Planar cell polarity gene expression correlates with tumor cell ...
Nonhematopoietic Tumor Cell Lines Express Stem Cell Factor and
Transcript of Nonhematopoietic Tumor Cell Lines Express Stem Cell Factor and

Nonhematopoietic Tumor Cell Lines Express Stem Cell Factor and Display c-kit Receptors
By Anne M. Turner, Krisztina M. Zsebo, Frank Martin, Fredrick W. Jacobsen, Larry G. Bennett, and Virginia C. Broudy
Human stem cell factor (SCF) acts in the presence of other growth factors t o stimulate the growth of primitive hemato- poietic progenitor cells. These effects are performed by activation of the SCF receptor, e-kit. Because of the potential use of SCF in patients undergoing chemotherapy and bone marrow transplantation, the effect of SCF on nonhematopoi- etic tumors requires investigation. To determine whether human tumor cell lines display c-kit receptors, we performed binding experiments with 1251-SCF on a breast carcinoma cell line (Du4475), a gastric carcinoma cell line (KAT0 Ill), a melanoma cell line (HlT144), as well as two small cell lung carcinoma cell lines (H69 and H128). The biologic effect of SCF on tumor cell lines was assessed by its ability t o stimulate tritiated thymidine uptake and to enhance colony growth in methylcellulose. The breast carcinoma cell line, Du4475, as well as two small cell lung carcinoma cell lines, H69 and H128, exhibit high-affinity c-kit receptors with approximate binding affinities of 40, 100, and 90 pmol/L, respectively. The number of high-affinity receptors per cell ranged from 700 t o 9,500. The gastric carcinoma cell line, as well as the melanoma cell line, showed trace binding of 1251-SCF. In the presence of SCF alone, or in combination with
TEM CELL FACTOR (SCF), a glycoprotein growth S factor, is the ligand for the tyrosine kinase receptor encoded by c-kit,’ In the presence of other growth factors, SCF stimulates the proliferation of hematopoietic progeni- tor cells, including burst-forming unit-erythroid (BFU-E) and colony-forming unit granulocyte-macrophage (CFU- GM), as well as more primitive progenitor^.^-^ Treatment of baboons with SCF results in increased numbers of circulat- ing leukocytes, reticulocytes, and lymphocytes, and an increase in marrow cellularity.5 The proliferative effect of SCF on early marrow progenitors makes it an attractive growth factor for clinical use. SCF may be useful to accelerate marrow recovery after high-dose chemotherapy or in the setting of bone marrow transplantation.
Human solid tumor cell lines have been shown to display receptors for hematopoietic growth The H69 and H128 lung carcinoma cell lines express high-affinity granu- locyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte-CSF (G-CSF) receptors?,1° The addition of GM-CSF or G-CSF resulted in increased growth of the H69 or H128 cells in vitro, indicating that the GM-CSF and
From the University of Washington, Seattle, WA; the Amgen Center,
Submitted October 3, 1991; accepted March 19,1992. Supported by National Institutes of Health Grant No. DIU1232 and
an American Cancer Society Faculty Research Award (KC.B.). Address reprint requests to Anne M. Tumer, MD, Division of
Hematology, RM-10, Department of Medicine, University of Washing- ton, Seattle, WA 981 95.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore by hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
Thousand Oaks, CA; and Amgen Diagnostics, Boulder, CO.
0 1992 by The American Society of Hematology. 0006-4971 /92/8002-0013$3.00/0
granulocyte-macrophage colony-stimulating factor or inter- leukin-3, there was less than a 17% increase in the colony growth of Du4475, H69, or H128 cell lines. Postulating that the lack of growth response could be secondary to endoge- nous SCF production by the tumor cell lines, we used an RNAse protection assay to determine whether the tumor cell lines contain SCF messenger RNA (mRNA). In addition, we tested tumor cell line supernatants for the presence of secreted SCF protein by enzyme immunoassay, and analyzed the tumor cell lines for membrane-bound SCF by indirect immunofluorescence. Our results show that the Du4475, H69, and H128 cell lines, as well as a melanoma cell line (HTT144), have multiple copies of SCF mRNA. Soluble SCF protein was detected in the cell supernatants in the Du4475 and H69 cell lines and SCF was found on the surface of all four cell lines. These data show that some human solid tumor cell lines display high-affinity c-kit receptors and produce SCF, which can be detected on the cell surface. These results suggest the possibility that autocrine production of SCF by e-kit receptor- bearing tumor cells may enhance cell growth in tumor cell lines. o 1992 by The American Society of Hematology.
G-CSF receptors on these tumor cells are functional and can transmit a proliferative signal. Because of the potential clinical use of SCF in patients with nonhematopoietic solid tumors, we investigated whether human tumor cell lines display receptors for SCF, and whether SCF can stimulate the growth of these cell lines. We identified high-affinity c-kit receptors on the Du4475 breast carcinoma cell line and on the H69 and H128 small cell lung carcinoma cell lines. These receptors are similar in molecular weight, binding affinity, and number of receptors per cell to c-kit receptors found in normal human marrow hematopoietic cells.ll However, exogenous SCF alone, or SCF in the presence of interleukin-3 (IL-3) or GM-CSF, did not induce significant proliferation of the tumor cell lines.
Because of the presence of high-affinity c-kit receptors on these tumor cell lines and the lack of a proliferative response to SCF, we investigated whether the tumor cells could produce endogenous SCF. We detected SCF-specific transcripts in the tumor cell lines using an RNAse protec- tion assay. The tumor cell lines displayed membrane-bound SCF and produced detectable quantities of soluble SCF. Our results show that several human tumor cell lines coexpress SCF and the c-kit receptor, and raise the question of constitutive activation of the c-kit receptors in these tumor cell lines.
MATERIALS AND METHODS
Purified recombinant human SCF (1-164) expressed in Escherichia coli was provided by Amgen (Thousand Oaks, CA). Recombinant human GM-CSF was expressed in BHK cells and was provided by Dr Ken Kaushansky (University of Washington). Recombinant human IL-3 was obtained from Genetics Institute (Cambridge, MA). Rat growth factor (media conditioned by Con-A-activated BALB/C spleen cells) was provided by Amgen.’*
The human small cell lung carcinoma cell lines H128 and H69, the human breast carcinoma cell line Du4475, the human
Cytokines.
Cells.
374 Blood, Vol80, No 2 (July 15), 1992: pp 374-381

SCF AND C-KTT IN TUMOR CELL LINES 375
gastric carcinoma cell line KATO 111, and the human glioblastoma cell line A172 were obtained from American Tissue Type Collec- tion (Rockville, MD). The human H'IT 144 melanoma cell line was obtained from Dr Alex Fefer (University of Washington). The human erythroleukemia cell line OCIMl13 was provided by Dr Thalia Papayannopoulou (University of Washington). The murine mast cell line MC/9 was obtained from Amgen. H128, H69, Du4475, and KATO I11 cells were maintained in RPMI 1640 (M.A. Bioproducts, Walkersville, MD) supplemented with 15% heat- inactivated fetal calf serum (FCS; Hyclone Laboratories, Logan, UT). The A172 cell line was maintained in Dulbecco's modified Eagle's medium (DMEM, GIBCO, Grand Island, NY) supple- mented with 10% FCS. H'IT 144 cells were maintained in McCoy's 5A media (GIBCO) supplemented with 10% FCS. OCIMl cells were maintained in Iscove's modified Dulbecco's medium (IMDM; GIBCO) supplemented with 10% FCS. MC/9 cells were main- tained in RPMI 1640 supplemented with 20% rat growth factor and 15% FCS.
Fresh melanoma cells were obtained from a skin biopsy from a patient with disseminated melanoma. This sample was provided by Dr Alex Fefer under a protocol approved by the Human Subjects Committee at the University of Washington. Human marrow cells were aspirated from the posterior iliac crest of consenting healthy adult volunteers, with the approval of the Human Subjects Commit- tee. Marrow mononuclear cells were isolated by Ficoll-hypaque density centrifugation.
Iodination of SCF. SCF was iodinated using the chloramine T method.14 The radiologic specific activity was determined by self-displacement analysis,15 and was generally 2,000 to 2,500 Ci/mmol. Iodination of SCF did not alter its receptor binding affinity.16 To assess the biologic activity of iodinated SCF, human marrow mononuclear cells (final concentration, 105/mL) were cultured in IMDM supplemented with 1.4% methylcellulose, 30% FCS, 1% bovine serum albumin (BSA, Reheis, Phoenix, AZ), penicillin/streptomycin, and erythropoietin (Epo) 1 U/mL17 with or without unlabeled SCF (5 ng/mL) or lzsI-SCF (5 ng/mL) as previously described.18 BFU-E colonies were counted after 14 days of incubation at 37°C. Unlabeled SCF and 1251-SCF increased the number of BFU-E colonies by 2.8- and 2.7-fold, respectively, in comparison to the number of BFU-E colonies found in the presence of Epo alone. These results indicate that SCF iodinated by the chloramine T method retains its biologic activity.
The cell lines were screened for the presence of c-kit receptors by incubating the cells with 0.3 nmol/L l=I-SCF with or without a 100-fold excess of unlabeled SCF in binding buffer consisting of RPMI 1640 supplemented with 1% BSA (Sigma, St Louis, MO), 50 mmol/L HEPES (pH 7.4), 0.1% sodium azide, and 10 pg/mL cytochalasin B (Sigma). After 1 hour of incubation at 37T, the cells were sedimented through phthalate oil (dibutyl phtha1ate:dinonyl phthalate 3.5:2.0) to separate cell- bound from free lZI-SCF,l6 and 1251 was measured in both the cell pellets and the supernatants using a Packard 5330 gamma counter (Packard Instrument Co, Downers Grove, IL). Receptor number and binding affinity were determined by incubating cells (generally 2 x lo5 cells/100 FL final volume) with varying amounts of lZI-SCF (10 pmol/L to 5 nmol/L) with or without a 100-fold excess of unlabeled SCF in binding buffer for 4 hours at 15°C. Under these conditions, saturation binding is achieved and internalization of lXI-SCF is minimized ( < 20%).19 All measurements were per- formed in duplicate or triplicate, and the data were analyzed using the ligand program.20
Colony formation by the tumor cell lines was quantitated by plating 5 x lo3 cells in 1 mL of 1.4% methylcellulose (Methocel; Dow Chemical Co, Midland, MI) in RPMI 1640 supplemented with 3% to 5% FCS and 1% BSA. Growth factors
Receptor quantitarion.
Colony assays.
were added to each plate before adding the methylcellulose mixture. SCF was used at a final concentration of 1, 10, or 100 ng/mL. GM-CSF and IL-3 were used at final concentrations of 10 ng/mL and 1 U/mL, respectively. Colonies ( > 50 cells/colony) were counted after 14 days of incubation at 37°C in 5% COz. All colony assays were performed in triplicate. For some colony assays, the cells were cultured under serum-deprived conditions in which FCS was replaced by deionized BSA (2 x low5 mol/L) BSA- adsorbed cholesterol (2 x low4 mol/L), iron-saturated human transferrin (low5), insulin (1.7 x mol/L), nucleosides (10 p,g/mL each), sodium pyruvate mol/L), and glutamine (2 X mol/L) as previously described.21 Chemicals used for serum-deprived cultures were obtained from Sigma.
The cells were washed twice in phosphate-buffered saline (PBS) and maintained in RPMI 1640 without FCS for 16 hours before tritiated thymidine uptake experiments. The cells were then plated at a final concentration of 1 x 104/mL in 96-well flat-bottomed microtiter plates (Costar, Cambridge, MA). SCF was added at a final concentration varying from 1.0 ng/mL to 1,OOO ng/mL. GM-CSF (10 ng/mL) or IL-3 (1 U/mL) were added alone or with SCF, and the cells were incubated for 72 hours at 37°C. Tritiated thymidine (Amersham, Arlington Heights, IL) was added at a concentration of 10 p,Ci/mL, and the incubation was continued for 4 hours at 37°C. The cells were then harvested onto glass microfiber filters (Whatman, Maidstone, UK) and counted in scintillation fluid (Ecolume; ICN, Iwine, CA) in a Packard Tri Carb 4530 scintillation counter. Tritiated thymidine uptake assays were performed in duplicate or triplicate. The ability of rat growth factor to stimulate tritiated thymidine uptake by the MC/9 cell line was used as a positive control. In additional experiments, tritiated thymidine uptake in the presence of 20% FCS was used as a positive control.
The SR-1 monoclonal antibody (MoAb) recog- nizes the human c-kit receptor and blocks binding of SCF to its receptor on hematopoietic cells.22 We investigated whether SR-1 also would also recognize the c-kit receptor on solid tumor cells. The tumor cells (2 x 105) were incubated with 0.3 nmol/L '=I-SCF in 100 p,L binding buffer with or without SR-1 ascites (final dilution, 1:1,000) for 4 hours at 15°C. Cell-associated lZI-SCF was quantitated as described above. To evaluate if the SR-1 blocked low-affinity binding sites in H69 and Du4475 cell lines, the cells were incubated in 2.0 nmol/L 1251-SCF with or without SR-1 ascites (final dilution, 1:500). To determine whether SR-1 would alter growth of the solid tumor cell lines, we performed colony assays. The cells were plated in methylcellulose as described above with or without SR-1 ascites (final dilution, 1:1,000 or 1:10,000).
Tumor cell line cells (lo7 cells) were incubated with 151-SCF (2.5 nmol/L) with or without a 200-fold excess of unlabeled SCF in 1 mL of binding buffer for 4 hours at 15°C to saturate cell surface SCF receptors. Crosslinking was performed using disuccinimidyl suberate (DSS; Pierce, Rockford, IL). DSS was added at a final concentration of 100 nmol/L and the incubation was continued for 30 minutes. The cells were washed twice in PBS at 4°C and then lysed in 1% Triton X-100 (Boehringer Mannheim, Indianapolis, IN), 2 mmol/L phenylmethylsulfonyl fluoride (PMSF Boehringer Mannheim), 10 p,mol/L pepstatin (Boehringer Mannheim), 10 mmol/L leupeptin (Boehringer Mannheim), 1 mg/mL aprotinin (Boehringer Mannheim), 2 mmol/L EDTA, and 1 mmol/L disopropyl fluorophosphate (Sigma) as described.1° The crosslinked receptor-ligand complexes were immunoprecipitated using rabbit polyclonal antihuman SCF anti- sera (Amgen) followed by Staph A (Pansorbin; Calbiochem, La Jolla, CA). The pellets were dissolved in 2X sodium dodecyl sulfate (SDS) sample buffer (0.125 mmol/L Tris HCI [pH 6.81, 4% SDS, 0.008% bromphenol blue, 20% glycerol) and subjected to electro-
Tritiated thymidine uptake experiments.
SR-1 antibody.
Afinity crosslinking.

376 TURNER ET AL
phoresis on a 7% polyacrylamide gel.u The gel was dried on a gel dryer (Hoefer SE1160 Gel Dryer, San Francisco, CA) and exposed to autoradiography film at -70°C for 7 days.
RNAse protection assay for SCF and c-kit messenger RNA ("A). RNA probes for SCF and c-kit proto-oncogene mRNA were produced by runoff transcription of cDNA regions cloned in pGEM vectors (Promega Biotech, Madison, WI) in the presence of 35S-UTP using materials and standard protocols provided by the manufacturer. Sense strand mRNA standards for quantitation of the assays were produced in a similar fashion using only tracer quantities of 35S-UTP.
Cells (4 x lo6) were washed in PBS and lysed by incubation at room temperature for 20 minutes in 1 mL 10 mmol/L Tris HCl(8.0 pH), 1 mmol/L EDTA, 20 mmol/L DTT, 100 kg/mL proteinase K, and 0.2% SDS. Samples (30 kL) of cell lysate or RNA standard were added to 70 pL of hybridization mix (50 kg/mL partially hydrolysed poly A, 20 mmol/L DTT, 4.8 mol/L sodium phosphate, pH 7.2, and 285,000 cpm/mL probe) and incubated at 84°C for 2 hours. After RNAse digestion for 20 minutes at 37°C using RNAse A (0.03 mg/mL) and RNAse T1 (5,000 U/mL), the sample was loaded onto a Sephacryl S200 Superfine gel filtration column (Sigma), and the void volume fraction containing hybridized, protected probe was counted. The quantity of gene-specific RNA in each sample was then calculated from a standard curve.
Culture media conditioned by the tumor cell lines was tested for the presence of soluble SCF by enzyme immunoassay (EIA) (Langley et al, in preparation). As a control, media that had not been used to culture the tumor cells was tested in parallel. The media were concen- trated (up to fourfold) in a centricon 10 microconcentrator (Amicon, Beverley, MA) before performing the EIA. Briefly, the EIA was performed using microtiter wells coated with affinity- purified rabbit anti-SCF polyclonal antibodies. Recombinant hu- man SCF (1-248) produced in Chinese hamster ovary (CHO) cells was used to generate a standard curve. Fifty microliters of SCF or media conditioned by the tumor cell lines was added to the wells. After an overnight incubation at room temperature, the signal- detecting antibody (the 7H6 anti-SCF MoAb covalently coupled to horseradish peroxidase) was added, and the incubation was contin- ued for 2 hours. The reaction was developed with peroxidase substrate, and absorbance at 450 nmol/L was read using a Molecular Devices Vmax Kinetic Microplate Reader (Molecular Device Corp, Menlo Park, CA). The sensitivity of the EIA for the detection of SCF is 0.04 ng/mL.
The cells (4 x 105) were washed twice in PBS with 0.2% BSA and then incubated with the 7H6 murine MoAb specific for human SCF (Amgen; final dilution of 7H6 ascites, 1:500) for 30 minutes at 4°C. Cells were labeled in parallel with an isotype-matched irrelevant murine MoAb (CllD8, anti-gp7O; obtained from Dr Janis Abko- witz, University of Washington). The cells were washed and incubated with phycoerythrin-conjugated goat antimouse IgG (Tago, Burlingame, CA) for 30 minutes at 4"C, washed, fixed in 1% paraformaldehyde, and analyzed in a Coulter Epics Elite cell sorter (Coulter, Hialeah, FL).
Enzyme immunoassay for soluble SCF protein.
Indirect immunofluorescence analysis for cell surjace SCF.
RESULTS
A number of human solid tumor cell lines were screened for SCF receptors by their ability to bind lz5I-SCF. The H128, H69, and Du4475 cell lines were found to display SCF receptors. In three experiments, the KAT0 I11 gastric carcinoma cell line and the H'IT 144 melanoma cell line showed trace binding of 1251-SCF; fresh human melanoma cells obtained
Human tumor cell lines display SCF receptors.
7
2 - .. n Z 3 0 m 1 -
2 4 6 8 10
'251-SCF BOUND (pM)
Fig 1. Scatchard analysis of '%SCF binding to Du4475 cells. Du4475 cells (2 x 10s) were incubated with 'zsI-SCF (10 pmol/L to 5 nmol/L) with or without a 100-fold excess of unlabeled SCF for 4 hours at 15°C. The data rbpresent the average of duplicate determina- tions and the results were analyzed using the ligand program. A second experiment gives similar results.
from a biopsy also bound IZI-SCF (one experiment, data not shown). Quantitative equilibrium binding experiments were performed with the Du4475, H69, and H128 cell lines. All three of these cell lines exhibited high-affinity SCF receptors; the Du4475 and H69 cell lines also displayed low-affinity receptors (Figs 1, 2, and 3). The Du4475 cell line displayed a high-affinity SCF receptor (kd 37 pmol/L) with - 700 receptors per cell and a low-affinity site (kd 1.8 nmol/L) with -4,000 receptors per cell (Fig 1). H69 small cell carcinoma cells expressed a high-affinity site (kd 37 pmol/L) with -1,200 receptors per cell, as well as the low-affinity site (kd 10 nmol/L) with - 17,000 receptors per
l2 1
j l
2 4 6 8 10 12 14
1 2 5 ~ - ~ ~ ~ BOUND (PM)
Fig 2. Scatchard analysis of '=I-SCF binding to H69 cells. The cells (2 x 10s) were incubated with '2sI-SCF as in Fig 1 and the data were analyzed by the ligand program. Two additional experiments gave similar results.

SCF AND C-KK IN TUMOR CELL LINES
X
m 9 16 . - 14 - w u 12 - U a . 10 -
h 181 \
377
Table 2. Effect of SCF on Colony Growth of Human Solid Tumor Cell Lines
No. of Colonies
Addition Du4475 H69 H128
I
0.4 0.8 1.2 1.6 2.0 2.4
'251-SCF BOUND (pM)
Fig 3. Scatchard enalysis of 'SI-SCF binding to H128 cells. The cells (2 x 10 ) were incubated with 12WXF as in Fig 1 and analyzed by the ligand program. Two additional experiments gave similar results.
cell (Fig 2). H128 cells express a single class of high-affinity SCF receptors (kd 90 pmol/L) with - 9,400 receptors per cell (Fig 3). The MoAb SR-1 blocked binding of 1251-SCF to all three cell lines (Table 1). Additionally, in the two cell lines with both high-affinity and low-affinity receptors (H69 and Du4475), SR-1 at a dilution of 1:500 blocked low- affinity (2 nmol/L) binding of lzsI-SCF as effectively as a 200-fold excess of unlabeled SCF.
Effects of SCF on the growth of human solid tumor cell lines. SCF, in the presence of other growth factors such as IL-3, GM-CSF, or Epo, exerts a proliferative effect on normal bone marrow cells in culture. To determine whether SCF stimulates the proliferation of tumor cell lines that display high-affinity SCF receptors, we performed colony assays and tritiated thymidine uptake experiments. When Du4475, H69, or H128 cells were cultured in the presence of SCF alone at concentrations ranging from 1 to 100 ng/mL, no increase in colony growth over that observed without exogenous SCF was observed (Table 2). The addition of GM-CSF or IL-3 either alone or in combination with SCF failed to increase colony number or size (Table 2). In the presence of 10% human serum, the number of Du4475 colonies increased 2.5-fold (186 colonies/5 X lo3
Table 1. SR-1 Blocks Binding of 1al-SCF to Tumor Cell Lines
cpm Bound
'z9-SCF 'z51-SCF Cell Line '261-SCF + Unlabeled SCF + SR-1 Antibody
Du4475 548 90 156 H69 58 1 163 201 H128 3,459 518 777
Celts (2 x lo5) were incubated with 0.3 nmol/L 1251-SCF with or without a 100-fold excess of unlabeled SCF or a 1 :1,000 dilution of SR-1 MoAb for 4 hours at 15°C. The results represent the average of duplicate determinations.
No growth factor 258 f 34 25423 358 f 54 SCF (1 ng) 236 f 51 241 2 32 417 f 146 SCF (10 ng) 231 f 38 228 f 35 345 f 1 1 SCF (100 ng) 211 f 30 216 2 28 345 f 35 GM-CSF 226 f 14 220 2 62 ND GM-CSF + SCF (10 ng) 212 f 4.9 270 2 37 ND IL-3 234f63 249 f 47 401 f 76 IL-3 + SCF (10 ng) 199 f 31 295 f 33 363
The cells were plated at a concentration of 5 x 103/mL in methylcellu- lose in RPMI 1640 supplemented with FCS, BSA, and the growth factors indicated. GM-CSF was used at a final concentration of 10 mg/mL. IL-3 was used at a final concentration of 1 UlmL. Colonies (> 50 cells) were counted on day 14. The data are presented as the average f standard deviation of triplicate plates. Where no standard deviation is given, the data are the average of duplicate plates. The results of one of three similar experiments are shown.
Abbreviation: ND, not done.
cells plated) over the number of colonies present when the cells were cultured in serum-deprived media (73 f 9 colo- nies/5 x 103 cells plated). In the presence of 10% FCS, there was a 29% increase in colony formation over cells grown in serum-derived media (95 colonies/5 x lo3 cells plated). The addition of 10 ng/mL SCF had no effect in the presence of human serum, calf serum, or serum-deprived media. SCF stimulated a modest increase in tritiated thymidine uptake in the Du4475 and H69 cell lines (Table 3). In the presence of GM-CSF, there was a modest increase in tritiated thymidine uptake in the H69 cell line in comparison to cells grown in the absence of growth factors. The magnitude of the change in tritiated thymidine uptake was small in comparison to the effect of 20% FCS on the tumor cell lines or the effect of rat growth factor on the
Table 3. Effect of SCF on DNA Synthesis in Human Solid Tumor Cell Lines
Tritiated Thvmidine Incorporation (cpm)
Du4475 H69 MC19
Experiment 1 No growth factor 1,630 f 297 1 ng SCF 2,459 f 176 10 ng SCF 1,835 f 289 100 ng SCF 3,402 f 46 GM-CSF 1,893 f 236 GM-CSF + 10 ng SCF 2,330 2 960 Rat growth factor 20%
Experiment 2 Serum-deprived 11,984 f 959
982 f 113 961 f 311
1,470 f 210 1,323 f 230 1,508 f 100
1 1 1 f 33
1,213 f 288
19,518 f 3,508
16,333 f 1,325 Serum-deprived
FCS 20%
The data are presented as the mean f standard deviation of triplicate samples. Where no standard deviation is given, the data are the average of duplicate determinations.
+ 10 ng SCF 15,905 16,491 2 1,105 42.724 f 1,721 35,384 f 1,980
*The results of one of three similar experiments are shown.

370 TURNER ET AL
1 2 3 4
200
97.4
Fig 4. Affinity crosslinking of '%SCF binding to the c4ir receptor. Lane 1, A172 glioblastoma cells; lane 2, H69 small cell lung carcinoma; lane 3, normal human marrow hematopoietic cells; lane 4, Du4475 bread carcinoma cells.
MC/9 cell line (Table 3). No effect of SCF on tritiated thymidine uptake by the H128 cell line was observed (two experiments, data not shown).
The addition of SR-1 ascites did not alter colony growth (three experiments) or tritiated thymidine uptake (one experiment) by thc Du4475, H69, or H128 cell lines (data not shown).
To determine the size of the c-kit receptor on the tumor cell lines and on normal human marrow cells, ILSI-SCF was
AfFniry crosslinking of lZ5I-SCF to the c-kit receptor.
30
25
20 a = Z @
E .- t 1 5 C P
a,"
z g * 10
5
A
50
40
10
Du4475 H69 H128 HTT144
Cell Type B
crosslinked to its receptor using DSS, and the IBI-SCF- receptor complex was precipitated with anti-SCF poly- clonal antibodies. The A172 glioblastoma cell line, which has been reported to display c-kit receptors of molecular weight 145 Kd.24 was crosslinked in parallel for comparison. Thc A172 and Du4475 cell lines displayed a major crosslinked band of molecular weight 157 Kd (Fig 4, lanes 1 and 2). A fainter band of similar molecular weight was found in the H69 cell line and normal human marrow hematopoietic cells (Fig 4, lanes 2 and 3). When the cells were crosslinked to '"'I-SCF in the presence of a 200-fold excess of unlabeled SCF, this band was absent (data not shown). After accounting for the contribution of the E coli-expressed SCF, the c-kit receptor size in these cells is estimated to be 139 Kd. In a separate affinity crosslinking experiment, the molecular weight of the c-kit receptor in the H128 cell line was found to be approximately 137 Kd (data not shown).
RNAse protection assays showed multiple copies of c-kit mRNA in the three cell lines showing high-affinity c-kit receptors (Fig SA). HTT, which showed trace binding of ILSI-SCF, had 0.4 copies of c-kit mRNA per cell. SCF mRNA was also found in all four cell lines tested (Fig 5B).
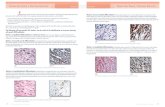
Soluble SCF protein was detected by EIA in media (RPMI 1640 supplemented with 3% FCS) in which Du4475, H69, or H128 cells had been cultured for 5 hours to 1 week (Table 4). Media that had not been exposed to the cells was tested in parallel, and lacked detectable SCF protein (Table 4). SCF was also found on the surface of the Du4475, H69, H128, and HTT cell lines by direct immunofluorescence analysis using an anti-SCF MoAb (Fig 6). The presence of cell surface SCF was not altered by incubating the tumor cell lines in serum-free media for 24 hours before immun- ofluorescent labeling, suggesting that SCF displayed on the cell surface is produced by the cells, rather than acquired from the serum used to culture the cells. The OCIMl
Detection of c-kit and SCF mRNA.
Detection of SCFproduction by tumor cell lines.
Fig 5. Number of copier per cell of c-ki, mRNA (A) or SCF mRNA (B) In the Du4475, H69, H128, and HTT
Du4475 H69 H128 HTT144 cell lines. Each sample was as- rayed twice and the results given
Cell Type are the average of two assays.

SCF AND c-K/r IN TUMOR CELL LINES 379
Table 4. Soluble SCF in Media Conditioned by Tumor Cell Lines
Incubation Time No. of SCF Cell Line (h) CelWlO-3 mL (nglmL)*
Du4475 5 139 0.1 24 282 0.3 48 231 0.6 120 176 1.2
H69 5 112 0.02 24 282 0.03 48 592 0.08 120 1,200 0.2 168 2,238 0.8
Media controlt 0-168 0 50.01
*The media were concentrated before performing the EIA; the data are presented as the quantity of SCF in the original unconcentrated media.
tRPMl 1640 supplemented with 3% FCS was incubated in tissue culture flasks in parallel to the flasks containing Du4475 or H69 cells.
human erythroleukemia cell line had no detectable SCF on the cell surface (data not shown).
DISCUSSION
This report shows the presence of high-affinity c-kit receptors on three nonhematopoietic human tumor cell lines. These receptors appear to be similar to c-kit receptors identified on normal human marrow cells in a number of respects. The breast carcinoma cell line, Du4475, as well as the two small cell lung carcinoma cell lines, H128 and H69, display high-affinity SCF receptors with a kd ranging from approximately 40 to 100 pmol/L. Normal human bone marrow mononuclear cells exhibit approximately 500 SCF receptors per cell, with a kd of 50 pmol/L.l1 Affinity crosslinking with the Du4475, H69, and H128 cell lines shows that the c-kit receptor has a molecular weight of 139 Kd, similar to the results obtained with normal human marrow cells. In addition to the structural similarities between the c-kit receptor found on normal marrow cells and on these tumor cell lines, the SR-1 antibody blocks binding of SCF to its receptor on both normal hematopoi- etic cells22 and on the tumor cell lines, indicating a degree of antigenic similarity as well.
In the presence of other growth factors, such as IL-3 or GM-CSF, SCF exerts a proliferative effect on early marrow progenitor^.^-^ Welham and Schrader have suggested that IL-3 may modulate display of c-kit receptors on factor- dependent cell lines and normal mast cells.z Incubation of marrow progenitors in the presence of low levels of IL-3 increased c-kit expression, whereas incubation in the pres- ence of high levels of IL-3 decreased c-kit protein. Unlike results with normal hematopoietic cells, SCF in the pres- ence of GM-CSF or IL-3 had a minimal effect on the growth of the tumor cell lines. Even at concentrations as high as 100 ng/mL, SCF did not have a substantial effect on tumor cell line growth in vitro under the conditions we used. The lack of a proliferative response to SCF may be explained by the endogenous production of SCF by these cell lines. SCF mRNA was found in each of the three cell lines (Du4475, H69, and H128) known to express high-affinity c-kit recep- tors. The number of copies of SCF mRNA per cell was
higher than that found in normal human marrow fibroblasts (5 copies of SCF mRNA/cell).% The tumor cell SCF mRNA is translated into an SCF protein product; mem- brane-bound SCF was detected on the cell surface by indirect immunofluorescence. In addition, soluble SCF was detected in media conditioned by the Du4475 or H69 cell lines. Studies of SId mutant mice show the critical impor- tance of the transmembrane form of SCF for hematopoie- sis, melanogenesis, and germ cell d e v e l ~ p m e n t . ~ ~ , ~ ~ The role of the soluble versus the transmembrane forms of human SCF is presently unknown.
The role that SCF and c-kit receptor may play in the immortalization of these tumor cell lines is unknown at the present time. The SR-1 antibody, although it effectively blocked IZI-SCF binding to its cell surface receptor, did not inhibit growth of the tumor cell lines. Previous experiments with normal human marrow cells have shown that SR-1, used at similar concentrations, blocks SCF-stimulated col- ony growth by 90%.22 It is possible that SCF binds to the c-kit receptor intracellularly in these tumor cell lines, thereby triggering cell growth. In this context, a truncated form of IL-3 has been shown to bind to cytoplasmic IL-3 receptors and confer growth factor independence on trans- fected ~e l l s .2~ In addition, Keating and Williams have described an internal autocrine loop involving the v-sis gene product and intracellular PDGF receptors?0 On the other hand, these tumor cell lines may have a mutation in their c-kit receptors resulting in abnormal signal transduction and possible constitutive activation of a mutant receptor. Such mutations have been described in the human CSF-1 receptor.31
In contrast to previously published result^,^,^^ we found that GM-CSF did not have a substantial effect on the growth of H128 or H69 small cell carcinoma cell lines as tested by tritiated thymidine uptake and methylcellulose colony assays. This change in response to GM-CSF may result from clonal evolution and loss of receptor display over time. A similar phenomenon was been described with the KG-1 and KG-la cell line^?^,^^
Steel and W mutant mice have abnormal skin pigmenta- ti0n,3~ suggesting that SCF is important for melanocyte development or migration.36 It is interesting to note that the human melanoma cell line HTT 144 had a paucity of c-kit receptors by 1251 binding assay and contained little c-kit mRNA. However, multiple copies of SCF mRNA were found in this tumor cell line and cell surface display of SCF was identified. Fresh human melanoma cells from a biopsy did show binding of S C F however, there was no prolifera- tive response to SCF in tissue culture. Murine melanocyte and melanoma cell lines have been found to contain c-kit RNA tran~cripts.3~
Tumor cell lines provide a convenient experimental model, but their growth characteristics may differ from those of solid tumors in vivo. We found specific binding of IzI-SCF to fresh human melanoma cells, and Sekido et a1 detected c-kit transcripts in a number of small cell lung carcinoma biopsy specimens as well as in cell lines.38 Strohmeyer et a1 recently described c-kit DNA and mRNA expression in human testicular germ cell tumors.39 Whether

380 TURNERETAL
6 4 ’
PE FLUORESCENCE
(ti
PE FLUORESCENCE
neoplastic tissues produce SCF in vivo remains to be determined. Our results show that three human solid tumor cell lines exhibit high-affinity c-kit receptors, contain SCF mRNA, and display membrane-associated SCF in vitro. Soluble SCF was detected in media conditioned by two of
B
Fig 6. The Du4475 and HlZ8 cell lines display membrane-associated SCF. The cell lines were labeled with the 7H6 anti-SCF MoAb (-) or with an irotype-matched control MoAb (---), and then with PE- conjugated goat antimouse IgG and analyzed in a Coulter Epics Elite Cell Sorter. (A) Du4475 cells. (B) H69 cells. (C) HI28 cells.
these cell lines. Exogenously added SCF did not signifi- cantly stimulate proliferation in these human tumor cell lines. These data suggest the possibility of an autocrine loop in which constitutively produced SCF may bind to the c-kit receptor and stimulate cell growth.
REFERENCES
1. Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu R-Y, Birkett NC, Okino KH, Murdock DC, Jacobsen FW, Langley KE, Smith KA, Takeishi T, Cattanach BM, Galli SJ, Suggs SV Stem cell factor is encoded at the S1 locus of the mouse and is the ligand for the c-lcit tyrosine kinase receptor. Cell 63:213,1990
2. McNiece IK, Langley KE, Zsebo KM: Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and Epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp Hematol19:226,1991
3. Broxmeyer HE, Cooper S, Lu L, Hangoc G, Anderson D, Cosman D, Lyman SD, Williams DE: Effect of murine mast cell growth factor (c-lcit proto-oncogene ligand) on colony formation by human marrow hematopoietic progenitor cells. Blood 772142, 1991
4. Bernstein ID, Andrews RG, Zsebo KM: Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+ lin- cells, and the generation of colony-forming cell progeny from CD34+ lin- cells cultured with interleukin-3, granu- locyte colony-stimulating factor, or granulocyte-macrophage colony- stimulating factor. Blood 772316,1991
5. Andrews RG, Bartelmez SH, Appelbaum FR, Bernstein ID, Knitter GH, Langley KE, Farrer D, Hendren W, Zsebo KM: Recombinant human SCF, a c-lcit ligand, stimulates hematopoiesis in primates. Blood 78:1975,1991
6. Dedhar S, Gadboury L, Galloway P, Eaves C: Human granulocyte-macrophage stimulating factor is a growth factor active on a variety of cell types of nonhematopoietic origin. Proc Natl Acad Sci USA 85:9253,1988 7. Berdel WE, Danhauser-Riedl S, Steinhauser G, Winton E F

SCF AND C-KIT IN TUMOR CELL LINES 381
Various human hematopoietic growth factors (interleukin-3, GM- CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood 73230,1989
8. Miyagawa K, Chiba S, Shibuya K, Piao Y-F, Matsuki S, Yokota J, Terada M, Miyazono K, Takaku F: Frequent expression of receptors for granulocyte-macrophage colony-stimulating factor on human nonhematopoietic tumor cell lines. J Cell Physiol 143:483,1990
9. Baldwin GC, Gasson JC, Kaufman SE, Quan SG, Williams RE, Avalos BR, Gazdar AF, Golde DW, DiPersio JF: Nonhe- matopoietic tumor cells express functional GM-CSF receptors. Blood 73:1033,1989
10. Avalos BR, Gasson JC, Hedvat C, Quan SG, Baldwin GC, Weisbart RH, Williams RE, Golde DW, DiPersio J F Human granulocyte colony-stimulating factor: Biologic activities and recep- tor characterization on hematopoietic cells on small cell lung cancer cell lines. Blood 4:851,1990
11. Abkowitz JL, Broudy VC, Zsebo KM, Martin FH: Evidence that Diamond-Blackfan anemia does not result from abnormalities of c-kit or its ligand. Blood 79:25,1992
12. Nabel G, Galli SJ, Dvorak AM, Carter H: Inducer T lymphocytes synthesize a factor that stimulates proliferation of clonal mast cells. Nature 291:332,1981
13. Papayannopoulou Th, Nakamoto B, Kurachi S, Tweeddale M, Messner H: Surface antigenic profile and globin phenotype of two new human erythroleukemia lines: Characterization and inter- pretations. Blood 72:1029,1988
14. Hunter WM, Greenwood F C Preparation of iodine 131 labeled human growth hormone of high specific activity. Nature 194:195,1962
15. Calvo JC, Radicella JP, Charreau EH: Measurement of specific radioactvities in labeled hormones by self displacement analysis. Biochem J 212259,1983
16. Broudy VC, Smith FO, Lin N, Zsebo KM, Egrie J, Bernstein ID: Blasts from patients with acute nonlymphocytic leukemia express functional receptors for stem cell factor. Blood (in press)
17. Broudy VC, Tait JF, Powell JS: Recombinant human eryth- ropoietin: Purification and analysis of carbohydrate linkage. Arch Biochem Biophys 265:329,1988
18. Broudy VC, Lin N, Brice M, Nakamoto B, Papayannopoulou T: Erythropoietin receptor characteristics on primary human erythroid cells. Blood 77:2583,1991
19. Broudy VC, Lin N, Egrie J, De Haen C, Weiss T, Papayanno- poulou Th, Adamson Jw: Identification of the receptor for erythropoietin on human and murine erythroleukemia cells and modulation by phorbol ester and dimethyl sulfoxide. Proc Natl Acad Sci USA 856513,1988
20. Munson DJ, Rodbard D: Ligand: A versatile computerized approach for characterization of ligand-binding systems. Anal Biochem 107:220,1980
21. Migliaccio G, Migliaccio AR: Cloning of human erythroid progenitors (BFU-E) in the presence of fetal bovine serum. Br J Haematol67129,1987
22. Broudy VC, Lin N, Zsebo KM, Birkett NC, Smith KA, Bemstein ID, Papayannopoulou Th: Isolation and characteriza- tion of a monoclonal antibody that recognizes the human c-kit receptor. Blood 79338,1992
23. Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophase T4. Nature 227:680,1970
24. Yarden Y, Kuang W-J, Yang-Feng T, Coussens L, Mune- mitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A Human proto-oncogene c-kit: A new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 63341,1987
25. Welham MJ, Schrader Jw: Modulation of c-kit mRNA and protein by hematopoietic growth factors. Mol Cell Biol 11:2901, 1991
26. Linenberger ML, Broudy VC, Martin FH, Jacobson FW, Bennett LG, Abkowitz J L Stem cell factor (SCF) production by human marrow fibroblasts. Blood 78:374a, 1991 (abstr, suppl 1)
27. Flanagan JG, Chan DC, Leder P: Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell 641025,1991
28. Brannan CI, Lyman SD, Williams DE, Eisenman J, Ander- son DM, Cosman D, Bedell MA, Jenkins NA, Copeland N G Steel-Dickie mutation encodes a c-kit ligand lacking transmem- brane and cytoplasmic domains. Proc Natl Acad Sci USA 88:4671, 1991
29. Dunbar CE, Browder TM, Abrams JS, Nienhuis A W COOH-terminal modified IL-3 is retained intracellularly and stimulates autocrine growth. Science 245:1493, 1989
30. Keating MT, Williams LT: Autocrine stimulation of intracel- lular PDGF receptors in v-sis transformed cells. Science 239:914, 1988
31. Roussel MP, Downing JR, Rettenmier CW, Sherr CJ: A point mutation in the extracellular domain of the human CSF-1 receptor (cfms proto-oncogene product activates its transforming potential). Cell 55979,1988
32. Ruff MR, Farrar WL, Pert CB: Interferon y and granulocyte/ macrophage colony-stimulating factor inhibit growth and induce antigens characteristic of myeloid differentiation in small-cell lung carcinoma cell lines. Proc Natl Acad Sci 83:6613,1986
33. Koeffler HP, Billing R, Lusis J, Sparkles R, Golde D W An undifferentiated variant derived from the human acute myeloge- nous leukemia cell line (KG-1). Blood 56:265,1980
34. Gasson JC, Kaufman SE, Weisbart RH, Tomonaga M, Golde DW High-affinity binding of granulocyte-macrophage col- ony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci USA 83:669,1986
35. Russell ES: Hereditary anemias of the mouse: A review for geneticists. Adv Genet 20357, 1979
36. Matsui Y, Zsebo KM, Hogan BLM: Embryonic expression of a haematopoietic growth factor encoded by the S1 locus and the ligand for c-kit. Nature 347:667,1990
37. Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A, Besmer P Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice- Evidence for an impaired c-kit kinase in mutant mice. Genes Dev 3:816,1989
38. Sekido Y, Obata Y, Ueda R, Hida T, Suyama M, Shimokata K, Ariyoshi Y, Takahashi T Preferential expression of c-kit protooncogene transcripts in small cell lung cancer. Cancer Res 51:2416,1991
39. Strohmeyer T, Peter S, Hartmann M, Munemitsu S, Acker- mann R, Ullrich A, Slamon DJ: Expression of the hst-1 and c-kit protooncogenes in human testicular germ cell tumors. Cancer Res 51:1811,1991