morbidity: Acute SDH is associated with high mortality and morbidity rates.
Simple SDH accounts for about half of all cases and implies that no parenchymal injury is present. Simple SDH is associated with a mortality rate of about 20%.
Complicated SDH accounts for the remaining cases and implies that parenchymal injury (eg, contusion or laceration of a cerebral hemisphere) is present. Complicated SDH is associated with a mortality rate of about 50%.
Age: The majority of SDHs are associated with age factors related to the risk of blunt head trauma. Certain age factors are related to more unusual variants of this disease.
SDH is more common in people older than 60 years. The elderly are predisposed to cerebral atrophy because they have less resilient bridging veins. Moreover, these veins can be damaged more easily in the elderly.
Since the adhesions existing in the subdural space are absent at birth and develop with aging, bilateral SDHs are more common in infants.
Interhemispheric SDHs are often associated with child abuse.
History:
Suspect acute subdural hematoma (SDH) whenever the patient has experienced a mechanism of moderately severe to severe blunt head trauma.
Patients generally lose consciousness, but this is not an absolute.
Chronic SDH is more difficult to anticipate, and about half of such cases offer no history of head trauma. Patients often present with progressive symptoms such as unexplained headache, personality changes, signs of increased ICP, or hemiparesis/plegia.
Any degree or type of coagulopathy should heighten suspicion of SDH.
Hemophiliacs can develop SDH with a seemingly trivial head trauma. An aggressive approach to diagnosis and immediate correction of the factor deficiency to 100% activity is paramount.
Alcoholics are prone to thrombocytopenia, prolonged bleeding times, and blunt head trauma.
o Maintain a high level of suspicion in this population.
o Promptly obtain a CT scan of the head when the degree of trauma is severe, focal neurologic signs are noted, or intoxication does not resolve as anticipated.
o In alcoholics, more than any other cohort, acute or chronic SDHs can be due to the deadly combination of repetitive trauma and alcohol-associated coagulopathies.
Patients on anticoagulants can develop SDH with minimal trauma and warrant a lowered threshold for obtaining a head CT scan.
Physical:
Physical examination of patients with head trauma should emphasize assessment of neurologic status using the GCS. Search for any focal neurologic deficits or signs of increased ICP.
Signs of external trauma alert the physician to the expected location of coup or contrecoup injuries on CT scan.
Any abnormality of mental status that cannot be explained completely by alcohol intoxication or the presence of another mind-altering substance should increase suspicion of SDH in the patient with blunt head trauma. Obtain an urgent CT scan.
GCS score less than 15 after blunt head trauma, in a patient with no intoxicating substance use (or impaired mental status baseline), warrants consideration of an urgent CT scan.
Presence of a focal neurologic sign following blunt head trauma is ominous and requires an emergent explanation.
Other Problems to be Considered:
DementiaBrain tumorSubdural empyema
Intracerebral pneumocephalus following a head injury: case report]
No Shinkei Geka. 2003; 31(4):425-9 (ISSN: 0301-2603)
Takinami K ; Hasegawa T ; Miyamori T ; Matumoto T ; Arakawa Y ; Sugino MDepartment of Neurosurgery, Toyama Municipal Hospital, 2-1 Imaizumihokubu-machi, Toyama-city, Toyama 939-8511, Japan.
We report a case of intracerebral pneumocephalus following a head injury. This condition is relatively rare, and only 14 cases, including the present case, have been reported to data. A 40-year-old man fell from a 3rd floor window on June 29, 1999. The patient was admitted to the hospital. Plain skull X-ray films revealed a left basal skull fracture, and CT scan revealed a small contusion at the left frontobasal lobe. The patient was treated conservatively. On July 16, he underwent an MRI, and a small contusion was revealed at the left frontobasal lobe. In addition, the brain appeared to have herniated into the ethmoidal sinus. On July 22, the patient underwent a CT scan, and a intracerebral pneumocephalus was revealed in the left frontal lobe. On August 2, an MRI was performed, and intracerebral pneumocephalus in the left frontal lobe and herniation of the brain into the ethmoidal sinus were noted. In addition, intracerebral pneumocephalus had increased. The patient was admitted to our hospital. Clinotherapy was performed, but intracerebral pneumocephalus increased. On August 9, the patient underwent surgery to repair the skull base. During surgery, it was noted that the left frontal contusion had adhered to the edge of the lacerated dura around the bone defect of the ethmoidal sinus. Following surgery, no
recurrence of pneumocephalus was noted. We conclude that once intracerebral air volume increases, early surgical repair should be carried out for intracerebral pneumocephalus. Meticulous MRI investigations of the lesion causing the intracerebral pneumocephalus should be conducted to select an appropriate operative procedure.
PreMedline Identifier: 12704824
Blow-out Fracture SIGNS AND SYMPTOMSPatients manifesting orbital blow-out fracture always present with a history of blunt ocular trauma. Blow-out fracture is usually caused by a large, low-velocity object, such as a fist or a ball. Sports-related injuries are common. If the trauma is recent, the patient may present with symptoms of pain, local tenderness and double vision. Complaints of an intense pressure feeling or swelling of the eye associated with nose blowing may also be reported.
Critical signs of recent blow-out fracture include:
edema and ecchymosis of the lid tissues restriction of ocular motility, especially with vertical movements orbital crepitus (subcutaneous emphysema)
hypoesthesia of the ipsilateral cheek, due to entrapment of the infraorbital nerve.
There may also be an associated nosebleed due to communication between the orbit and maxillary sinus. Orbital edema initially surrounds and displaces the globe, in some cases causing the eye to appear proptotic. However, as the swelling subsides, the eye is likely to drop down and back, becoming enophthalmic. Associated traumatic uveitis and/or hyphema may be noted as well.
In some cases, patients suffering orbital blow-out fracture initially ignore treatment, and may present long after the initial inflammatory manifestations of trauma have subsided. In these patients, evaluation reveals only relative enophthalmos and motility restriction, usually in upgaze, and possible infraorbital hypoesthesia.
PATHOPHYSIOLOGYBlow-out fracture may result in cases of abrupt trauma to the eye by any object >5cm in diameter. Because the orbital rim is very strong, the forces of blunt trauma are reflected back, compressing the eye and creating a tremendous increase in pressure within the orbit.
Since the larger bones which comprise the orbit contain sinuses, the orbital walls are at great risk for fracture; should the trauma be of sufficient force, these walls can literally "blow out." The medial wall (ethmoid bone) is occasionally affected. But most commonly, the orbital floor (the superior aspect of the maxillary bone) sustains the damage. In cases of floor fractures, the eye may partially drop down into the maxillary sinus, causing enophthalmos and entrapment of the inferior rectus or inferior oblique muscle.
This entrapment leads to a tethering effect, resulting in a limited downgaze ability and, more notably, an inability toward upgaze in the affected eye. While this situation can be surgically corrected in the early stages, prolonged entrapment leads to fibrosis of the muscle(s) and permanent motility impairment. Associated medial wall fractures may induce damage to the medial rectus muscle and/or the lacrimal apparatus, but this is uncommon.
In most cases, these fractures result in orbital emphysema, creating a direct communication between the ethmoid sinus and the orbit. This produces the feeling of pressure within the orbit when the patient attempts to blow his/her nose. The greatest risk to consider with medial wall fractures is orbital cellulitis, secondary to sinus infection, should pathogenic organisms within the sinus invade the post-tarsal eyelid.
MANAGEMENTAll cases of blunt ocular trauma with resultant crepitus or motility restriction warrant orbital imaging studies. Computed tomography (CT scan) is the procedure of choice. CT is better at imaging the bony structures of the orbit than either plain skull films (X-ray) or MRI. Obtain both axial and coronal scans.
Should you discover a floor fracture with associated herniation of the orbital contents, consider surgical intervention. Generally, surgery is only for patients with recent trauma who manifest significant diplopia in primary gaze or downgaze, or in cases of cosmetically unacceptable enophthalmos. Most oculoplastic specialists will wait 10 to 14 days following the
trauma to allow for resolution of the associated edema and hemorrhage. The treatment consists of surgical resection of the periosteum and repair of the fracture, utilizing a bone graft or synthetic material such as silicon or Teflon.
Long-standing entrapment of the extraocular muscles leads to fibrosis and irreversible scarring; intervention to improve motility after four weeks is typically unsuccessful. Initiate prophylactic antibiotic therapy immediately in the event of associated medial wall fractures with orbital emphysema, or if there is any suspicion of ethmoid damage. A broad spectrum oral preparation such as cephalexin or erythromycin (250-500mg QID) may be used for 10 to 14 days. Surgical repair of the medial wall is unnecessary in uncomplicated ethmoid fractures, since the condition resolves spontaneously in three to four weeks.
CLINICAL PEARLS
Cases of orbital blow-out fracture do not constitute an emergency, however, accurate diagnosis and management of the associated ocular manifestations is paramount.
One test that is very helpful in differentiating muscle entrapment in orbital fracture from other muscle or nerve complications is the forced duction test. Entrapped muscles will resist forced movements with a forceps or even a cotton-tipped applicator. Again, this test should ideally be performed after resolution of the orbital swelling.
Check for crepitus by palpating the bony rim of the orbit or lid-small bubbles of air will "pop" when compressed. For long-standing fractures in which the patient experiences diplopia in downgaze, but is not a surgical
candidate, consider incorporating unilateral prism correction into the patient's reading glasses.
UNCAL HERNIATION - A condition that occurs when the brain is under abnormally increased pressure. The increased intracranial pressure forces the brain downward inside the skull. This results in typical neurologic manifestations (coma, paralysis, and a unilateral dilated pupil). May occur secondary to head injury, primary or metastatic brain tumor, bacterial meningitis, and brain abscess. Brain herniations may involve different portions of the brain such as the cerebellum (cerebellar herniation), uncus (uncal herniation), and transtentorial herniation of the cerebrum.
Artificial Tears
Pronunciation (ar ti FISH il tears)
U.S. Brand Names Akwa Tears® [OTC]; AquaSite® [OTC]; Bion® Tears [OTC]; HypoTears [OTC]; HypoTears PF [OTC]; Isopto® Tears [OTC]; Liquifilm® Tears [OTC]; Moisture® Eyes [OTC]; Moisture® Eyes PM [OTC]; Murine® Tears [OTC]; Murocel® [OTC]; Nature's Tears® [OTC]; Nu-Tears® [OTC]; Nu-Tears® II [OTC]; OcuCoat® [OTC]; OcuCoat® PF [OTC]; Puralube® Tears [OTC]; Refresh® [OTC]; Refresh® Plus [OTC]; Refresh® Tears [OTC]; Teargen® [OTC]; Teargen® II [OTC]; Tearisol® [OTC]; Tears Again® [OTC]; Tears Naturale® [OTC]; Tears Naturale® Free [OTC]; Tears Naturale® II [OTC]; Tears Plus® [OTC]; Tears Renewed® [OTC]; Ultra Tears® [OTC]; Viva-Drops® [OTC]
Synonyms Hydroxyethylcellulose; Polyvinyl Alcohol
Generic Available Yes
Canadian Brand Names Teardrops®
Pharmacologic Category Ophthalmic Agent, Miscellaneous
Use Ophthalmic lubricant; for relief of dry eyes and eye irritation
Pregnancy Risk Factor C
Warnings/Precautions Individual product formulations may include (as an active ingredient) benzalkonium chloride, polyvinyl alcohol, carboxymethylcellulose, hydroxymethylcellulose, hydroxypropyl methylcellulose, propylene glycol, dextran 70, or polysorbate 80. Refer to product labeling for specific ingredients.
Adverse Reactions 1% to 10%: Ocular: May cause mild stinging or temporary blurred vision
Dosage Use as needed to relieve symptoms, 1-2 drops into eye(s) 3-4 times/day
Patient Information Wash hands thoroughly; if irritation or condition worsens or persists for longer than 3 days, discontinue use; do not touch tip of container to any surface; close immediately after use
Nursing Implications Not for use with soft contact lenses
Dental Health: Effects on Dental Treatment No effects or complications reported
Dental Health: Vasoconstrictor/Local Anesthetic Precautions No information available to require special precautions
Mental Health: Effects on Mental Status None reported
Mental Health: Effects on Psychiatric Treatment None reported
Dosage Forms Solution, ophthalmic: 15 mL and 30 mL dropper bottles
International Brand Names Teardrops® (CA)
Viscotears® Liquid GelCarbomer
Please read this carefully before you start to use your eye drops. This leaflet provides a summary of the information available on the drops.
If you have any questions or are not sure about anything ask your doctor or pharmacist.
What you should know about Viscotears Liquid Gel
The name of your eye drops is Viscotears Liquid Gel. The active ingredient is carbomer 2.0 mg/g (0.2 %).
It also contains cetrimide, sorbitol, sodium hydroxide and water.
The tube contains 10ml of liquid gel. This is one of a group of medicines called Artificial Tears.
Product Licence Holder:Novartis Pharmaceuticals UK LtdFrimley Business ParkFrimley, CamberleySurrey, GU16 7SRUK
Manufacturer: Dr. Mann-PharmaBerlin, Germany.
Viscotears Liquid Gel is a substitute for tear fluid for the management of dry eyes and for unstable tear film.
Before you use the eye drops
Consult your doctor before you use the drops if you are pregnant or breast feeding or if you are allergic to any of the ingredients of Viscotears Liquid Gel.
Consult your doctor before you use the drops if you are suffering from any other eye disorder or if you are using any other medicines in your eyes.
Do not drive a motor vehicle or operate machinery if your vision becomes blurred immediately after you have inserted the drops. Wait until your vision clears.
Dosage
The usual dosage is as follows:
Adults: Put one drop in each eye 3 or 4 times each day.
Children: Only to be used as directed by a doctor.
Your doctor may advise you to use this medicine in a different way, so follow your doctor's directions about when and how to use your medicine and read the label. Your pharmacist may also help if you are not sure.
How to use your eye drops
1. Wash your hands 2. If you wear contact lenses, remove them before using the drops and do not
replace for at least 30 minutes. 3. Hold the tube vertically (see figure1) and squeeze it gently, until a small
drop forms. 4. Tilt your head backwards (see figure 2). 5. Rest one hand on your cheek and gently pull down your lower eye lid (see
figure 3). 6. Look upwards. 7. Insert one drop. 8. Blink a few times to spread the liquid gel evenly over your eye. 9. Wipe away any excess gel from around the eyelids. 10. Repeat for your other eye.
NOTE: Do not touch your eye with the tip of the dropper.
Follow these instructions carefully. Consult your pharmacist if there is anything you do not understand.
Side effects which may occur
Eye drops sometimes cause unwanted effects in some people.
These effects are often mild and may wear off after a few day's treatment. If they are severe or last for more than a few days, tell your doctor. Also if your eye drops upset you in any way, tell your doctor.
Effects which may occur are:
A mild burning sensation in the eye which quickly passes. Blurring of vision after insertion of the drop, which will gradually clear.
Do not drive or operate machinery if your vision is blurred.
Storage
Store Viscotears Liquid Gel well away from children.
Do not store above 25° C.
Do not use the eye drops after the expiry date shown on the tube (EXP).
Do not use the eye drops for longer than one month after opening.
This leaflet does not contain the complete information about Viscotears Liquid Gel. If you have any questions or are not sure of anything ask your doctor or pharmacist who have access to additional information.
This leaflet only applies to Viscotears Liquid Gel and not to any other artificial tears product.
Date of Preparation: March 2002