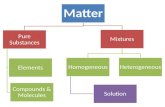
Lab 4 Notes Solutions. are homogeneous mixtures of two or more substances consist of a solvent...
-
Upload
neal-shields -
Category
Documents
-
view
219 -
download
0
Transcript of Lab 4 Notes Solutions. are homogeneous mixtures of two or more substances consist of a solvent...

Lab 4 Notes
Solutions

Solutions
• are homogeneous mixtures of two or more substances
• consist of a solvent (larger amount, usually a liquid) and solutes (smaller amount, usually a solid)
• solutes are uniformly dispersed within the solvent

Concentration of Solution - Molarity
Molarity M = moles/liter 1 M = 1 mole/L 1 mole of NaCl has the mass of
Na: 22.99 g/mole + Cl: 35.45 g/moleNaCl: 58.44 g/mole
How to make a solution of 1M NaCl: Mass out 58.44 g then dissolve in 1 L water

Concentration of Solutions - %
% (m/v) is another frequently used concentration measure
mass of solute__ x 100%
volume of solution

Types of Solution

Water
• is the most common solvent.
• is a polar molecule.
• forms hydrogen bonds with solutes

Diffusion
Passive movement of substances from an area of high concentration to an area of low concentration

Osmosis
Diffusion of water across a semipermeable membrane Water flows form from an area of lower
solute concentration (hypotonic) to area of higher solute concentration (hypertonic), assume the solute cannot move
Isotonic: both areas have the same concentration
Semipermeable small molecules can go through, large molecules cannot

Dialysis
Filtration of solutions
through a selectively permeable membrane. dialysis membranes let water and certain
solutes (those that have the right size) move across the membrane to equalize
Example: membranes of kidney let water, waste molecules, salt and sugar move across (sugar is reabsorbed!), but blood cells are too big