Structure of atom ppt
-
Upload
lekshmisg91 -
Category
Science
-
view
453 -
download
9
description
Transcript of Structure of atom ppt


PREPARED BY
LEKSHMI.S.G
PHYSICAL SCIENCE
REG NO: 13357003
BNVCTE,THIRUVALLAM


All matter is made up of atoms.Atoms of an element are identical.Each element has different atoms.Atoms can engage in a chemical reactions.Atoms can neither be created nor be destroyed.Atoms are indivisible.
Atomic Theory
John Dalton
(1776-1884)

atom has a positively charged central part (nucleus)
Electrons are distributed around nucleus
Mass of an atom is concentrated at nucleus.
Compared with total volume of an atom, the volume of nucleus is meager
Rutherford’s Atomic Model
Ernest Rutherford (1871-1937)

Solar system Rutherford‘s atom model
Planetary model of atom

Bohr's Model of the Atom
Niels Bohr
1913

Bohr's Model of the Atom
electrons orbit the nucleus like planets orbit the sun

Bohr's Model of the Atom
electrons fills the orbits closest to the nucleus
e.g. fluorine:#P = 9
#e- = 9
#N = 10
9P10N

each orbit can hold a specific maximum number of electrons
Shell maximum no: of electrons
1 - K
2 - L
3 - M
4 - N
Bohr's Model of the Atom
2
8
18
32

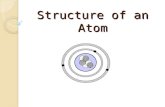
Bohr’s Model
Nucleus
Electron
Orbit
Energy Levels

What is the structure of an atom?
• Nucleus - center of the atomHome of Protons and NeutronsHas a positive charge
• ProtonHas a relative mass of 1Has a positive (+) chargeDetermines the atomic numberFound inside the nucleus

What is the structure of an atom?
• NeutronHas no (0) chargeHas a relative mass of 1Found inside the nucleus

What is the structure of an atom?
• ElectronHas a negative (-) chargeFound outside the nucleus
• Rutherford atom model - electrons are around the nucleus
• Bohr model – electrons are in specific energy levels called shells

How are p, n, e related?
• No: protons = No: electrons
• No: protons = atomic number
• No: protons + No:neutrons = mass number
• No: neutrons=mass no: - atomic no:

ATOMI C STRUCTUREATOMI C STRUCTURE
the number of protons in an atom
the number of protons and neutrons in an atom
HeHe22
44Atomic mass
Atomic number
number of electrons = number of protons