Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ...
Transcript of Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ...
![Page 1: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/1.jpg)
Corporate Presentation
Midatech Pharma plcAugust 2017
![Page 2: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/2.jpg)
DisclaimerTHIS PRESENTATION MAY NOT BE COPIED OR REPRODUCED IN ANY FORM, FURTHER DISTRIBUTED OR PASSED ON, DIRECTLY OR INDIRECTLY, TO ANY OTHER PERSON, OR PUBLISHED, IN WHOLE OR IN PART, FOR ANY PURPOSE. IN PARTICULAR, THIS PRESENTATIONAND ITS CONTENTS ARE NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION IN OR INTO OR FROM THE UNITED STATES, CANADA, AUSTRALIA, SOUTH AFRICA OR JAPAN OR ANY JURISDICTION WHERE SUCH DISTRIBUTION IS UNLAWFUL. ANY FAILURE TO COMPLYWITH THESE RESTRICTIONS MAY CONSTITUTE A VIOLATION OF APPLICABLE SECURITIES LAWS.This presentation does not constitute or form part of any offer or invitation to sell or issue, or any solicitation of any offer to purchase or subscribe for, any securities of the Company, nor shall it or any part of it nor the fact of its distribution form the basis of, or berelied on in connection with, any contract commitment or investment decision in relation thereto.The information contained in this presentation has been prepared by Midatech Pharma plc ("Midatech" or the "Company"). It has not been fully verified and is subject to material updating, revision and further amendment. This presentation has not been approvedby an authorised person in accordance with Section 21 of the Financial Services and Markets Act 2000 (“FSMA”) and therefore it is being delivered for information purposes only to a very limited number of persons and companies who are persons who haveprofessional experience in matters relating to investments and who fall within the category of person set out in Article 19 of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or are high net worth companies within themeaning set out in Article 49 of the Order or are otherwise permitted to receive it. Any other person who receives this presentation should not rely or act upon it. By accepting this presentation and not immediately returning it, the recipient represents andwarrants that they are a person who falls within the above description of persons entitled to receive the presentation. This presentation is not to be disclosed to any other person or used for any other purpose.Please note that the information in this presentation has yet to be announced or otherwise made public and as such constitutes inside information for the purposes of Article 14 of the Market Abuse Regulation (596/2014/EU) and the Criminal Justice Act 1993. Youshould not therefore deal in any way in the securities of the Company until after the formal release of an announcement by the Company as to do so may result in civil and/or criminal liability.Panmure Gordon (UK) Limited ("Panmure Gordon") is acting in the provision of corporate finance business to the Company, within the meaning of the Financial Conduct Authority’s Conduct of Business Sourcebook (“COBS”), and no-one else in connection with theproposals contained in this Presentation. Accordingly, recipients should note that Panmure Gordon is neither advising nor treating as a client any other person and will not be responsible to anyone other than the Company for providing the protections afforded toclients of Panmure Gordon under the COBS nor for providing advice in relation to the proposals contained in this presentation.While the information contained herein has been prepared in good faith, neither the Company nor any of its shareholders, directors, officers, agents, employees or advisers give, have given or have authority to give, any representations or warranties (express orimplied) as to, or in relation to, the accuracy, reliability or completeness of the information in this presentation, or any revision thereof, or of any other written or oral information made or to be made available to any interested party or its advisers (all suchinformation being referred to as “Information”) and liability therefore is expressly disclaimed. Accordingly, neither the Company nor any of its shareholders, directors, officers, agents, employees or advisers take any responsibility for, or will accept any liabilitywhether direct or indirect, express or implied, contractual, tortious, statutory or otherwise, in respect of, the accuracy or completeness of the Information or for any of the opinions contained herein or for any errors, omissions or misstatements or for any loss,howsoever arising, from the use of this presentation. In particular, unless expressly stated otherwise, the financial information contained in this presentation relates to the Company and its subsidiary undertakings. To the extent available, the industry and marketdata contained in this presentation has come from official or third party sources. Third party industry publications, studies and surveys generally state the data contained therein have been obtained from sources believed to be reliable, but that there is noguarantee of the accuracy or completeness of such data. While the Company believes that each of these publications, studies and surveys has been prepared by a reputable source, the Company has not independently verified the data contained therein. In addition,certain of the industry and market data contained in this presentation come from the Company’s internal research and estimates based on the knowledge and experience of the Company’s management in the market in which the Company operates. While theCompany believes that such research and estimates are reasonable and reliable, their underlying methodology and assumptions, have not been verified by any independent source for accuracy or completeness and are subject to change without notice. Accordingly,undue reliance should not be placed on any of the industry or market data contained in this presentation.Neither the issue of this presentation nor any part of its contents is to be taken as any form of commitment on the part of the Company to proceed with any transaction and the right is reserved to terminate any discussions or negotiations with any prospectiveinvestors. In no circumstances will the Company be responsible for any costs, losses or expenses incurred in connection with any appraisal or investigation of the Company. In furnishing this presentation, the Company does not undertake or agree to any obligationto provide the recipient with access to any additional information or to update this presentation or to correct any inaccuracies in, or omissions from, this presentation which may become apparent.This presentation should not be considered as the giving of investment advice by the Company or any of its shareholders, directors, officers, agents, employees or advisers. In particular, this presentation does not constitute an offer or invitation to subscribe for orpurchase any securities and neither this presentation nor anything contained herein shall form the basis of any contract or commitment whatsoever. Each party to whom this presentation is made available must make its own independent assessment of theCompany after making such investigations and taking such advice as may be deemed necessary. In particular, any estimates or projections or opinions contained herein necessarily involve significant elements of subjective judgment, analysis and assumptions andeach recipient should satisfy itself in relation to such matters.This presentation and the information contained herein are not an offer of securities for sale and are not for publication and or distribution in the United States or to any US person (within the meaning of Regulation S under the United States Securities Act of 1933,as amended (the “Securities Act”)) or in Canada, Australia, South Africa or Japan or any jurisdiction where such offer or distribution is unlawful. Any failure to comply with this restriction may constitute a violation of United States securities laws.The securities of the Company have not been registered under the Securities Act and may not be offered or sold in the United States or to any US person unless the securities are registered under the Securities Act or an exemption therefrom is available.Certain statements in this presentation may constitute “forward-looking statements” within the meaning of legislation in the United Kingdom and/or United States. In some cases, you can identify forward-looking statements by the use of words such as “may,”“could,” “expect,” “intend,” “plan,” “seek,” “anticipate,” “believe,” “potential,” “estimate,” “predict,” “potential,” or “continue” or the negative of these terms or other comparable terminology. You should not place undue reliance on forward-looking statementsbecause they involve known and unknown risks, uncertainties and other factors that are, in some cases, beyond our control and that could materially affect actual results, the acquisition, levels of activity, performance, or achievements. Any forward-lookingstatements are based on currently available competitive, financial and economic data together with management’s views and assumptions regarding future events and business performance as of the time the statements are made and are subject to risks anduncertainties. We wish to caution you that there are some known and unknown factors that could cause actual results to differ materially from any future results, performance or achievements expressed or implied by such forward-looking statements. Referenceshould be made to those documents that Midatech shall file from time to time or announcements that may be made by Midatech in accordance with the London Stock Exchange AIM Rules for Companies (“AIM Rules”), the Disclosure and Transparency Rules(“DTRs”) and the rules and regulations promulgated by the US Securities and Exchange Commission, which contains and identifies other important factors that could cause actual results to differ materially from those contained in any projections or forward-lookingstatements. These forward-looking statements speak only as of the date of this presentation. All subsequent written and oral forward-looking statements by or concerning Midatech are expressly qualified in their entirety by the cautionary statements above. Exceptas may be required under the AIM Rules or the DTRs or by relevant law in the United Kingdom or the United States, Midatech does not undertake any obligation to publicly update or revise any forward-looking statements because of new information, future eventsor otherwise arising.
2
![Page 3: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/3.jpg)
A rapidly growing international specialty pharmaceutical company
UK-based public company (LON: MTPH / Nasdaq: MTP)• >100 employees across Europe and the US• Diversified strategy and sources of revenue with innovative R&D pipeline• Highly experienced pharma management team
Fully integrated R&D capabilities with three platform technologies: “right time, right place”• 1 Q-Sphera™ - “printed” sustained-release particles• 2 GNP - gold nanoparticle carrier system• 3 Nano-inclusion solubilisation• MTP technology, based on known therapeutic agents, avoids NCE risk• At least 2clinical trials planned in 2017
Full service US Sales and Marketing• Sales channel to commercialise high value pipeline• Currently six marketed products: potential aggregate peak sales of up to $50 million• Double-digit top-line growth and break even expected over the next 12 months• Q-Octreotide, the lead pipeline product, expected to file in 2018
Organisation
R&DPipeline
US Commercial
3
![Page 4: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/4.jpg)
Highly Experienced Pharma Management Team
4
Rolf StahelNon-executive Chairman
Dr. Jim Phillips, MB, ChB, MBAChief Executive Officer
Nick Robbins-Cherry, ACA, MBAChief Financial Officer
Dr. Craig Cook, MD, MBAChief Operating Officer
Rob Rainey, FCA, MBA, MAHead of Corporate Development
David BenharrisPresident, Midatech Pharma US
![Page 5: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/5.jpg)
Overview and value proposition
5
Midatech GroupStrong R&D product
pipeline3 technology platforms
Gold nanoparticleLiver cancerBrain cancer
Immunotherapy
Nano inclusionBrain cancer
Sustained releaseOncology
Commercialisationcapability
Portfolio of cancer supportive care products and sales channel
ZuplenzGelclairOravig
Soltamox
Future value upside lies in innovative, drug delivery, built on Midatech’s three proprietary platform technologies
US operation generates revenue and provides the sales channel when pipeline products reach market
![Page 6: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/6.jpg)
Midatech’s international R&D, manufacturing and sales facilities
6
Midatech US CommercialRaleigh, North Carolina36 staff, including 20 reps
Midatech Corp HQ and GNP R&DOxford, UK22 staff
Midatech Q-Sphera SR R&DCardiff, UK16 staff
Midatech ManufacturingBilbao, SpainDevelopment-scale manufacturing facility30 staff
![Page 7: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/7.jpg)
7
Innovative platform technologies: driving significant future valueQ-Sphera™ Gold Nano Particle (GNP) Nano Inclusion (NI)
Technologies
Sustained Delivery Targeted Delivery Local Delivery
ApplicationsBrain Cancer
Adults Carcinoid
CancerAcromegaly Oncology Immuno-Oncology Brain Cancer
Children
Liver Cance
r
Brain Cancer
Vaccines Tumour Macrophages
Development Programmes
1. MTX110 DIPG1. MTD119 Liver Cancer (HCC)2. MTX102 Diabetes Vaccine
1. MTD201 Q-Octreotide 2. MTD202 OpsiSporin
![Page 8: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/8.jpg)
Development pipeline: 2 programmes entering the clinic in 2017
8
RESEARCH PRECLINICAL CLINICAL / REGULATORY PHASES
Immunotherapy
Cancer
Ophthalmology
Q-Octreotide carcinoid/acromegaly MTD201
Glioblastoma
Opsisporin to be out-licensed
Liver hepatocellular carcinoma MTD119
Immuno-oncology vaccine
Immuno-oncology TAM
Development of multiple high-value, targeted therapies for major diseases with unmet medical need
DIPG pontine glioma MTX110/MTR111 named patient basis + clinical trials
Uses gold nanoparticle technology
Uses polymer microsphere technology Uses nano-inclusion technology
Type 1 diabetes vaccine MTX102 to be out-licensed
Development timelines are company estimates
![Page 9: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/9.jpg)
Research and developmentDriving value for shareholders
![Page 10: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/10.jpg)
10
Nanotech Gold Nanoparticle GNP for targeted delivery & enhanced biodistribution
Drug conjugates eliminated via the kidneys and liverDesigned to release the active compound inside the cell
Internal GMP manufacturing facility
1.4 - 1.8nm
Smallest particles in biomedical use: 10x-20x
smaller than peers
SOLUBILITY
THERAPEUTICS
Ultra-small gold nanoparticles are bio-inert, non-toxic, non-immunogenic; do not generate immune-response
EXCRETABILITY
SCALABILITY
Multivalency Size
Small size ~1.5nm and defined charge allows transport to disease sites that are otherwise very difficult to reach
Enable the transport of water insoluble and lipid soluble compounds to disease sites
RELEASABILITY
SOLUBILITY
TARGETING
THERAPEUTICS
MOBILITY TARGETING
THERAPEUTICS COMPATIBILITY
CORONA
LINKER
SULPHUR LAYER
NOBLE METAL CORE
Multivalency - enables binding of several targeting and therapeutic agents to a single nanoparticle
Payloads conjugated to form small (~5nm) medicines for targeted delivery
+
THERAPEUTICS
![Page 11: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/11.jpg)
11
Q-Sphera – printed microspheres for Sustained Release
PROPRIETARY MICROSPHERE PLATFORMPrecision encapsulation platform enabling tuneable sustained drug release for chronic
diseases:• Emulsion-free synthesis with product monodispersity
• Precise control over particle size, morphology, release kinetics• High drug loading, minimal burst release, essential to development of safe & effective therapies
ADVANTAGES• Tox: non-toxic and well tolerated• Particle size: allows fine gauge needles • PK: easily tuned to range dosing regimes• PK: highly linear reproducible kinetics• PK: each sphere the same, low variability• PK: predictable degradability, 3–6 months• Class 3 solvents: no solvent-related tox• High drug loading: small injectable mass• High API purity: minimal impurities• Microbial and endotoxin control: sterility
assurance via terminal sterilisation and very low endotoxin levels
MICROPARTICLES• Encapsulate drugs into micron sized
particles - (diameter ~25μm)• Compatible with small molecules -
peptides, oligonucleotides, proteins• Tuneable using biodegradable
polymers
”PRINTING” DRUGS AT SCALE• Innovative adaptation of inkjet
technology to enable “printing” of uniform drug-containing microparticles
• Printing ‘000,000 particles per second
![Page 12: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/12.jpg)
Q-Octreotide for Acromegaly and Carcinoidentering human trials in 2017
12
Long-acting formulation of Octreotide acetate for treatment of carcinoid (cancer) and acromegaly
Streamlined manufacturing process• Terminal sterilisation avoids aseptic manufacture• Printed particles avoid the need to sieve and fraction• Knock-on benefits to other Q-Sphera™ projects
Product benefits vs Sandostatin® LAR®• Fast and simple to reconstitute reducing errors and wastage• Smaller needle and injection volume improves patient experience
Commencing clinical registration studies• Formulation complete• Human studies to commence in 2H 2017• 505b2 submission in the US expected in late 2018
Commercialisation and market potential• c.$100m, representing c.5% of a $2bn a year market in first line
SSAs, and less than 10% of Sandostatin® LAR’s $1.6bn+ annual sales
• Partner in EU and RoW, use MTP US to market in the USA
1
2
3
4
5
Incorporating Midatech’sQ-Sphera™ technology
Plasma octreotidein Rabbits vs Sandostatin LAR
Plasma IGF1in Rabbits vs Sandostatin LAR
Sandostatin® and LAR® are registered trademarks of Novartis
![Page 13: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/13.jpg)
Direct administration of Midatech Nanotherapeutics into the tumour through Convection Enhanced Delivery (CED):• Delivers therapeutic constructs via a series of catheters fixed
directly into the substance of the tumours
Compassionate use/named patient programme • Five patients treated with MTX110 to date in UK and US• First patient continues to be infused monthly (10 infusions to date)
Clinical / Regulatory• High level of regulatory support received in 2017• Planning for US and EU studies at advanced stage – estimated start
H2 2017• Potential for Orphan Drug designation and paediatric extensions
Ultra rare childhood brain tumour• c.1,000 cases / year worldwide• Median survival 9 months; universally fatal• No treatment options currently available
Commercialisation and market potential• Panobinostat API licensed from Novartis June 2017• Product could be commercially available as early as 2019• Market estimate $50-100m globally
1
2
3
4
5
Incorporating Midatech’snano-inclusion technology
MTX110 for DIPGin compassionate use, and entering human trials in 2017
13
CED Infusion port
![Page 14: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/14.jpg)
MTD119 for HCCIND-enabling underway
14
Currently in IND-enabling phase• Construct selected, incorporating DM1 (used in ADCs)• Plan to file for first-in-human study in Q4 2017• Orphan Designation applied for
Manufacturing• Manufacturing scale up underway to GMP
Commercialisation and market potential• Co-promote with Midatech’s own sales force in the USA• Global partner sought to co-develop this program• HCC market expected to be worth $550m by 2024 (ex-China)• Sorafenib is the only current approved product but limited
efficacy and too toxic for most patients
Compelling animal data• GNP-conjugation enables otherwise lethal doses of DM1 to be
administered• Clear dose responses• GNP-targeted chemo suppresses tumour growth, and more
effective than current standard of care (Sorafenib)
2
3
4
Incorporating Midatech’sgold nanoparticle technology
1
GNP conjugation improves tolerance to an otherwise lethal dose of DM1in mouse model with human HCC xenograft
GNP-drug more effective than SoC Sorafenib at tumour volume reductionin mouse model with human HCC xenograft
![Page 15: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/15.jpg)
15
Commercial platform and products
![Page 16: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/16.jpg)
Midatech Pharma US (MTPUS): fully integrated commercial platformMTPUS commercial operations• MTPUS has an effective and established commercial platform to sell and
market our current oncology supportive care portfolio (6 products)
• 20 reps + 5 field sales managers with access and established relationships in the highest prescribing oncology markets
• Established through acquisition of DARA BioSciences, Inc. for $24 million and of the complementary product Zuplenz for $3.75 million
MTPUS commercial strategy• Leverage oncology platform and access to acquire complementary products
that enhance portfolio and increase revenues
• 2,400 primary call points
• Utilise commercial operations to:
• Acquire additional products and launch internally developed pipeline products• Fund on-going R&D programmes from long-term cash flows
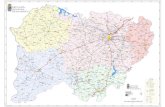
16
= Midatech US sales territories
![Page 17: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/17.jpg)
Product portfolio
• For post-chemo radiotherapy anti-nausea and vomiting product
• The 5HT3 market in the US is about $10bn / 20m prescription per annum, just a 0.25% market share would give sales of $25m/pa
• Covered by 11/13 commercial insurers (>250m population)
• Competitive pricing $500/pack (range of competition $250-$1,800)• Superior support and presentation• Zero patient co-pay program• May be taken with or without water and
dissolves in seconds• No stickiness from a patch or gritty
sensation from a tablet
17
• Indicated for oral mucositis (caused by chemotherapy or radio therapy), with approx. 400,000 annual sufferers in the US• Expansion study starting in 2017
• Lead product in uncompetitive environment (compounded magic mouthwash)
• Covered by 11/13 commercial insurers (>250m population)
• Superior support and mechanism of action vs. magic mouthwash• Zero patient co-pay program• 3rd year of sales in 2016
• Estimated peak annual sales potential c.$10m
• Buccal tablet for treatment of oral “thrush” associated with radio/chemo therapy and in HIV patients
• Covered by 11/13 commercial insurers (>250m population)
• Over 4m rx written annually for localised treatment
• 0.1% market share = c.$3m annual sales (targeting 0.3%-0.5% market share)
• Superior support and presentation• Zero patient co-pay program• Once-daily, local treatment• Does not interrupt eating or drinking
• Annual sales potential c.$10m
![Page 18: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/18.jpg)
Summary
![Page 19: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/19.jpg)
2016 financial highlights• Total gross revenues for the year up 510% to £9.21m (2015: 844% to £1.51m)
• Statutory revenue for the year up 718% to £6.38m (2015: 2,500% to £0.78m)
• £17.61m cash and deposits at 31 December 2016 (2015: £16.18m)
• Net loss after tax of £20.16m (2015: £10.10m) with net cash inflow in the year of £0.97m (2015: £14.17m outflow)
• Tax credit receivable of £1.44m (2015: £1.20m)
• Entered into a senior secured £6 million loan agreement with Silicon Valley Bank in Q1 2017
• October 2016 Gross £16.7m Equity Raise completed in London
19
![Page 20: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/20.jpg)
Anticipated near-term events/ news flow
20
• Q-Octreotide: human studies to commence in Q3 2017, for potential 2019 approval
• HCC/ MTD119: IND-enabling, orphan status and planned IND submission for 2018
• Expansion (Ph4) study for Gelclair in bone marrow transplant in USA
• DIPG/ MTX110: commence pivotal studies in the USA and Europe in 2H 2017
• Further M&A/ in & out-licensing opportunities anticipated
![Page 21: Midatech Corporate Presentation standard 070817 v2 Corporate presentation www... · ] o ] u d,/^WZ ^ Ed d/KED zEKd KW/ KZZ WZK h /E Ez&KZD U&hZd, Z /^dZ/ hd KZW ^^ KE U /Z d>zKZ/E](https://reader030.fdocuments.net/reader030/viewer/2022020214/5aa067477f8b9a76178df449/html5/thumbnails/21.jpg)
Summary
21
Midatech Pharma is an international specialty pharma company• Focused on development and commercialisation of multiple high-value, targeted therapies for
major diseases with unmet medical needs• Own product launches from 2019• US commercial arm has six products in oncology treatment and supportive care, driving revenue
growth
On-track for execution of three-pronged strategy for growth and value creation• R&D - developing novel pre-clinical and clinical portfolio pipeline• Commercial – grow and leverage our US commercial capability• M&A/ BD - seeking attractive acquisition targets, products and technology
1
Leading proprietary platform technologies• GNP (Gold nanoparticles ) – targeted delivery• Q-Sphera - sustained-release• Nano-inclusion – local delivery• Targeted delivery and release of existing therapeutics to the “right place” at the “right time”
2
3