Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in...
Transcript of Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in...
![Page 1: Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in food industry due to its low cost and available equipment [15]. The process involves](https://reader036.fdocuments.net/reader036/viewer/2022063015/5fd242aa7539492f3d4f595c/html5/thumbnails/1.jpg)
M
A
wanodM(©
K
1
icntaettifca
IT
h2
Available online at www.sciencedirect.com
ScienceDirectHOSTED BY
Food Science and Human Wellness 3 (2014) 175–182
icro-encapsulation of Pacific white shrimp oil as affected by emulsificationcondition
Sirima Takeungwongtrakul a, Soottawat Benjakul a,∗, Aran H-kittikun b
a Department of Food Technology, Faculty of Agro-Industry, Prince of Songkla University, Hat Yai, Songkhla 90112, Thailandb Department of Industrial Biotechnology, Faculty of Agro-Industry, Prince of Songkla University, Hat Yai, Songkhla 90112, Thailand
Received 29 August 2014; received in revised form 28 October 2014; accepted 11 December 2014
bstract
Micro-encapsulation of shrimp oil using the mixture of whey protein concentrate (WPC) and sodium caseinate (SC) (1:1, w/w) as a wall materialas carried out. The impact of core/wall material ratios (1:2 and 1:4, w/w) and homogenizing pressures (13.79 and 27.58 MPa) on characteristics
nd stability of emulsion was investigated. The size of emulsion oil droplets decreased with increasing homogenizing pressure (P < 0.05) but wasot influenced by core/wall material ratio (P > 0.05). During the extended storage, particle size, flocculation factor (Ff) and coalescence index (Ci)f all emulsions sharply increased, especially in emulsions prepared at 13.79 MPa with a core/wall material ratio of 1:2 (P < 0.05). After sprayrying, micro-encapsulated shrimp oil (MSO) prepared at 13.79 MPa with a core/wall material ratio of 1:2 had the larger size than others (P < 0.05).
SO prepared using a core/wall material ratio of 1:4 with homogenizing pressure of 27.58 MPa exhibited higher encapsulation efficiency (EE)51.3%–52.8%) than others. Thus, both core/wall material ratio and homogenizing pressure directly affected micro-encapsulation of shrimp oil. 2014 Beijing Academy of Food Sciences. Production and hosting by Elsevier B.V. All rights reserved.
cabumiabiptWc
mstu
eywords: Shrimp oil; Emulsion; Pressure; Encapsulation; Spray drying
. Introduction
Hepatopancreas, a byproduct generated from the manufactur-ng of hepatopancreas-free whole shrimp, has been reported toontain high content of n-3 PUFA and astaxanthin [1]. However,-3 PUFA are easily oxidized due to their high degree of unsa-uration. As a consequence, off-flavour compounds [2] as wells toxic products [3] are formed. To lower such a deterioration,ncapsulation can be as a key technology in delaying or inhibi-ing oxidation and masking undesirable odours and flavours inhe final product [4]. Furthermore, the process converts the oilnto a free flowing powder, which can be easily handled and usedor nutraceuticals and/or food fortification. Micro-encapsulationan be defined as a process, in which tiny droplets, namely core,re surrounded by a coating of micro-encapsulating agent. This
∗ Corresponding author at: Department of Food Technology, Faculty of Agro-ndustry, Prince of Songkla University, Hat Yai, Songkhla 90112, Thailand.el.: +66 74 286334; fax: +66 74 558866.
E-mail address: [email protected] (S. Benjakul).Peer review under responsibility of Beijing Academy of Food Sciences.
fie[o
ttp://dx.doi.org/10.1016/j.fshw.2014.12.001213-4530/© 2014 Beijing Academy of Food Sciences. Production and hosting by E
oating wall can be made of a variety of food grade materi-ls and can protect the entrapped core by providing a physicalarrier against environmental conditions [5]. Protein is widelysed in the preparation of emulsion and serves as coating wallaterial during micro-encapsulation process. In recent years, the
nterest in milk proteins as encapsulating agents has increasednd the concept of using milk products as wall materials haseen established [6]. The wall material must have emulsify-ng properties and be capable of dehydration. The milk proteinroducts including sodium caseinate and whey protein concen-rate have excellent emulsifying and dehydration properties [7].
hey protein and sodium caseinate act as the emulsifier, whichan stabilize the emulsion before drying [8].
Additionally, emulsification is one of the critical steps inicro-encapsulation of oils by spray-drying, as the emulsion
tability and droplet size play a key role in the encapsula-ion efficiency during and after the process [9]. To ensure theniform distribution of oil droplet, homogenization with suf-cient pressure for emulsification has been widely used in
mulsion preparation and encapsulation in the food industry10,11]. The advantage of high pressure homogenization overther technologies is that strong shear and cavitation forceslsevier B.V. All rights reserved.
![Page 2: Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in food industry due to its low cost and available equipment [15]. The process involves](https://reader036.fdocuments.net/reader036/viewer/2022063015/5fd242aa7539492f3d4f595c/html5/thumbnails/2.jpg)
1 nce a
eHtietacdiaTdmnhfdooc
2
2
csLI
2s
nSdwp(Pista(
2
ohss(
emeherwa4ETfl
2ap
2
ccs(SaaTendTa
2w(Pdcvd
2td(t
F
C
76 S. Takeungwongtrakul et al. / Food Scie
fficiently decrease the diameter of the original droplets [12].igh pressure homogenization induces significant changes in
he interfacial protein layer because of the considerable increasen interaction between adsorbed proteins at the interface of themulsion [13]. Several factors such as process conditions, pro-ein concentration and oil volume fraction have been reported toffect the properties of emulsion [14]. Spray-drying is the mostommon micro-encapsulation technology used in food industryue to its low cost and available equipment [15]. The processnvolves the atomization of emulsions into a drying mediumt a high temperature, resulting in very fast water evaporation.he micro-encapsulated oil was reported to have higher oxi-ation stability during the extended storage [16]. Although theicro-encapsulation of several oils or lipids has been reported,
o information regarding the micro-encapsulation of shrimp oilas been reported. Due to the differences in composition of oilsrom different sources, emulsification conditions prior to sprayrying can be varied and determine the characteristics of thebtained powder. Thus, this study aimed to investigate the impactf homogenization at varying pressure levels and the ratio ofore/wall material on characteristics of encapsulated shrimp oil.
. Materials and methods
.1. Chemicals
Sodium azide (NaN3) was purchased from Fluka Chemi-al (Buchs, Switzerland). Sodium dodecyl sulphate (SDS) andodium caseinate were procured from Sigma Chemical Co. (St.ouis, MO, USA). Whey protein concentrate was obtained from
.P.S. International Co., Ltd. (Bangkok, Thailand).
.2. Collection of hepatopancreas from Pacific whitehrimp
Hepatopancreas of Pacific white shrimp (Litopenaeus van-amei) with the size of 50–60 shrimps/kg was obtained from theea wealth frozen food Co., Ltd., Songkhla province, Thailanduring July and August 2013. Pooled hepatopancreas (3–5 kg)as placed in a polyethylene bag. The bag was imbedded in aolystyrene box containing ice with a sample/ice ratio of 1:2w/w) and transported to the Department of Food Technology,rince of Songkla University, Hat Yai, Songkhla within approx-
mately 2 h. The sample was stored at −18 ◦C until use, but thetorage time was not longer than 1 month. Prior to oil extrac-ion, hepatopancreas was thawed using running water (25 ◦C)nd ground in the presence of liquid nitrogen using a blenderPhillips, Guangzhou, China) for 30 s.
.3. Extraction of oils from hepatopancreas
Oil was extracted from hepatopancreas following the methodf Sachindra et al. [17] with some modifications. The prepared
epatopancreas (20 g) was homogenized with 90 mL of coldolvent mixtures (isopropanal:hexane, 50:50, v/v (4 ◦C)) at thepeed of 9500 rpm using an IKA Labortechnik homogenizerModel T25 basic, Selangor, Malaysia) for 2 min at 4 ◦C. Theworo
nd Human Wellness 3 (2014) 175–182
xtract was filtered using a Whatman filter paper No. 4 (What-an International Ltd., Maidstone, England). The residue was
xtracted with cold solvent mixtures for another two times. Theexane fractions were pooled and repeatedly washed with anqual quantity of 1% NaCl in order to separate the phases andemove traces of polar solvents. Hexane fraction was then addedith 2–5 g of anhydrous sodium sulphate, shaken very well,
nd decanted into a round-bottom flask through a Whatman No. filter paper. The solvent was evaporated at 40 ◦C using anYELA rotary evaporator N-1000 (Tokyo Rikakikai, Co. Ltd,okyo, Japan) and the residual solvent was removed by nitrogenushing. The obtained oil was used for micro-encapsulation.
.4. Characteristics of shrimp oil-in-water emulsion asffected by core/wall material ratio and homogenizingressure
.4.1. Preparation of shrimp oil-in-water emulsionAqueous stock solution (20%, w/w) of whey protein con-
entrate and sodium caseinate (1:1, w/w) in deionized waterontaining 0.03% (w/w) sodium azide was prepared, andtirred overnight using a magnetic stirrer at room temperature28–30 ◦C). The solution obtained was used as ‘wall material’.hrimp oil was added into the solution at different ratios (1:2nd 1:4, core/wall material). The mixtures were homogenizedt a speed of 10,000 rpm for 3 min using a homogenizer (Model25 basic, IKA Labortechnik, Selangor, Malaysia). The coarsemulsions were then passed through a Microfluidics homoge-izer (Model HC-5000, Microfluidizer, Newton, MA, USA) atifferent pressure levels (13.79 and 27.58 MPa) for four passes.he emulsions were stored at room temperature and taken fornalyses at day 0, 1, 7 and 14 as follows:
.4.1.1. Droplet size. Droplet size distribution of emulsionsas determined using a ZetaPlus zeta potential analyzer
Brookhaven Instruments Corporation, Holtsville, NY, USA).rior to analysis, emulsion was diluted with 1% (w/v) sodiumodecyl sulphate (SDS) solution in order to dissociate floc-ulated droplets. The surface-weighted mean (d32) and theolume-weighted mean particle diameter (d43) of the emulsionroplets were measured as described by Palazolo et al. [18].
.4.1.2. Flocculation and coalescence. To determine floccula-ion factor (Ff) and coalescence index (Ci), the emulsions wereiluted with distilled water in the presence and absence of 1%w/v) SDS. Ff and Ci were calculated using the following equa-ions:
f = d43−SDS
d43+SDS
i = d43+SDS,t − d43+SDS,in × 100
d43+SDS,in
here d43+SDS and d43−SDS are the volume weight distributionf the emulsion droplets in the presence and absence of 1% SDS,espectively. d43+SDS,in is the initial volume weight distributionf the emulsion droplets in the presence of 1% SDS; d43+SDS,t
![Page 3: Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in food industry due to its low cost and available equipment [15]. The process involves](https://reader036.fdocuments.net/reader036/viewer/2022063015/5fd242aa7539492f3d4f595c/html5/thumbnails/3.jpg)
nce a
ip
2dpComiζ
a
2
swm8pTco
2miItNwsfluw
S2fhLmlrc
E
w
2utLdm
ewa
2amtw41
2
dusc
3
3
3
wphamstsfadflwssdidirtrsfi
ds
S. Takeungwongtrakul et al. / Food Scie
s the volume weight distribution of the emulsion droplets in theresence of 1% SDS at the designated storage time.
.4.1.3. ζ-Potential. The electrical charge (ζ-potential) of oilroplets in the emulsions was determined using a ZetaPlus zetaotential analyzer (Model ZetaPALS, Brookhaven Instruments,o., Holtsville, NY, USA) at room temperature. The shrimpil-in-water emulsions were diluted 250-fold prior to measure-ents. The diluted emulsions were mixed thoroughly and then
njected into the measurement chamber of the instrument. The-potential of each individual sample was calculated from theverage of five measurements on the diluted emulsion.
.4.2. Preparation of micro-encapsulated oilEmulsions were subjected to drying using a laboratory scale
pray-dryer (LabPlant Ltd., LabPlant SD-05, Huddersfield, UK)ith a 1.5 mm diameter nozzle. The emulsion was fed into theain chamber through a peristaltic pump. Feed flow rate was
.08 mL/min; drying air flow rate was 4.3 m/s and compressor airressure was 0.28 MPa. Air inlet temperature was 180 ± 2 ◦C.he outlet temperature was controlled at 90 ± 2 ◦C. Moistureontents of all samples were in the range of 2.92%–3.28%. Thebtained powder was determined as follows:
.4.2.1. Encapsulation efficiency (EE). The surface oil waseasured by adding 15 mL of hexane to 2 g of powder and shak-
ng with a vortex mixer (G-560E, Vortex-Genie 2, Scientificndustries, Inc., Bohemia, NY) for 2 min at room tempera-ure. The solvent mixture was then filtered through a Whatmano. 1 filter paper and the collected powder on the filter paperas rinsed three times with 20 mL of hexane [19]. The filtrate
olution containing the extracted oil was transferred to a cleanask, which was left to evaporate and then was dried at 60 ◦Cntil constant weight was obtained. The surface oil (SO) contentas calculated based on the extracted oil [20].The total oil was determined using the method described by
hahidi and Wanasundara [21]. 5 g of powder was dissolved in5 mL of a 0.88% (w/v) KCl solution. Then 50 mL of chloro-orm and 25 mL of methanol were added. The mixture was thenomogenized using a high-speed mixer (Model T25 basic, IKAabortechnik, Selangor, Malaysia) for 5 min at 9500 rpm. Theixture was transferred to a separation funnel; the chloroform
ayer was separated and then evaporated using a rotary evapo-ator at 60 ◦C to recover the oil. Total oil (TO) content was thenalculated.
EE was calculated as follows:
E =(
TO − SO
TO
)× 100
here TO is the total oil content and SO is the surface oil content.
.4.2.2. Powder size. Powder size distribution was measuredsing a laser light diffraction instrument (Laser Scattering Spec-
rometer Mastersizer model MAM 5005, Malvern Instrumentstd., Worcestershire, United Kingdom). The powder sample wasispersed in 99.5% ethanol and the particle distribution wasonitored during five successive readings. The particle size wasbwet
nd Human Wellness 3 (2014) 175–182 177
xpressed as the volume-weighted mean particle diameter (d43),hich is the mean diameter of a sphere with the same volume,
nd is generally used to characterize a particle [22].
.4.2.3. Powder morphology. Powder morphology was evalu-ted by a scanning electron microscopy (SEM). Powder wasounted on a bronze stub and sputter-coated with gold (Sput-
er coater SPI-Module, West Chester, PA, USA). The specimenas observed using a scanning electron microscope (Quanta00, FEI, Eindhoven, Netherlands) at an acceleration voltage of5 kV with magnifications of 3000×.
.5. Statistical analysis
All experiments were run in triplicate. All analyses were con-ucted in five replications. Statistical analysis was performedsing one-way analysis of variance (ANOVA). Mean compari-on was carried out using Duncan’s multiple range test. For pairomparison, T-test was used [23].
. Results and discussion
.1. Characteristics of shrimp oil-in-water emulsion
.1.1. Emulsion droplet size.Particle size of shrimp oil droplet in emulsions containing
hey protein concentrate and sodium caseinate (1:1, w/w) pre-ared with different ratios of core/wall material (1:2 and 1:4) andomogenizing pressure levels (13.79 and 27.58 MPa) expresseds the surface-weighted mean (d32) and the volume-weightedean particle diameter (d43) was monitored during 14 days of
torage at room temperature (Table 1). The d32 is directly relatedo specific surface area. The smaller d32 contributes to the higherpecific surface area, which offers the increase in protein loadsor adsorbing at interface of emulsions [24]. d43 can be useds the index of coalescence and flocculation. The increase in43 reflects the association of individual droplets into largerocs [24]. The d32 is more influenced by the small particles,hereas d43 is highly influenced by larger ones [25]. The emul-
ion made with core/wall material ratio of 1:2 and 1:4 with theame homogenizing pressure had no differences in the d32 and43 of emulsions at 0 day of storage. During the storage, thencreases in d32 and d43 were noticeable in all samples up toay 14 (P < 0.05), suggesting the aggregation of droplets. Dur-ng the storage time, the core/wall material ratio of 1:4 couldetard the increase in particle size in emulsion more effectivelyhan that of 1:2, regardless of homogenizing pressure used. Theesult suggested that the high amount of wall material must beufficient so that oil droplets are surrounded by thick and stronglm during emulsification.
With the same ratio of core/wall material, the decrease in both32 and d43 of droplets was obtained when homogenizing pres-ure increased (P < 0.05). This decrease in oil droplet size might
e related to the greater turbulence and shear forces associatedith increased homogenizing pressure applied [26]. Homog-nization includes two steps: firstly high shear stress leadso droplet deformation which increases their specific surface
![Page 4: Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in food industry due to its low cost and available equipment [15]. The process involves](https://reader036.fdocuments.net/reader036/viewer/2022063015/5fd242aa7539492f3d4f595c/html5/thumbnails/4.jpg)
178 S. Takeungwongtrakul et al. / Food Science and Human Wellness 3 (2014) 175–182
Table 1Particle size of droplets in shrimp oil emulsions containing whey protein and sodium caseinate with different core/wall material ratios and homogenizing pressuresduring the storage.
Core/wall material ratio (w/w) Pressure used (MPa) Storage time (day) d32 (nm) d43 (nm)
1:2 13.79 0 169.12 ± 0.51Ad 188.64 ± 1.02Ad
1 171.64 ± 0.32Ac 192.86 ± 0.22Ac
7 239.02 ± 1.18Ab 269.47 ± 1.37Ab
14 334.00 ± 2.42Aa 377.84 ± 2.12Aa
27.58 0 167.73 ± 0.54Bd 186.00 ± 0.57Bd
1 171.84 ± 0.70Ac 191.76 ± 0.34Bc
7 222.29 ± 0.62Bb 247.81 ± 1.24Bb
14 293.42 ± 2.24Ba 328.26 ± 2.63Ba
1:4 13.79 0 168.20 ± 0.57Ac 188.54 ± 1.45Ad
1 170.16 ± 2.55Ac 190.49 ± 0.37Ac
7 198.61 ± 0.56Ab 223.06 ± 0.87Ab
14 262.54 ± 1.43Aa 295.85 ± 1.76Aa
27.58 0 167.09 ± 0.34Bd 185.19 ± 1.34Bd
1 170.73 ± 1.54Ac 189.76 ± 0.25Bc
7 193.48 ± 1.24Bb 216.75 ± 1.11Bb
14 244.35 ± 2.45Ba 273.57 ± 2.68Ba
Data are expressed as mean ± SD (n = 5).L ateriaU ll ma
aecpasiti[e[st[hsswe
3
oiDeCbgCtci
p1AeTe
cpuWSeose1awm
3
cidtohr
owercase letters in the same column within the same pressure and core/wall mppercase letters in the same column within the same storage time and core/wa
rea up to disruption. Then the new interface is stabilized bymulsifiers [27]. This process causes the modification of proteinonformation, particularly globular protein. Those proteins oreptides with more exposed hydrophobic domains likely adsorbst increasing droplet interface more effectively. Adsorption ofodium caseinate and whey protein concentrate surroundingnterfacial oil droplet provided steric hindrance [28]. The reduc-ion of emulsion droplets size, which generally represents anncreased stability, results in greater retention of core material29,30]. Emulsion droplet size has a pronounced effect on thencapsulation efficiency of core materials during spray drying31]. The micro-encapsulation of emulsion with small dropletize confers the advantages in terms of emulsion stability, reten-ion of oil in the dried powder and less extractable surface oil32]. During the storage, d32 and d43 of emulsion prepared usingigher pressure (27.58 MPa) had the slower rate of increase inize, compared with emulsion prepared using the lower pres-ure (13.79 MPa). The results indicated that emulsion preparedith higher homogenizing pressure was more stable during the
xtended storage time.
.1.2. Flocculation and coalescenceFlocculation factor (Ff) and coalescence index (Ci) of shrimp
il-in-water emulsion prepared under different conditions dur-ng 14 days of storage at room temperature are shown in Table 2.uring storage, all emulsion samples had the increase in Ff, how-
ver the rate of change varied. Similar results were found fori. Emulsion underwent flocculation when the repulsive forcesetween the drops were not too strong and if adhesion ener-ies were large enough, the adhesion could be promoted [33].
oalescence occurs when two or more oil droplets approachogether and join together to form a larger one after the interfa-ial membrane is ruptured. The process is irreversible and resultsn the instability of emulsion [34]. With the same homogenizing
tdcp
l ratio indicate significant difference (P < 0.05).terial ratio indicate significant difference (P < 0.05).
ressure, the emulsion with core/wall material ratio of 1:2 and:4 had the similar Ff and Ci at day 0 and 1 of storage (P > 0.05).fter the first day of storage, Ff and Ci increased (P < 0.05),
specially for emulsion with the core/wall material ratio of 1:2.he result indicated that the amount of wall material must benough to stabilize the oil droplets in emulsion.
When comparing Ff and Ci of emulsion with the sameore/wall material ratio subjected to different homogenizingressures, no differences in Ff and Ci were found in emulsionsing 13.79 and 27.58 MPa at 0 and 1 day of storage (P > 0.05).ith the increasing storage time, Ff and Ci increased (P < 0.05).
uch changes were more pronounced in emulsion with homog-nizing pressure of 13.79 MPa and the core/wall material ratiof 1:2. The result was related well with droplet size of emul-ion (Table 1), showing the highest change in d32 and d43 inmulsion with homogenizing pressure of 13.79 MPa with the:2 of core/wall material ratio during the storage. Based on Ffnd Ci, emulsion with the highest stability could be preparedhen homogenizing pressure was 27.58 MPa and the core/wallaterial ratio was 1:4.
.1.3. ζ-Potentialζ-Potential of shrimp oil-in-water emulsions as affected by
ore/wall material ratio and homogenizing pressures is shownn Table 2. Zeta-potential is the potential difference between theispersion medium and the stationary layer of fluid attached tohe dispersed droplet. This value can be related to the stabilityf emulsion [14]. All emulsion samples had ζ-potential valuesigher than −45 mV at 0 day of storage. Negatively chargedesidues on oil droplet governed by wall material surrounding
he shrimp oil droplets mostly contributed to repulsion betweenroplets, thereby lowering coalescence. At day 0 and 1, nohange in ζ-potential was observed (P > 0.05). Subsequently, ζ-otential of all samples decreased, especially emulsions with the![Page 5: Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in food industry due to its low cost and available equipment [15]. The process involves](https://reader036.fdocuments.net/reader036/viewer/2022063015/5fd242aa7539492f3d4f595c/html5/thumbnails/5.jpg)
S. Takeungwongtrakul et al. / Food Science and Human Wellness 3 (2014) 175–182 179
Table 2Flocculation, coalescence and ζ-potential of droplets in shrimp oil emulsions containing whey protein and sodium caseinate with different core/wall material ratiosand homogenizing pressures during the storage.
Core/wall material ratio (w/w) Pressure used (MPa) Storage time (day) Flocculation factor (Ff) Coalescence index (Ci) ζ-Potential (mV)
1:2 13.79 0 0.98 ± 0.05Ac – −46.76 ± 0.75Ac
1 1.00 ± 0.04Ac 2.24 ± 0.31Ac −46.71 ± 1.21Ac
7 1.37 ± 0.02Ab 42.85 ± 2.42Ab −37.64 ± 2.11Ab
14 1.93 ± 0.10Aa 100.30 ± 3.46Aa −31.26 ± 1.07Aa
27.58 0 0.84 ± 0.10Ac – −46.84 ± 1.14Ac
1 0.87 ± 0.09Ac 2.89 ± 0.31Ac −46.56 ± 0.73Ac
7 1.07 ± 0.02Bb 33.23 ± 2.42Bb −37.15 ± 0.54Ab
14 1.45 ± 0.10Ba 76.49 ± 3.46Ba −31.01 ± 0.89Aa
1:4 13.79 0 0.96 ± 0.05Ac – −46.67 ± 0.69Ac
1 0.97 ± 0.05Ac 2.03 ± 0.71Ac −46.27 ± 0.93Ac
7 1.11 ± 0.03Ab 18.30 ± 2.52Ab −44.66 ± 0.85Ab
14 1.45 ± 0.03Aa 56.90 ± 3.23Aa −38.15 ± 0.61Aa
27.58 0 0.92 ± 0.06Ab – −47.83 ± 1.78Ac
1 0.92 ± 0.02Ab 2.47 ± 0.64Ac −46.32 ± 1.00Ac
7 0.94 ± 0.04Bb 17.04 ± 1.55Ab −43.41 ± 1.19Ab
14 1.15 ± 0.02Ba 47.72 ± 2.21Ba −37.94 ± 1.32Aa
Data are expressed as mean ± SD (n = 5).L ateriaU ll ma
cdtToatrElatetTyos[hooba
3
jw
3
f
sbmftmcirfptedTwtmhhiolfp
rEcEwresult indicated that the emulsification condition (core/wall
owercase letters in the same column within the same pressure and core/wall mppercase letters in the same column within the same storage time and core/wa
ore/wall material ratio of 1:2. The layers of protein surroundingroplets might undergo aggregation via ionic interaction duringhe extended storage as indicated by the change in zeta potential.he insufficient electrostatic repulsion might lead to the devel-pment of flocculation and coalescence. Emulsions exhibitingbsolute ζ-potential higher than +30 mV or lower than −30 mVend to be electrostatically stable, whilst emulsions within theange of −30 to 30 mV tend to coagulate or flocculate [35].mulsion with core/wall material ratio of 1:2 had low abso-
ute ζ-potential value, regardless of homogenizing pressure. As result, there was no enough force to prevent the moleculeso align together. During the storage, change in ζ-potential ofmulsion with the core/wall material ratio of 1:2 was higherhan that of 1:4 for both homogenizing pressure levels used.he results suggested that the core/wall material ratio of 1:4ielded the emulsion with higher stability. In general, high valuef ζ-potential means better stability because of the mutual repul-ion between the electrical double layers of macromolecules36]. When comparing ζ-potential of all samples with bothomogenizing pressure levels, no differences in ζ-potential werebserved at both core/wall material ratios (P > 0.05). Particle sizef the resulting emulsions and emulsion stability were governedy zeta potential surrounding droplets, which was more likelyssociated with the charge of protein films at interface.
.2. Characteristics of micro-encapsulated shrimp oil
Emulsions with different processing conditions were sub-ected to spray drying. The powder containing oil as the coreas characterized.
.2.1. Encapsulation efficiency (EE)EE of micro-encapsulated shrimp oil prepared using the dif-
erent core/wall material ratios and homogenizing pressures is
mip
l ratio indicate significant difference (P < 0.05).terial ratio indicate significant difference (P < 0.05).
hown in Table 3. EE reflects the degree of protection affordedy the wall material to oil droplets [26]. EE varied from a mini-um value of 14.65% to a maximum value of 52.05%. EE values
rom 0% to 95% were reported [37–40]. This was dependent onhe type and composition of wall material, the ratio of core/wallaterial, the drying process used, and the stability and physico-
hemical properties of the emulsions. The highest EE was foundn the sample prepared from emulsion with core/wall materialatio of 1:4 and homogenizing pressure of 27.58 MPa. The sur-ace oil was lower with coincidental increase in EE in samplerepared from emulsion with core/wall material ratio of 1:4 thanhose of 1:2. This result was in agreement with Rodea-Gonzálezt al. [9] who reported that EE was increased and surface oilecreased when core/wall material increased from 1:2 to 1:3.he core material is embedded as micro-particles within theall material. As the ratio of core/wall material is increased,
he ability of the wall material to retain and protect the coreaterial is increased [41], resulting in a lower surface oil and
igher EE. The surface oil represents non-encapsulated oil andas been used as an important parameter determining the qual-ty of encapsulated products. Non-encapsulated oil is prone toxidation, thus leading to the development of off-flavour andower acceptability of the product [42]. Furthermore, the sur-ace oil increases the wettability but lowers dispersibility of theowders [43].
The powder made from the emulsion having a core/wall mate-ial 13.79 and 27.58 MPa showed the similar surface oil andE (P > 0.05). For the powder prepared from emulsion withore/wall material ratio of 1:4, the lower surface oil and higherE was noticeable when homogenizing pressure of 27.58 MPaas implemented, compared with those of 13.79 MPa. This
aterial ratio and homogenizing pressure) had the markednfluence on encapsulation as well as the characteristic ofowder.
![Page 6: Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in food industry due to its low cost and available equipment [15]. The process involves](https://reader036.fdocuments.net/reader036/viewer/2022063015/5fd242aa7539492f3d4f595c/html5/thumbnails/6.jpg)
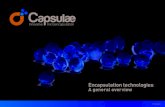
180 S. Takeungwongtrakul et al. / Food Science and Human Wellness 3 (2014) 175–182
F ulsiow nifica
3
wpreiHccsepda
harfiaaasccu
TPs
C
1
1
DLU
ig. 1. Surface morphology of micro-encapsulated shrimp oil prepared from em/w) with different core/wall material ratios and homogenizing pressures (mag
.2.2. Powder particle sizePowder particle size of the encapsulated shrimp oil produced
ith the different core/wall material ratios and homogenizingressures is shown in Table 3. Powders had the average diameteranging from 9.14 to 10.18 �m, except the powder prepared frommulsion having core/wall material ratio of 1:2 and homogeniz-ng pressure of 13.79 MPa, which had the diameter of 13.05 �m.omogenizing pressure had no effect on size of powder when
ore/wall material ratio of 1:4 was used. The result indicated thatore/wall material ratio of 1:4 was sufficient to align themselvesurrounding shrimp oil droplets prior to spray drying. How-ver, there was difference in EE between both homogenizing
ressures. Thus, higher pressure more likely dissociated the oilroplets, in which wall material could occupy rapidly and acteds wall of droplets. This was evidenced by high EE when high3
e
able 3owder size, surface oil, total oil and encapsulation efficiency of different encapsulaodium caseinate with different core/wall material ratios and homogenizing pressures
ore/wall material ratio (w/w) Pressure used (MPa) Powder size,
:2 13.79 13.05 ± 1.1227.58 9.74 ± 0.32
:4 13.79 9.55 ± 0.2727.58 9.66 ± 0.52
ata are expressed as mean ± SD (n = 3).owercase letters in the same column within the same core/wall material ratio indicappercase letters in the same column within the same pressure indicate significant di
n containing a mixture of whey protein concentrate and sodium caseinate (1:1,tion: 3000×). F = fused together.
omogenizing pressure (27.58 MPa) was used. Finney et al. [44]nd Jafari et al. [29] reported that particle size had no impact onetention of some volatile and oil, as solid in emulsion, was suf-ciently high. When comparing the size of shrimp oil emulsionsnd powder, an increase in powder particle size was observedfter spray drying process. The increased powder size partlyrose from coalescence of oil droplet in emulsion during thepray drying process [45]. Additionally, the wall generated alsoontributed to the increased diameter. The diameter of micro-apsules depends on homogenization parameters, the materialssed and conditions of the spray drying process [46].
.2.3. Powder morphologySEM microphotographs of the powders produced from
mulsion with the different core/wall material ratios and
ted shrimp oil powders prepared from emulsions containing whey protein and.
d4,3 (�m) Surface oil (w/w%) Encapsulation efficiency (%)
aA 23.86 ± 0.47aA 14.65 ± 1.38aB
bA 23.51 ± 1.53aA 14.96 ± 0.39aB
aB 12.42 ± 0.28aB 31.50 ± 1.81bA
aA 9.01 ± 1.23bB 52.05 ± 0.71aA
te significant difference (P < 0.05).fference (P < 0.05).
![Page 7: Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in food industry due to its low cost and available equipment [15]. The process involves](https://reader036.fdocuments.net/reader036/viewer/2022063015/5fd242aa7539492f3d4f595c/html5/thumbnails/7.jpg)
nce a
heeepdccloaaeca[otaidvic
4
wChHsodmectwscsf
A
PTTU
R
[
[
[
[
[
[
[
[
[
[
[
[
[
[
S. Takeungwongtrakul et al. / Food Scie
omogenizing pressures are shown in Fig. 1. All powdersxhibited similar surface morphologies. Powders prepared frommulsions with core/wall material ratio of 1:2 using both homog-nizing pressure levels appeared to be agglomerated. Clumps ofarticles were observed (Fig. 1A and B). This was plausiblyue to high surface oil levels (Table 3), which made the parti-les stick together. For powders prepared from emulsion withore/wall material ratio of 1:4 with both homogenizing pressureevels (Fig. 1C and D), a higher level of surface indentation wasbtained, compared with those prepared from emulsion with
core/wall material ratio of 1:2. The different morphologiesnd surface irregularities were governed by composition of themulsion, droplet size, and temperature during the drying pro-ess [47]. Wrinkles or dimples on the surface were observed inll powders. These results are consistent with Klinkesorn et al.40] and Ahn et al. [48], who detected wrinkles on the surfacef the particles in spray-dried powder. Wrinkles were attributedo the results of mechanical stresses induced by uneven dryingt different parts of the droplets during the early stages of dry-ng [49], to the movement of the moisture during the surfacerying period [50], and to the effect of a surface tension-driveniscous flow [49]. Wrinkles of the particle followed by an incip-ent expansion may induce changes in the size of particles andauses the broken wall material [51].
. Conclusion
Shrimp oil was encapsulated from emulsion stabilized byhey protein concentrate and sodium caseinate (1:1, w/w).ore/wall material ratio and homogenizing pressure directlyad the impact on emulsion and resulting encapsulated powder.igher homogenizing pressure reduced droplet size of emul-
ion. Emulsification at 27.58 MPa with a core/wall material ratiof 1:4 yielded the emulsion with the highest stability during 14ays of storage. After spray drying, emulsion with high core/wallaterial ratio and homogenizing pressure rendered the micro-
ncapsulated shrimp oil powder with higher EE. Thus, shrimp oilould be encapsulated using a mixture of whey protein concen-rate and sodium caseinate (1:1, w/w) as encapsulating agentsith core/wall material ratio of 1:4 and homogenizing pres-
ure level of 27.58 MPa, followed by spray-drying. However, theonditions used in the present study still showed the low encap-ulation efficiency, the improvement of encapsulation processor shrimp oil is still required.
cknowledgements
This work was supported by the Higher Education Researchromotion and National Research University Project ofhailand, Office of the Higher Education Commission. TheRF Senior Research scholar program and Prince of Songklaniversity were also acknowledged.
eferences
[1] S. Takeungwongtrakul, S. Benjakul, A. H-Kittikun, Lipids fromcephalothorax and hepatopancreas of Pacific white shrimp (Litopenaeus
[
nd Human Wellness 3 (2014) 175–182 181
vannamei): compositions and deterioration as affected by iced storage,Food Chem. 134 (2012) 2066–2074.
[2] W. Kolanowski, D. Jaworska, J. Weißbrodt, Importance of instrumental andsensory analysis in the assessment of oxidative deterioration of omega-3long-chain polyunsaturated fatty acid-rich foods, J. Sci. Food Agric. 87(2007) 181–191.
[3] M.D. Guillén, A. Ruiz, Oxidation process of oils with high content oflinoleic acyl groups and formation of toxic hydroperoxy- and hydroxyalke-nals. A study by 1H nuclear magnetic resonance, J. Sci. Food Agric. 85(2005) 2413–2420.
[4] R.V. Tonon, C.R.F. Grosso, M.D. Hubinger, Influence of emulsion compo-sition and inlet air temperature on the microencapsulation of flaxseed oilby spray drying, Food Res. Int. 44 (2011) 282–289.
[5] G. Gallardo, L. Guida, V. Martinez, et al., Microencapsulation of linseedoil by spray drying for functional food application, Food Res. Int. 52 (2013)473–482.
[6] A. Goula, K. Adamopoulos, A method for pomegranate seed application infood industries: seed oil encapsulation, Food Bioprod. Process. 90 (2012)639–652.
[7] M.K. Keogh, B.T. O’ Kennedy, Milk fat microencapsulation using wheyproteins, Int. Dairy J. 9 (1999) 657–663.
[8] H. Singh, X.Q. Zhu, A. Ye, Lipid encapsulation, US Patent ApplicationEP1,876,905 A1 (2008).
[9] D.A. Rodea-González, J. Cruz-Olivares, A. Román-Guerrero, et al., Spray-dried encapsulation of chia essential oil (Salvia hispanica L.) in wheyprotein concentrate–polysaccharide matrices, J. Food Eng. 111 (2012)102–109.
10] S. Schultz, G. Wagner, K. Urban, et al., High-pressure homogenizationas a process for emulsion formation, Chem. Eng. Technol. 27 (2004)361–368.
11] W. Ding, N.P. Shah, Effect of homogenization techniques on reducing thesize of microcapsules and the survival of probiotic bacteria therein, J. FoodSci. 74 (2009) M231–M236.
12] J. Perrier-Cornet, P. Marie, P. Gervais, Comparison of emulsification effi-ciency of protein-stabilized oil-in-water emulsions using jet, high pressureand colloid mill homogenization, J. Food Eng. 66 (2005) 211–217.
13] S.H. Lee, T. Lefèvre, M. Subirade, et al., Effects of ultra-high pressurehomogenization on the properties and structure of interfacial protein layerin whey protein-stabilized emulsion, Food Chem. 113 (2009) 191–195.
14] B. Wang, D. Li, L.J. Wang, et al., Effect of concentrated flaxseed protein onthe stability and rheological properties of soybean oil-in-water emulsions,J. Food Eng. 96 (2010) 555–561.
15] A. Gharsallaoui, G. Roudaut, O. Chambin, et al., Applications of spray-drying in microencapsulation of food ingredients: an overview, Food Res.Int. 40 (2007) 1107–1121.
16] P. Calvo, Á.L. Castano, M. Lozano, et al., Influence of the microencap-sulation on the quality parameters and shelf-life of extra-virgin olive oilencapsulated in the presence of BHT and different capsule wall compo-nents, Food Res. Int. 45 (2012) 256–261.
17] N. Sachindra, N. Bhaskar, N. Mahendrakar, Recovery of carotenoids fromshrimp waste in organic solvents, Waste Manage. 26 (2006) 1092–1098.
18] G.G. Palazolo, P.A. Sobral, J.R. Wagner, Freeze–thaw stability of oil-in-water emulsions prepared with native and thermally-denatured soybeanisolates, Food Hydrocoll. 25 (2011) 398–409.
19] E. Bae, S. Lee, Microencapsulation of avocado oil by spray drying usingwhey protein and maltodextrin, J. Microencapsul. 25 (2008) 549–560.
20] S.M. Jafari, E. Assadpoor, Y. He, et al., Encapsulation efficiency of foodflavours and oils during spray drying, Dry. Technol. 26 (2008) 816–835.
21] F. Shahidi, U.N. Wanasundara, Oxidative stability of encapsulated sealblubber oil ACS Symposium Series, ACS Publications, 1995, pp. 139–153.
22] E.C. Frascareli, V.M. Silva, R.V. Tonon, et al., Effect of process conditionson the microencapsulation of coffee oil by spray drying, Food Bioprod.Process. 90 (2012) 413–424.
23] R.G.D. Steel, J.H. Torrie, Principles and Procedures of Statistics, vol. 8,
McGraw-Hill Book Co., Inc., NY, 1960, pp. 6–8.24] E. Hebishy, M. Buffa, B. Guamis, et al., Stability of sub-micron oil-in-wateremulsions produced by ultra high pressure homogenization and sodiumcaseinate as emulsifier, Chem. Eng. 32 (2013) 1813–1818.
![Page 8: Micro-encapsulation of Pacific white shrimp oil as ... · micro-encapsulation technology used in food industry due to its low cost and available equipment [15]. The process involves](https://reader036.fdocuments.net/reader036/viewer/2022063015/5fd242aa7539492f3d4f595c/html5/thumbnails/8.jpg)
1 nce a
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
[
82 S. Takeungwongtrakul et al. / Food Scie
25] H. Bengtsson, E. Tornberg, Physicochemical characterization of fruit andvegetable fiber suspensions. I: Effect of homogenization, J. Texture Stud.42 (2011) 268–280.
26] S.A. Hogan, B.F. McNamee, E.D. O’Riordan, et al., Microencapsulat-ing properties of sodium caseinate, J. Agric. Food Chem. 49 (2001)1934–1938.
27] J. Floury, A. Desrumaux, J. Lardieres, Effect of high-pressure homog-enization on droplet size distributions and rheological properties ofmodel oil-in-water emulsions, Innov. Food Sci. Emerg. Technol. 1 (2000)127–134.
28] C.C. Sánchez, J.M.R Patino, Interfacial, foaming and emulsifying char-acteristics of sodium caseinate as influenced by protein concentration insolution, Food Hydrocoll. 19 (2005) 407–416.
29] S.M. Jafari, E. Assadpoor, B. Bhandari, et al., Nano-particle encapsulationof fish oil by spray drying, Food Res. Int. 41 (2008) 172–183.
30] A. Soottitantawat, H. Yoshii, T. Furuta, et al., Microencapsulation by spraydrying: influence of emulsion size on the retention of volatile compounds,J. Food Sci. 68 (2003) 2256–2262.
31] A. Soottitantawat, F. Bigeard, H. Yoshii, et al., Influence of emulsion andpowder size on the stability of encapsulated d-limonene by spray drying,Innov. Food Sci. Emerg. Technol. 6 (2005) 107–114.
32] S.J. Risch, G.A. Reineccius, Spray-dried orange oil: effect of emulsionsize on flavor retention and shelf stability, Flavor Encapsul. 370 (1988)67–77.
33] D. Langevin, S. Poteau, I. Hénaut, et al., Crude oil emulsion propertiesand their application to heavy oil transportation, Oil Gas Sci. Technol. 59(2004) 511–521.
34] Z. Long, Q. Zhao, T. Liu, et al., Influence of xanthan gum on physical char-acteristics of sodium caseinate solutions and emulsions, Food Hydrocoll.32 (2012) 123–129.
35] Y. Wang, D. Li, L.J. Wang, et al., Effects of high pressure homogenizationon rheological properties of flaxseed gum, Carbohydr. Polym. 83 (2011)489–494.
36] J. Acedo-Carrillo, A. Rosas-Durazo, R. Herrera-Urbina, et al., Zeta poten-tial and drop growth of oil in water emulsions stabilized with mesquite
gum, Carbohydr. Polym. 65 (2006) 327–336.37] M.Y. Baik, E. Suhendro, W. Nawar, et al., Effects of antioxidants andhumidity on the oxidative stability of microencapsulated fish oil, J. Am.Oil Chem. Soc. 81 (2004) 355–360.
[
nd Human Wellness 3 (2014) 175–182
38] N. Hardas, S. Danviriyakul, J. Foley, et al., Accelerated stability studiesof microencapsulated anhydrous milk fat, LWT – Food Sci. Technol. 33(2000) 506–513.
39] T.C. Kha, M.H. Nguyen, P.D. Roach, Effects of spray drying conditionson the physicochemical and antioxidant properties of the Gac (Momordicacochinchinensis) fruit aril powder, J. Food Eng. 98 (2010) 385–392.
40] U. Klinkesorn, P. Sophanodora, P. Chinachoti, et al., Characterization ofspray-dried tuna oil emulsified in two-layered interfacial membranes pre-pared using electrostatic layer-by-layer deposition, Food Res. Int. 39 (2006)449–457.
41] M. Hojjati, S.H. Razavi, K. Rezaei, et al., Spray drying microencapsulationof natural canthaxantin using soluble soybean polysaccharide as a carrier,Food Sci. Biotechnol. 20 (2011) 63–69.
42] S. Drusch, S. Berg, Extractable oil in microcapsules prepared by spray-drying: localisation, determination and impact on oxidative stability, FoodChem. 109 (2008) 17–24.
43] C. Vega, E.H.J. Kim, X.D. Chen, et al., Solid-state characterization ofspray-dried ice cream mixes, Colloids Surf. B 45 (2005) 66–75.
44] J. Finney, R. Buffo, G. Reineccius, Effects of type of atomization andprocessing temperatures on the physical properties and stability of spray-dried flavors, J. Food Sci. 67 (2002) 1108–1114.
45] S. Drusch, Y. Serfert, M. Scampicchio, et al., Impact of physicochemicalcharacteristics on the oxidative stability of fish oil microencapsulated byspray-drying, J. Agric. Food Chem. 55 (2007) 11044–11051.
46] W. Kolanowski, M. Ziolkowski, J. Weißbrodt, et al., Microencapsulationof fish oil by spray drying – impact on oxidative stability. Part 1, Eur. FoodRes. Technol. 222 (2006) 336–342.
47] C.S. Handscomb, M. Kraft, Simulating the structural evolution of dropletsfollowing shell formation, Chem. Eng. Sci. 65 (2010) 713–725.
48] J.H. Ahn, Y.P. Kim, E.M. Seo, et al., Antioxidant effect of natural plantextracts on the microencapsulated high oleic sunflower oil, J. Food Eng.84 (2008) 327–334.
49] T. Sheu, M. Rosenberg, Microstructure of microcapsules consisting ofwhey proteins and carbohydrates, J. Food Sci. 63 (1998) 491–494.
50] D. Walton, The morphology of spray-dried particles a qualitative view, Dry.
Technol. 18 (2000) 1943–1986.51] L. Alamilla-Beltrán, J. Chanona-Pérez, A. Jiménez-Aparicio, et al.,Description of morphological changes of particles along spray drying, J.Food Eng. 67 (2005) 179–184.