How important is tryptophan in human health?cognitive disorders, anxiety, or neurodegenerative...
Transcript of How important is tryptophan in human health?cognitive disorders, anxiety, or neurodegenerative...

See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/318541580
How important is tryptophan in human health?
Article in Critical Reviews in Food Science and Nutrition · February 2019
DOI: 10.1080/10408398.2017.1357534
CITATIONS
61READS
6,096
5 authors, including:
Some of the authors of this publication are also working on these related projects:
The Toxicology of Mercury: Current Research and Emerging Trends View project
Cancer and Nutrition View project
Joanna Kałużna-Czaplińska
Lodz University of Technology
77 PUBLICATIONS 991 CITATIONS
SEE PROFILE
Paulina Gątarek
Lodz University of Technology
12 PUBLICATIONS 139 CITATIONS
SEE PROFILE
Max Stanley Chartrand
DigiCare Research Foundation
176 PUBLICATIONS 766 CITATIONS
SEE PROFILE
Geir Bjorklund
Council for Nutritional and Environmental Medicine (CONEM)
296 PUBLICATIONS 4,366 CITATIONS
SEE PROFILE
All content following this page was uploaded by Max Stanley Chartrand on 07 September 2017.
The user has requested enhancement of the downloaded file.

How important is tryptophan in human health?
Joanna Ka»u_zna-Czapli�nskaa, Paulina Gatareka, Salvatore Chirumbolo b, Max Stanley Chartrandc,and Geir Bjørklund d
aDepartment of Chemistry, Institute of General and Ecological Chemistry, Lodz University of Technology, Lodz, Poland; bDepartment of Neurologicaland Movement Sciences, University of Verona, Italy; cDigiCare Behavioral Research, Casa Grande, AZ, USA; dCouncil for Nutritional and EnvironmentalMedicine, Mo i Rana, Norway
ABSTRACTTryptophan (Trp) is an amino acid and an essential component of the human diet. It plays a crucial role inmany metabolic functions. Clinicians can use Trp levels in the course of diagnosing various metabolicdisorders and the symptoms associated with those diseases. Furthermore, supplementation with thisamino acid is considered in the treatment of depression and sleep disorders, mainly due to the Trprelationship with the synthesis of serotonin (5-HT) and melatonin. It is also used in helping to resolvecognitive disorders, anxiety, or neurodegenerative diseases. Reduced secretion of serotonin is associatedwith autism spectrum disorder, obesity, anorexia and bulimia nervosa, and other diseases presentingperipherals symptoms. The literature strongly suggests that Trp has a significant role in the correctfunctionality of the brain-gut axis and immunology. This information leads to the consideration of Trp asan essential dietary component due to its role in the serotonin pathway. A reduced availability of Trp indiet and nutraceutical supplementation should be considered with greater concern than one mightexpect. This paper constitutes a review of the more salient aspects gleaned from the current knowledgebase about the role of Trp in diseases, associated nutritional disorders, and food science, in general.
KEYWORDSTryptophan; serotonin;metabolic disorder;neurodegenerative disease;food technology; human diet
Introduction
The role of the essential amino acid tryptophan (Trp) is gainingin interest relative to dietary and nutritional sciences. Recentresearch has demonstrated that this amino acid exerts a protec-tive action in the intestine, as it contributes to the enhancedexpression of the tight junction proteins claudin-3 and zonulaoccludens (ZO-1) in the jejunum of experimental animals (Liuet al., 2017). Its fundamental importance appears mostly in itsrelationship with serotonin, which is important in food-to-nutrition synthesis. Past research has suggested a direct connec-tion between serotonin production and the available circulatingTrp, recently proposed as a hallmark as a possible marker ofpsychiatric serotonin-related disorders (Comai et al., 2016).From a food technology viewpoint, the importance of Trp inhuman physiology would suggest recommendations and guide-lines by worldwide experts to supplement or enrich foods withTrp. Due to the complexity of the relationship of serotonin andmelatonin and circadian rhythms, it’s hard to ascertain or attri-bute the needed levels of Trp in a given diet (Hulsken et al.,2013; Silva et al., 2017).
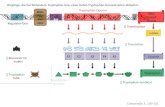
However, several researchers have suggested that Trp sup-plementation in a daily diet might improve pharmacotherapyin some diseases (Figure 1). Because of the tryptophan hascomparatively low tissue storage and their concentration in thebody is low, compared to other amino acids, for healthy nutri-tion are needed only small amounts (Richard et al., 2009).Some food products containing tryptophan are presented in
Table 1(Rambali et al., 2002; Richard et al., 2009; USDA FoodComposition Databases, 2017). The recommended daily dosefor adults is estimated to be between 250 mg and 425 mg, whichresults in a dietary intake of 3.5 to 6.0 mg/kg of body weight perday (Richard et al., 2009).
Tryptophan is an essential component of the diet. It plays akey role in protein synthesis, and is a precursor of biologicallyactive compounds such as serotonin, melatonin, quinolinicacid, kynurenic acid, tryptamine, and also coenzymes impor-tant for electron transfer reaction (redox balance of metabo-lism), such as nicotinamide adenine dinucleotide (NADC).This compound, which are final product tryptophan metabo-lism, might be produced from ingested tryptophan but alsovitamin B3 (niacin) (Richard et al., 2009; de Figueiredo et al.,2011; Palego et al., 2016). To people health is detrimental bothdeficiency and excessive intake. Tryptophan has been used totreat variety disorders, but in most countries has been with-drawn. During the treatment of tryptophan preparations havebeen observed undesirable symptoms including a variety ofpulmonary, cutaneous, and neurologic symptoms, and alsoeosinophilia-myalgia syndrome, and disease associated withmuscle pain. Many different diseases and disorders have beenlinked with tryptophan and its metabolites (Table 2). Anincreased metabolism of Trp, or adverse effects of low Trp suchas decreased absorption or intake, have been observed in differ-ent types of pathology. It should be mention disease and disor-ders such as premenstrual syndrome (PMS) (tryptophan plays
CONTACT Joanna Ka»u_zna-Czapli�nska [email protected] Department of Chemistry, Institute of General and Ecological Chemistry, Lodz Uni-versity of Technology, Zeromskiego 116, 90–924 Lodz, Poland.© 2017 Taylor & Francis Group, LLC
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITIONhttps://doi.org/10.1080/10408398.2017.1357534
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

a role in increased activation of Trp catabolism), pellagra(caused by a deficiency of niacin which precursor is Trp),chronic kidney disease (is observed alterations in Trp metabo-lism in the case of kynurenine pathway) (Karu et al., 2016), coe-liac disease (availability of Trp to the brain is low, especially insubjects with depression), Parkinson’s disease (large neutralamino acid compete with Trp), mental disorders (reducedavailability of Trp what is consequence is low level of serotonin)(Sainio, Pulkki, and Young, 1996; Russo et al., 2009), sleep dis-orders (abnormal level of melatonin, which is synthesize fromserotonin) (Kaczor and Skalski, 2016), schizophrenia (dysfunc-tional serotonin transmission), bulimia and anorexia (depletionof Trp) (Sainio, Pulkki, and Young, 1996; Russo et al., 2009).
It is well known that Trp is only available through the die-tary process, as its precursors allow gut microflora to synthesizethe essential amino acid in humans. Tryptophan can be metab-olized through the methoxyindole and kynurenine pathways.The kynurenine pathway, which takes up about 95% of the bio-logically available Trp, is controlled by the rate-limitingenzymes indoleamine 2,3-dioxygenase (IDO) and Trp 2,3-diox-ygenase (TDO).
Stress hormones and Trp induce TDO synthesis and activa-tion, while IDO can be induced by pro-inflammatory, inter-feron-gamma (IFN-g), tumor necrosis factor-alpha (TNF-a),and Th-1 type cytokines, i.e. during an innate response of theimmune system. IDO suppresses the activity causing the induc-tion of TDO, and vice versa, while the ratio of kynurenine
(products) to Trp (substrate) gives information about IDOactivity. Upregulation of the IDO activity caused by chronicinflammation of the immune system could be a major factor inthe initiation and propagation of obesity and associated meta-bolic syndrome (Mangge et al., 2014).
Tryptophan supplementation could promote synthesis andneurotransmission of serotonin. Moreover, it may be effectivein treating disorders of serotonin deficiency by increasing theprecursor for 5-HT synthesis and normalizing its release(Haleem, 2012). Levels of Trp, and hence its circulating bio-availability, does not seem to be directly linked to cognitionand mood improvements, as recent reports suggest that excessof Trp impairs cognition, rather than improving it (Hulskenet al., 2013). On the other side, a deficiency in Trp, caused bymalnutrition, may affect the central and peripheral serotoniner-gic pathways, although further nutrition-derived hormonalmolecules may rescue some of this deficiency (Patrick andAmes, 2014).
With serotonergic dysfunction have been associated withsymptoms of panic, depression, aggression and suicidality.Because the serotonin system is involved in the various psychi-atric disorders, but not only, serotonin system is also involvedin the regulation of satiety, it can be concluded that the activityof serotonin can be important in the pathophysiology of eatingdisorders such as anorexia nervosa. A fundamental concern fornutritionists and food technologists is to focus on the role ofTrp in neurological and immune disorders, to achieve a deepawareness and knowledge of the risks and potentials associatedwith the supplementation use of this amino acid. The aim ofthis paper is based on the currently existing and very recent lit-erature to present a more focused viewpoint relative to the roleof Trp in diseases associated with human nutritional disorders.
1. Tryptophan and irritable bowel syndrome
One of the most common alimentary tract illnesses in humansis irritable bowel syndrome (IBS). This disease also constitutesa significant social problem. IBS is a bowel function disorder.Pain associated with defecation and defecation frequency orstool consistency characterizes this disease. Still unclear is etio-pathology of the illness. Pathological factors include, amongothers, disturbances in the functions of serotonin at this level of
Figure 1. Tryptophan and different diseases.
Table 1. Tryptophan amount per 100 g in common foods.
Tryptophan (mg)
milk 42eggs 165wheat flour 110sausage 93potato 28chees 325beef 230banana 10soybeans 160bread, oat bran, toasted 140chia seeds, dried 440chicken, breast, skinless, boneless, meat only 400cocoa 290
2 J. KAºU _ZNA-CZAPLI�NSKA ET AL.
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

the digestive process ( _Zelowski et al., 2013). IBS is a functionalgastrointestinal disease, because these disorders arise from dys-function of the organ, excluding morphological changes withinit (Fitzgerald et al., 2008).
There are four clinical forms of this syndrome: IBS withconstipation (IBS-C), IBS with diarrhea (IBS-D), mixed IBS(IBS-M) and unsubtyped IBS (Longstreth et al., 2006).Throughout the world, about 10–20% of adolescents and adultsexhibit symptoms associated with IBS (Fitzgerald et al., 2008).Irritable bowel syndrome is more common in women than inmen. The disease develops most often in the third decade of life(Drossman et al., 2002). The factors causing this diseaseinclude, among others, environmental, genetic, depression,inflammatory predispositions, and chronic stress (Fitzgeraldet al., 2008; _Zelowski et al., 2013).
Fitzgerald et al. (2008) examined patients with IBS andhealthy, of comparable age and body mass index (BMI) andsex-matched controls. They observed the higher concentrationof kynurenine in the blood of patients with IBS in comparisonwith the control group and positive correlation between thekynurenine/tryptophan (Kyn/Trp) ratio and IBS symptomseverity (Fitzgerald et al., 2008).
Keszthelyi et al. (2012) demonstrated a relationship betweenthe amount of serotonin, synthesized in the brain, and theamount of Trp supplied to the body with diet. Their random-ized placebo-controlled study suggested that the sudden short-age of the precursor of serotonin obtained by administering anamino acid-enriched beverage lacking Trp to patients resultedin the dramatic reduction of the concentration of serotonin inthe blood, as well as, a decrease in the level of 5-hydroxyindole-acetic acid in urine. In contrast, there was no change in the con-centration of these compounds in the intestinal mucosa. Theresearchers concluded that 5-HT synthesis in the brain is highlydependent on the availability of Trp in plasma, which is influ-enced by the competitive uptake of other large neutral aminoacids (LNAAs) and Trp across the blood-brain barrier(Keszthelyi, 2012).
Other scientists measured serum serotonin concentration inindividuals with irritable bowel syndrome, compared with con-trol group, and also evaluated the urine concentration of 5-hydroxyindole acetic acid (5-HIAA), which is a metabolite ofserotonin. They examined participants aged 19–50 years,including healthy subjects, patients with predominant constipa-tion (IBS-C) and patients with predominant diarrhea (IBS-D).The results pointed to a reduction of serotonin concentrationin patients with IBS-C and IBS-D. In all patients with IBS, adecrease in urinary excretion of 5-hydroxyindole acetic acidwas observed. These results indicate that disturbed metabolismof serotonin could play a role in the pathogenesis of functionalbowel diseases (Moskwa et al., 2007).
Studies conducted by Atkinson et al. (2006), showed thatpatients with IBS-D (aged 19–52 years) have a higher concen-tration of serotonin in the blood than healthy patients, both inthe fasting and during the postprandial time. However, the dif-ferences were not detected as IBS with constipation (IBS-C,aged 19–52 years). Food intake significantly increased levels ofserotonin in the blood of patients with IBS with diarrhea, withrespect to healthy subjects (n D 35, aged 18–46 years). Theresults assessed the concept that an impaired release might
characterize IBS-C, whereas IBS-D is characterized by reducedserotonin reuptake (Atkinson et al., 2006).
Reports by Dunlop et al. (2005) indicated an increase in thepostprandial levels of serotonin in the blood of patients with adiarrhea form of IBS (IBS-D) and a significant reduction inpatients with constipation-predominant IBS (IBS-C), comparedto healthy controls (Dunlop et al., 2005). Dunlop et al. (2005)examined 15 patients with IBS-D, 15 patients with IBS-C and15 healthy control participants. This study compared postpran-dial serotonin release and mucosal serotonin metabolism invarious types of IBS. The results demonstrated that patientswith IBS-C showed impaired postprandial serotonin release.
Houghton et al. (2003) examined 39 female patients withIBS-D aged 19–52 years, and 20 healthy females aged 20–46 years. Obtained data suggested that postprandial symptom-atology could be connected with increased plasma serotoninconcentration in IBS-D patients (Houghton et al., 2003; Dunlopet al., 2005). Furthermore, sleep disorders are frequently associ-ated with women affected by IBS. In this circumstance, it hasbeen observed that a reduction in the early nighttime ratio ofmelatonin: Trp may be related to the altered sleep status in IBScases (Heitkemper et al., 2016).
Current therapy of IBS should involve Trp biology andmetabolism, either by improving diet panels, herbal therapyand/or using pharmacotherapeutic drugs able to prevent orreduce Trp catabolism and chemical degradation (Grundmann,Yoon and Moshiree, 2010; Catanzaro et al., 2014; Shi et al.,2015). At the same time, the controversial role of the excess ofTrp that has been reported in past studies on gut mucosa can-not yet be dismissed (Madara and Carlsso, 1991).
2. Obesity, overweight and tryptophan metabolism
In the last four decades, obesity has increased dramaticallythroughout the world. In 1980 the number of obese and over-weight people were 857 million, whereas by 2013 this numberhad increased to 2.1 billion (Youssef, 2015). Obesity is a verycomplex, multifactorial metabolic disorder, which is oftenrelated to an immune-mediated systemic inflammation of theadipose tissue and to insulin resistance and hyperlipoproteine-mia, where a major role is exerted by NF-kB (Catrysse and VanLoo, 2017). The basic determinants of obesity can be bothover-nutrition and lack of physical exercise. Simple reasoningon a diet should suggest that the excessive intake of food mighteven lead to an excess intake of Trp precursors and of food-derived Trp.
Furthermore, Trp is responsible for the calorie intake regula-tion (Mangge et al., 2014). Recent data suggests that obesity isassociated with altered Trp and tyrosine (Tyr) metabolism(Strasser, Berger, and Fuchs, 2015). As previously reported,these compounds also play a role in neuropsychiatric symp-toms (Andr�e et al., 2014). As the primary pathway of Trpmetabolism is the kynurenine pathway, and indoleamine-2,3-dioxygenase (IDO) is the first enzyme of the pathway, theseproinflammatory molecules that stimulate IDO may cause orexacerbate obesity (Andr�e et al., 2014; Mangge et al., 2014;Strasser, Berger, and Fuchs, 2015). Yet, apparently contradic-tory issues do exist, particularly regarding activity of Trp basedon its circulating levels in obese subjects, or in those
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 3
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

circumstances where metabolic syndrome may have conse-quences in other anatomic regions of the human body (Oxenk-rug, 2013; Mangge et al., 2014; Oxenkrug, 2015; Yu et al., 2017).
In overweight adults, Strasser, Berger, and Fuchs, 2015)investigated the effect of a two-week caloric restriction weightloss diet (CRWLD) on the circulating levels of leptin, on furtherinflammatory biomarkers and assessing the short-term dietaryeffects of Trp and inflammatory biomarkers in overweightadults (Strasser, Berger, and Fuchs, 2015), researchers found animpairment in the biosynthesis of serotonin from its naturalprecursor. This may be related to the increased susceptibilityfor mood disorders and carbohydrate craving observed in thestudy. Leptin is a hormonal peptide, produced by adipocytes,which is correlated to body fat homeostasis and satiety, as itcontributes in regulating food intake and energy balance butthe functionality of which is also strongly associated with theneurological activity (Zhou et al., 1997; Van Doorn et al.,2017). High levels of leptin characterize most of the patientswith obesity (L€onnqvist et al., 1995; Hundal et al., 2000; Savinoet al., 2013), suggesting the involvement of a peripheral andcentral resistance. Recent papers showed that a reduction ofbody weight has a dramatic impact on the circulating levels ofleptin (Klempel and Varady, 2011; Musil et al., 2015). In theresearch carried on by Strasser, Berger, and Fuchs (2015), thereduction of leptin concentrations in the circulation canimprove insulin sensitivity, blood pressure, and blood lipid lev-els. Concentrations of Trp and Kyn decreased significantly by15 and 17% for the low caloric diet (LCD) group and by 21 and16% for the very low-calorie diet (VLCD) group, while leptinwas reduced by 46% (Strasser, Berger, and Fuchs, 2015).
Reducing body weight by increasing metabolic activity andaccelerating the onset of satiety may involve a serotoninergic-driven mechanism. For this reason, it might be very useful inthe treatment of obesity to consider the supplementation ofTrp during caloric restriction diet (Yu et al., 2017). Also,increased availability of Trp can increase the production ofserotonin and reduce the symptoms of depression in peoplestruggling with overweight (Oh, Park, and Kim, 2016). Thus,Trp supplementation could prove very useful in the treatmentof uncontrolled weight gain or prevent neuropsychiatric symp-toms (Strasser, Berger, and Fuchs, 2015).
Despite the fact that weight loss in obese patients showed animprovement or prevention of changes in the ratio of theintake/bioavailable Trp and other signals related to obesity thatactivates the immune system and inflammation, in obesepatients with bariatric surgical intervention a reduction in thisratio or of immune markers was not found (Brandacher et al.,2007). Current literature reports several studies dealing withobesity and Trp metabolism, which are often related toimmune-mediated inflammation, with notorious differencesbetween juveniles and adults (Mangge et al., 2014; Reininghauset al., 2014; Raheya et al., 2015).
In a study from a group with Mangee et al. (2014), 527 par-ticipants aged between 10–65 years were analyzed. Resultsshowed that Kyn serum levels and Kyn/Trp ratio to over-weight/obese adults (age from 18, to 65 years), significantlyincreased in comparison to controls. Data for ow/ob juvenilemales (age �18 years) showed decreased Kyn/Trp ratio valuescompared to controls. Furthermore, juveniles fulfilling the
criteria of the metabolic syndrome exhibited constant Kyn/Trpratio and Kyn, whereas adults with MetS had significantlyincreased Kyn and Kyn/Trp ratio. Trp serum levels decreasedin adult ow/ob females but were not markedly different fromnormal weighted patients in the ow/ob groups or between ow/ob group with or without MetS (Mangge et al., 2014).
The results from these researchers suggested that Trpmetabolism and obesity vary significantly between juvenilesand adults. This indicates that early onset low-grade inflamma-tion, which can be found in obese adolescents, is different fromadults, and that juveniles are more likely to suffer from a pro-cess driven by a Th2-mediated response, contrarily to obeseadults, where a Th1 immune mechanism is prevalent. Thesefacts have potential clinical significance, because, first, conser-vative treatment of obesity through lifestyle changes includemore prevalent physical activity during childhood and adoles-cence, and can prevent the critical transition to more aggressiveimmunology before there is irreversible clinical damage. Sec-ond, a simple analytical determination of the concentration ofKyn/Trp may provide more reliable diagnostic evidence of thepresence of Th-1 proinflammatory markers regardless of age,particularly in obese patients (Mangge et al., 2014).
Furthermore, scientists have indicated that potential patho-genic links do exist between serotonin levels, chronic immuneactivation and in decreased IDO-mediated Trp in obesity (Ritzeet al., 2015; Ritze et al., 2016). Immune activation and systemicinflammation are associated with obesity comorbidity, while atthe same time it is connected with synthesized and releasedproinflammatory cytokines (like TNF-a, INF-g, hormones-leptin and others) in adipose tissue. IDO is inducible by IFN-g;and is also involved in the regulation of immune responses anddegraded Trp to form N-formyl kynurenine, which subse-quently can convert to niacin. Furthermore, IDO can reduceTrp plasma levels in morbidly obese patients. Serotonin pro-duction may be reduced by Trp metabolic changes, and thiscan in turn contribute to depression, mood disturbances, andimpaired satiety leading to increased caloric uptake and finallyobesity.
Obesity has shown to be associated with a reduced concen-tration of Trp in the plasma, independently from dietary intakeor weight reduction (Namkung et al., 2015; Zhang et al., 2015).As stated earlier, Trp is a precursor for the biosynthesis of 5-hydroxytryptamine (5HT, serotonin). Serotonin is a neuro-transmitter and biochemical regulator, which contributes tosatiety and hunger balance. Gustatory information during theact of eating is transmitted to the nucleus accumbent, which istypically considered the reward center. This leads to the releaseor the up-regulation of serotonin and opiates, which are calledthe “reward mediators”. Likewise, appetite-controlling neuronsare connected to specialized brain regions (Halford and Blun-dell, 2000).
Overeating and obesity are the results of diet including pal-atable food, where the time spent eating will be prolonged dueto suppressed satiety (Brandacher et al., 2007). In this context,serotonin as neurotransmitter may be involved in the controlof food intake, which is a satiety signal (Halford and Blundell,2000). Moreover, this process is responsible for the inhibitionof the expression of neuropeptides Y, which occur in the hypo-thalamus, through depressing hunger and control of body
4 J. KAºU _ZNA-CZAPLI�NSKA ET AL.
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

Table2.
Summaryofstud
iesregardingam
ount
oftryptoph
anandits
metabolitesinbody
fluids
invario
usdiseases
ordisorders.
No.
Disease
ordisorder
Metabolites
Stud
ypopu
latio
nSex
Sample
Relatio
nto
disease
Levelofm
etabolite
Methods
Reference
1IrritableBowel
Synd
rome(IBS)
kynu
renine
(Kyn)
tryptoph
an(Trp)
IFN-g
41IBS33
controls
female
plasma
Increase
ofTrpalongtheKyncatabolic
pathway
caused
increasedsensivity
ofthe
IDOto
IFN-g
andassociationbetweenIFN-
gandKyn/Trpratio
contrib
uteto
serotonergicdysfun
ction,viadeficit5
-HT,
which
may
explaingeneratio
ngastrointestinalsymptom
andincrease
incidenceofanxietyanddepression.
Kyn"T
rp$
Kyn/Trp"
IFN-g
$Trp,Kyn-
HPLCIFN-g-
electrochemiluminesc
ence
multip
lexsystem
Fitzgerald
etal.2008
2IrritableBowel
Synd
rome(IBS)
kynu
renicacid(KYN
A)qu
inolinicacid(QA)
37IBS20
controls
both
serum
Controlofthe
gutm
otilityandenteric
neurnalexcitabilityisinvolved
balance
betweenqu
inolinicandkynu
renicacid.
KYNA#Q
A"
HPLC
Wollnyetal.
2006
3IrritableBowel
Synd
rome(IBS)
tryptoph
an(Trp)
serotonin(5-HT)5-
hydroxyind
oloacetic
acid(5-HIAA)
14IBS14
controls
both
plasma
Serotonergicmodulationby
ATDaaffects
visceralperceptio
nandcogn
ition
inIBS
andcontrol.
Trp#5
-HIAA#
HPLC
Kilkensetal.
2004
4IrritableBowel
Synd
rome(IBS)
serotonin(5-HT)5-
hydroxyind
oloacetic
acid(5-HIAA)
23IBS-C23
IBS-D25
controls
both
serum
urine
Metabolismof5-HTandsecretionmay
bedisturbedinirritablebowelsynd
rome
(IBS).D
isturbed
metabolismofserotonin
probablyplay
aroleinpathogenesisof
functio
nalbow
eldiseases.
5-HT"5
-HIAA#
ELISA
Moskw
aetal.
2007
5IrritableBowel
Synd
rome(IBS)
tryptoph
an(Trp)
8IBS-C10
IBS-D11
control
both
plasma
Rise
leveloftryptophanaffectson
gastrointestinalsymptom
sinIBSandalso
decreasesanxietysymptom
s.
ATDa :Trp#A
TIb:Trp
#HPLC
Shufflebotham
etal.2006
6IrritableBowel
Synd
rome(IBS)
serotonin(5-HT)
29IBS-C55
IBS-D35
controls
both
plasma
Modulatingofdifferent
5-HTreceptorsare
involvinginIBS.Redu
ced5-HTreup
take
connectedwith
IBS-D,impairedrelease
may
belinkedwith
IBS-C.
IBS:C:5-HT#I
BS-D:5-HT
"HPLC
Atkinson
etal.
2006
7IBS-D
serotonin(5-HT)
39IBS-D20
controls
female
plasma
Symptom
exacerbatio
nfollowingmeal
ingestioninpatientswith
IBS-Dis
conn
ectedwith
increasedlevelsofplasma
5-HT,togetherwith
aredu
ctionin5-HT
turnover.
5-HT"
HPLC
Hough
ton
etal.2003
8IrritableBowel
Synd
rome(IBS)
serotonin(5-HT)5-
hydroxyind
oloacetic
acid(5-HIAA)
15IBS-C15
IBS-D15
controls
both
plasma
IBS-Cpatientsshow
impairedpostprandial5-
HTrelease.
IBS-C:5-HT"5
-HIAA#
HPLC
Dunlopetal.
2005
9Ch
ronicKidn
eyDisease
(CKD
)tryptoph
an(Trp)10
metabolitesofTrpc
27both
serum
Declineinkidn
eyfunctio
nisassociated
with
metabolismof
tryptoph
anviathe
kynu
renine
pathway,w
ithoutevident
eliminationoftryptoph
anmetabolismvia
the5-HTpathway.
KYNA"w
ereassociated
with
#cognitive
functio
nIAA"w
ascorrelated
with
anxietyand
depression
LC-M
S/MS
Karu
etal.
2016
10Obesity
tryptoph
an(Trp)Trp
/LNAA
dratio
9obese8controls
both
plasma
BrainTrpup
take
iscorrelated
with
the
plasmaTrp/LN
AAratio
.Thisdeterm
ine
brainserotoninsynthesis.Serotonin-
mediatedregu
latio
nof
food
intake
may
contrib
uteto
bluntedTrp/LN
AA,w
hich
response
tocarbohydrateintake
inthe
obese.
Trp#
HPLC
Caballero
etal.1988
(Continuedon
nextpage)
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 5
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

Table2.
(Continued)
No.
Disease
ordisorder
Metabolites
Stud
ypopu
latio
nSex
Sample
Relatio
nto
disease
Levelofm
etabolite
Methods
Reference
11Obesity
tryptoph
an(Trp)
kynu
renine
(Kyn)
Kyn/Trpratio
359ow
/obe
212
controls
both
serum
Indu
ctionof
theTrp-Kynpathway
are
associated
todevelopm
ento
fthe
metabolicsynd
romeinobesity.O
besity
andTrpmetabolismdiffersbetween
juvenilesandadults.
adult:Trp#
HPLC
Mangg
eetal.
2014
Kyn"
Kyn/Trp"
juvenile:Trp
"Kyn#
Kyn/Trp#
12Obesity
tryptoph
an(Trp)
kynu
renine
(Kyn)
Kyn/Trpratio
27overweigh
t11
obese
both
serum
Disturbed
metabolism
ofTrpinfluentson
biosynthesisofserotoninandmight
beassociated
with
increasedcarbohydrate
cravingandsusceptib
ilityform
ood
disturbances.
VLCD
fLCDg
HPLC
Strasseretal.
2015
Trp
##
Kyn
##
Kyn/Trp
$$
13Obesity/Depressiontryptoph
an(Trp)
973individu
als
both
plasma
LowerTrplevelsinoverweigh
t/obesewom
ansugg
eststhatlowTrp(lowserotonin
synthesis)may
contrib
uteto
either
vulnerabilityto
depression
inobese
wom
enor
vulnerabilityto
obesity
indepressedwom
en.
ow/obe
wom
enmen
HPLC
Rahejaet
al.
2015
Trp
#"
14Type
2Diabetes
(T2D
)tryptoph
an(Trp)
kynu
renine
(Kyn)
kynu
renicacid
(KYN
A)
30T2D24
controls
both
plasma
IncreasedplasmalevelsofKynandKYNAof
T2Dpatientsmight
confirm
“kynurenine
hypothesis”ofinsulin
resistance
andits
progressionto
T2D.
Trp"K
yn"K
YNA"
GC-MS
Oxenkrug,
2015
15BipolarD
isorder
(BD)
kynu
renine
(Kyn)K
yn/
Trpratio
78BD
(54overweigh
t,24
norm
alweigh
t),
156controls(76
overweigh
t,80
norm
alweigh
t)
both
serum
IncreasedlevelofkynurenineandKyn/Trp
ratio
intheoverweigh
tpatientswith
BDcouldbe
connectedbetweenshort
perio
dsof
euthym
iaandworsening
ofillnesscourse
inoverweigh
tpatientswith
BD.
Overweigh
tpatients
with
BD:Kyn
"Kyn/
Trp"
HPLC
Reiningh
aus
etal.2014
16An
orexiaNervosa
(AN)
tryptoph
an(Trp)
32acAN
h32
recANi32
controls
female
plasma
InacAN
hpatientsobserved
lowerTrplevels,
which
also
influenceindiminished5-HT.
Redu
cedavailabilityofthe5-HTmay
accountfor
thepoor
response
totreatm
entA
Npatients.
acAN
hrecANi
HPLC
Ehrlich
etal.
2009
Trp
##
17An
orexiaNervosa
(AN)
5-hydroxyind
oloacetic
acid(5-HIAA)
14AN
10controls
female
cerebrospinal
fluid(CSF)
Centralnervous
system
serotoninergic
metabolismisassociated
with
weigh
tloss
andmalnutrition
inAN
.
5-HIAA#
GC-MS
Kaye
etal.
1988
18An
orexiaNervosa
(AN)
tryptoph
an(Trp)
serotonin(5-HT)
LNAA
dTrp/LN
AAratio
42AN
42controls
both
plasma
Decreaseindepressive
symptom
sand
anxiety,which
encoun
teredinthecourse
ofre-fe
edinginAN
may
bebio-availability
oftryptoph
an.
Trp#5
-HT#L
NAA
d#
Trp/LN
AA#
HPLC
Gauthieretal.
2014
19An
orexiaNervosa
(AN)
tryptoph
an(Trp)LNAA
d
Trp/LN
AAratio
13AN
21controls
female
plasma
Mooddisturbances
have
been
connected
with
redu
ceserotonergicfunctio
n.InAN
individu
alsobserved
redu
cedcentral
serotoninmetabolism(braintryptoph
anavailabilitydecreased).
Trp:afterp
roteinmeal"
aftercarbohydrate
meal#
Trp/LN
AA:
afterp
roteinmeal#
aftercarbohydrates
meal"
HPLC
Schw
eiger
etal.1986
6 J. KAºU _ZNA-CZAPLI�NSKA ET AL.
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

20Bu
limia(BN)and
Anorexia
Nervosa
(AN)
tryptoph
an(Trp)LNAA
d
Trp/LN
AAratio
13BN
10AN
15controls
female
plasma
Decreased
Trp/LN
AAratio
may
cause
consequences
such
asdisturbances
ofmoodandneuroend
ocrin
eregu
latio
nin
ANindividu
als.
AN:Trp/LNAA
#vs
control
HPLC
Schreiber
etal.1991
BN:Trp/LNAA
ratio
$vs
controls
21An
orexiaNervosa
(AN)
tryptoph
an(Trp)
serotonin(5-HT)Trp/
LNAA
ratio
19AN
12controls
both
blood
Inpathologycouldbe
involved
allm
easured
biologicalindicesexcept
5-HT.AN
isassociated
with
impu
lsivity
andanxiety.
5-HT"t
otalTrp#f
ree
Trp#t
otalTrp/LN
AA#
HPLC
Askenazy
etal.
1998
22An
orexiaNervosa
(AN)
tryptoph
an(Trp)
serotonin(5-HT)5-
hydroxytryptophan
(5-HTP)
16AN
25controls
female
serum
Basedon
thesestud
iescanbe
distingu
ished
twodifferent
subg
roup
sofAN
patients.
One
ofgroupcharacterized
byamarkedly
lowerTrpleveland
high
erlevelsof
5-HTP
and5-HT.
Trp#5
-HT#
HPLC
Comaiet
al.
2010
5-HTP
"
23An
orexiaNervosa
(AN)
tryptoph
an(Trp)
20AN
20controls
—serum
HighlevelofTrp
may
triggerA
NbecauseTrp
isaprecursoro
f5-HT.Serotoninis
responsibleform
oodregu
latio
n,andit
high
levelm
aycausedepression
and
decreasedeatin
gwhich
leadsto
AN.
Trp"
HPLC
Naureen
etal.
2014
24Bu
limiaNervosa
(BN)
tryptoph
an(Trp)Trp/
LNAA
ratio
22BN
16controls
female
plasma
Participantswith
BNcanbe
morevulnerable
tothemoodloweringeffectsofATDa .
Acutechangesin5-HTactivity
arelinked
with
moodBN
subjects.
Trp"
fluorometric
methodof
DenklaandDew
eyKaye
etal.
2000
25Au
tism(ASD
)tryptoph
an(Trp)
37ASD28
controls
adults
—plasma
Abnorm
alTrp-serotoninmetabolisminthe
brainmight
beresponsibleforthe
clinical
manifestations
andbehavioral
abnorm
alities
ofautism.H
ighfree
Trp
levelisresponsibleforlow
ermental
developm
entand
hyperactivity.
totalTrp
$free
Trp"
fluorometric
methodof
DenklaandDew
eyHoshino
etal.
1986
12controlschild
26Au
tism(ASD
)tryptoph
an(Trp)
20ASDadults
both
plasma
Changesinbehavior
(increasing
whirling
,bang
ing,hittingself,rocking,andtoe
walking
)aretriggeredby
depletingTrpin
adultp
atientswith
ASD.
—HPLC
McDougle
etal.1996
27Au
tism(ASD
)tryptoph
an(Trp)
55ASDchildren44
controls
both
plasma
DecreaselevelofTrp
inASDcouldimpair
serotoninsynthesis,andthislead
toa
worsening
inbehavior
inASDsubjects.
LowerlevelofTrp
might
bedu
eto
redu
cedproteinintake
and/or
dysfunction
insynthesizing
proteininto
aminoacidsin
thedigestivetract.
Trp#
HPLC-MS/MS
Adam
setal.
2011
28Au
tism(ASD
)tryptoph
an(Trp)
138ASDchildren138
controls
both
plasma
LowerlevelofTrp
may
deterio
ratio
ninthe
behavior
ofautistic
children.Trpandother
LNAA
dcompeteforb
rainserotonin
synthesisandwhenislowlevelofTrp
then
islowbrainserotoninsynthesis.
Trp#
HPLC
Naushad
etal.
2013
29Au
tism(ASD
)tryptoph
an(Trp)
33ASDchildren21
controls
both
urine
Abnorm
alTrp-serotoninmetabolisminthe
brainmight
beresponsibleforthe
worsening
ofautistic
symptom
s.Redu
celevelofTrp
may
causeincreasedirritability
andmild
depression.
Trp#
GC-MS
Ka»u_ zna-
Czapli� nska
etal.2010
30Au
tism(ASD
)tryptoph
an(Trp)
14ASDchildren10
controls
both
urine
LowerlevelofTrp
may
lead
totheworsening
ofautistic
symptom
s(increasedirritability
andmild
depression).
Trp#
GC-MS
Ka»u_ zna-
Czapli� nska
etal.2014
(Continuedon
nextpage)
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 7
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

Table2.
(Continued)
No.
Disease
ordisorder
Metabolites
Stud
ypopu
latio
nSex
Sample
Relatio
nto
disease
Levelofm
etabolite
Methods
Reference
31Au
tism(ASD
)tryptoph
an(Trp)
48ASD53
controls
both
urine
Abnorm
alam
inoacidmetabolism(includ
ing
Trpmetabolism),increasedoxidative
stress,and
alteredgu
tmicrobiom
esin
ASD.
Trp#
UPLC/MS/MSGC-MS
Mingetal.
2012
32Parkinson’sDisease
(PD)
tryptoph
an(Trp)
kynu
renine
(Kyn)
Kyn/Trpratio
22PD
11controls
both
serum,cerebrospinal
fluid(CSF)
IncreasedTrpdegradationinperip
heral
bloodinPD
patientssubstantiatesthatin
thisdiseaseparticipateimmunological
abnorm
alities.Interferon-mediatedIDO
activity
isresponsibleforthe
increasedTrp
degradationrateas
isevidentb
ythe
increasedKyn/Trpratio
s.
Trp#K
yn#K
yn/Trp
"HPLC
Widneretal.
2002
33Parkinson’sDisease
(PD)
tryptoph
an(Trp)
20PD
20controls
both
cerebrospinalfluid
(CSF)
Degradatio
nofTrpcanlead
tothegeneratio
nof
3-HKA
,acompoun
dleadingto
increasedoxidativestressinpreclinicalPD
stud
ies.
Trp#
GC-TO
FMS
Trup
petal.
2014
34Parkinson’sDisease
(PD)
3-hydroxykynurenine(3-
HK)
48PD
57controls
—cerebrospinalfluid
(CSF)
3-HKlinkedwith
potent
excitotoxicity
properties.Blockproductio
nof3-HK
(through
Trpcatabolism)allows
neuroprotectivestrategy
andtherapeutic
interventio
nagainst3
-HKform
ation.
3-HK"
UHPLC-MS/MSGC-MS
Lewitt
etal.
2013
35Parkinson’sDisease
(PD)
tryptoph
an(Trp)
92PD
65controls
both
urine
Degradatio
nofTrpmay
beconnectedwith
theactivated
cell-mediatedimmune
response
typicalofP
D.
Trp#
GC-MSLC-M
SLuan
etal.
2015
36Alzheimer’sDisease
(AD)
tryptoph
an(Trp)
kynu
renine
(Kyn)
Kyn/Trpratio
21AD
20controls
—serum
IncreasedTrpdegradationinAD
patientsis
associated
with
sign
sofachronicimmune
activation,whileincreasedKyn/Trpwas
associated
with
redu
cedcogn
itive
performance.
Trp#K
yn"K
yn/Trp
"HPLC
Widneretal.
1999
37Alzheimer’sDisease
(AD),
Hun
tington’s
disease(HD)
tryptoph
an(Trp)
kynu
renine
(Kyn)
Kyn/Trpratio
24AD
12HD
—serum
System
icchronicimmun
eactivationin
patientswith
ADandHDisassociated
with
sign
ificant
degradationofTrp,which
ismostlikelydu
eto
activationof
IDOby
immunologicstimuli.
Trp#K
yn#K
yn/Trp
"HPLC
Widneretal.
2000
38Alzheimer’sDisease
(AD)
tryptoph
an(Trp)
kynu
renine
(Kyn)
Kyn/Trpratio
43AD
both
serum
Increasedbloodconcentrationof
Kyn/Trpis
associated
with
immuneactivationand
inflam
mationrepresentcriticalfactorsin
thepathogenesisofAD
.
Trp#K
yn"K
yn/Trp
"HPLC
Wissm
ann
etal.2013
39Alzheimer’sDisease
(AD)
tryptoph
an(Trp)
16AD
17controls
both
plasma
AcuteTrpdepletionhadno
effecton
cortisol
secretionforsub
jectswith
ADandhealthy
controls.
Trp#
HPLC
Porter Marshall,
and
O’Brien
2002
40Alzheimer’sDisease
(AD)
kynu
renicacid(KYN
A)19
AD20
controls
both
cerebrospinalfluid
(CSF)
Nosign
ificant
alteratio
nsinCSFKYNAlevels
inAD
patientscomparedto
controls.In
ADtheinconsistencyofKYNAalteratio
nscouldbe
becauseof
theheterogeneity
ofthedisease.
KYNA$
KYNA"f
emale
ADvs
maleAD
HPLC
Wennstr€ om
etal.2014
8 J. KAºU _ZNA-CZAPLI�NSKA ET AL.
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

41SleepDisorders
(SD)
melatonin
94individu
als
male
saliva
Thecombinedinterventio
non
breakfast,
morning
sunlight
andevening-lighting
seem
sto
beeffectiveto
keep
high
ermelatoninsecretionatnigh
t.Higherlevel
ofmelatoninisresponsiblefore
asyonset
ofthenigh
tsleep
andhigh
erqu
ality
ofsleep.
melatonin"i
nG3vs
G1
andG2j
ELISA
Wadaet
al.
2013
42Delayed
Sleep
Phase
Synd
rome
(DSPS)
melatonin
56individu
als
both
saliva
Exam
inationof
themelatoninsecretion
profilecanrevealseveralkey
differences
betweenindividu
alswith
andwith
out
circadianrhythm
disrup
tions.
Thetim
eofmelatonin
secretionare
sign
ificantlydelayed
inDSPSpatients.
ELISA
Rahm
anetal.
2009
43SleepDeprivation
tryptoph
an(Trp)
serotonin(5-HT)
109individu
als
—plasma
Theincreasedlevelsof
5-HTandTrpmay
explaintheantid
epressiveeffectofacute
sleepdeprivation.
Trp"5
-HT"
LC-M
SDaviesetal.
2014
$no
differences
inlevelsmetabolite
betweensubjectswith
disease/disorder
andcontrols
"increaselevelofm
etabolite
insubjectswith
disease/disordercomparedto
controls
#decreaselevelofm
etabolite
insubjectswith
disease/disordercomparedto
controls
a ATD
-AcuteTryptoph
anDepletio
nbATI-AcuterTryptoph
anIncrease,sub
jectsconsum
edan
aminoaciddrinkthateithercontaining
2.3gTrp
c 10metabolitesofTrp:
serotonin(5-HT)
5-hydroxy-3-indoleaceticacid(5-OHIAA)
kynu
renine
(Kyn)
kynu
renicacid(KYN
A)qu
inolinicacid
xanthurenicacid
quinaldicacid
3-OHanthranilic
acid
indoxylsulfate
indole-3-acetic
acid(IA
A)dLN
AA-Large
NeutralAm
inoAcids
e ow/ob-
overweigh
t/obesesubjects
f VLCD-V
eryLowCaloric
Diet
gLCD-Low
Caloric
Diet
h acAN-p
articipantswith
acuteanorexianervosa(AN)
i recAN-p
articipantswerepreviouslytreatedforanorexianervosa(AN)
j G1-no
interventio
nG2-have
protein-richfoodsandvitaminB-6-richfoodsatbreakfastand
sunlight
exposureafterb
reakfast
G3-thesamecontentasG2andincand
escent
light
exposureatnigh
t
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 9
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

adiposity (Wurtman and Wurtman, 1995; Manousopoulouet al., 2016). Also, it has been found that serotonin specificallyregulates fat and/or carbohydrate intake (Blundell and Lawton,1995; Bray, 2001).
3. Anorexia nervosa and bulimia nervosa
Eating disorders (EDs) are widespread and serious diseasesthroughout the world, with a chronic course and potentiallyfatal outcome (Winkler et al., 2016). Genetic and environmen-tal factors contribute to the development of many complex eat-ing disorders (Haleem, 2012). The most common eatingdisorders may include anorexia nervosa (AN) and bulimianervosa (BN). Both diseases are disorders of considered to arisefrom unknown etiology. They usually begin during adolescencein women, but may also be seen in men (Becker et al., 2003;Kaye et al., 2005, 2008; Haleem, 2012).
A study conducted by Haleem (2012) suggested that femalesare more vulnerable to food restriction, which may start with achronic deficiency in Trp levels and bioavailability. Monoamin-ergic neurotransmitters such as serotonin (5-HT), noradrena-line (NA) and dopamine (DA) contribute to the regulationfeeding behavior, while the accessibility of precursor aminoacids in the blood powerfully influences the synthesis of thosemonoamines in the brain (Ehrlich et al., 2009).
The most common symptoms of eating disorders arerestricted eating, body image distortions, and denial of emacia-tion, binge-purge behaviors, and resistance to treatment. Theyare also characterized by aberrant patterns of weight regulationand feeding behavior, but also by different perceptions towardsshape and body weight, dysphoric mood exhibit behaviors,such as perfectionism and obsessive–compulsiveness. AN andBN are relapsing and often are chronic disorders, whereas ANhas the highest death rate compared to other psychiatricdisorders.
Patients with AN accompanied an obsession with bodyweight and inexplicable fear of weight gain, even in the face ofthe increasing destruction of the body, characteristically exhibitmotor restlessness and excessive exercise (Kaye, Gendall, andStrober, 2001). The main role in behavioral changes observedin a patient with anorexia nervosa presents anomalies occurringin the serotoninergic pathway. In AN an individual’s serotoninlevel is involved in almost all the behavioral changes. Patientswith AN show higher frequency of compulsive exercising rela-tive to those with BN patients (Haleem, 2012). After a period offood restriction, the sufferer usually emerges with BN. Theymay or may not have been linked with weight loss. After surfeitfollowed by self-induced vomiting or different way compensa-tion of surfeit, people suffering from BN also have a fear ofweight gain and distorted view of their body shape. Individualswith BN are impulsive, and often sensation seeking, whereasindividuals with AN tend toward emotional expressiveness andconstriction of affect, and may exhibit great constraint.
The above-described characteristics of anorexia nervosa andbulimia nervosa often begin in childhood, are premorbid andoften persist even after recovery. This would suggest that suchbehavior caused by underfeeding is not secondary. Dysregula-tion of impulse control and appetite or mood in AN and BNcontributes modified brain serotonin function. The occurrence
of AN precedes the disturbance of neuronal serotonin modula-tion, which contributes to premorbid symptoms of inhibition,anxiety, and obsessionality. In patients with BN, it has beenobserved that dietary depletion of Trp is associated with Trp-associated mood irritability and increased food intake, which iscaused by dysfunction of serotonergic tone (Kaye et al., 2005,2008). Because Trp is the precursor to serotonin, Trp deficitcould significantly alter serotoninergic neurotransmission. Inthe refeeding, the Trp/LNAA ratio increases, as it associatedwith a decrease in depressive symptoms. This fact provides anargument for a possible impact of the AN mood symptomswith the serotoninergic pathway through a normalization ofthe biological markers. The increase in the Trp/LNAA ratio ispossible by the intake of related essential amino acids. Thetransport of Trp is predictive through the blood-brain barriertowards the cerebrospinal fluid (CSF) in the evaluation of theTrp/LNAA ratio. Then, Trp can be used for the synthesis ofcerebral serotonin. In these particular ways, the serotoninergictransfer, which leads to a decrease in depressive symptoms, isrestored (Gauthier et al., 2014). For all patients with EDs, com-mon features include dysfunctional cognizance relating toshape and weight and result in restrained eating behaviors.
In the literature, there is lots of evidence of anxiety, appe-tite dysregulation, extremes of impulse control and obses-sional behaviors, caused by disturbances 5-HT in thosesuffering from EDs. Enhancement of the brain serotoninrelease, which can affect appetite regulation, can determinemeal consumption, depending on the amount of protein andcarbohydrate in the meal. Carbohydrate consumption causesdepletion of the large neutral amino acids valine, leucine,isoleucine, phenylalanine and tyrosine. This LNAA competeswith Trp for uptake into the brain. Such elevates the plasmaTrp/LNAA ratio, thereby the amount of Trp in the brain,causing rapid synthesis and release of 5-HT. In contrast, adiet rich in proteins can block those effects, resulting in thelarge amounts of LNAA in the blood (Fernstrom et al., 1979;Kaye et al., 2005, 2008). The results of many studies indicatea decrease in plasma of the Trp/LNAA ratio and Trp levelsin patients with acute underweight (Schreiber et al., 1991;Askenazy et al., 1998; Ehrlich et al., 2009; Comai et al., 2010;Gauthier et al., 2014).
A broader viewpoint is shown by Gauthier et al. (2014),where they show links between serotonin biomarkers, nutri-tional status and psychological states in anorexia nervosa con-jointly. For the first time, they were able to highlight the role ofthe low level of Trp in plasma, blood serotonin, and LNAA andTrp/LNAA ratio with malnutrition (Gauthier et al., 2014).They also found a positive correlation between anxiety, depres-sion score, and total blood serotonin levels in a group of ANindividuals classified as an impulsive. These results are consis-tent with the results obtained by other teams. Acute Trp deple-tion in AN patients was found to lead to an increase in anxiety.Through the restrictive dietary behaviors, individuals maydecrease cerebral serotonin synthesis. Additionally, reduced 5-HT concentrations in hypothalamic causes hyperactivity, whichintensifies behaviors leading to weight loss. The emergence ofBN symptoms and amplified impulsivity appear to be related tolow serotonin levels. This may explain the frequent overlapbetween the restrictive forms of AN and the bulimic state, and
10 J. KAºU _ZNA-CZAPLI�NSKA ET AL.
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

behaviors are alternating between these eating disorders(Gauthier et al., 2014).
4. Autism spectrum disorder
Autism spectrum disorder (ASD) is a developmental, multi-factorial disorder, characterized by symptoms that evolvewith age adversely affecting the development of the child(Bara, Bucciarelli, and Colle, 2001). The incidence of ASDhas grown worldwide by 600% since the 1970s. In the US,currently at least one of 68 children has ASD (Zablotskyet al., 2015; Christensen et al., 2016). Patients with ASDdemonstrate the problem with interacting and exhibit littleinterest in others and lack of social awareness (Ka»u _zna-Czapli�nska and B»aszczyk, 2012). Etiology and pathogenesisof ASD are not still fully known. However, evidence pointsto nutritional deficiencies or overloads, complex geneticinteractions, maternal age and health state, exposure tochemicals or viruses, heavy metal toxicity, immunologicaloverload from early vaccinations, certain food additives,and dysfunctional immune systems or allergies. Deficienciesin the levels of amino acids occur for many children withdevelopmental disorders.
In recent years, observations relative to metabolic bio-markers have shed light on the influence of amino acids on var-ious developmental disorders. In some cases, the neurologicalfunction could be specified just by studies of amino acids. Someresearchers have suggested that a pivotal role in ASD might befound with Trp metabolism. A metabolite of Trp is the neuro-transmitter serotonin (5-HT), which is, among other things,responsible for regulating humor and behavior, and also facili-tating calmness, feeling of well-being, relaxation, personal secu-rity, concentration, and self-confidence. Hence, reducedserotonin levels have been demonstrated to influence manydevelopmental disorders. For this reason, it is reasonable toposit a connection between escalation of autistic symptoms andabnormal levels of serotonin (Adams and Holloway, 2004).Likewise, a dysfunctional serotonergic system could be involvedwith ASD. As stated above, Trp is converted into serotonin inthe brain, where it competes for transport with nine other large,neutral amino acids (LNAA) (Beretich, 2009).
Many children with ASD exhibit a deficiency in Trp due tosignificant food selectivity and self-imposed diet restriction.This often leads to reduced levels of serotonin and a worseningof autistic behaviors. The literature mentions that urinaryexcretion of Trp might be caused by a low concentration of die-tary proteins. In 1986, research suggested a link between someproblems in children with ASD and abnormal Trp metabolism.The results indicated that free plasma Trp levels were evaluatedin ASD children compared to healthy children and adult con-trols (Hoshino et al., 1986). Therefore, biochemical abnormali-ties were associated with a significantly lower level of Trp inurine.
More recently, researchers examined 54 children aged4–10 years, 33 ASD children (4 female and 29 males) and 21normal children as healthy controls (8 females and 13 males)—the gender ratios in the subjects under study, incidentally, wereabout the same as found in other studies. The ASD childrenwere divided into a group of 10 children with ASD on the
restricted diet low casein and gluten, and 23 ASD childrenwithout restricted diet. The highest values of Trp in urine wereobserved in control group. Significantly lower concentrationlevels of Trp were reported in the samples from 23 ASD chil-dren that were on the restricted diet. Low levels of Trp mightalso cause intensification of the symptoms of ASD, such asincreased irritability and mild depression (Ka»u_zna-Czapli�nska,Michalska, and Rynkowski, 2010). McDougle et al. (1993) havereceived similar results about low diet in Trp. The researchersalso suggested that by depleting Trp in an adult with ASD theymight induce significant changes in behavior, which were notseen in that control group such as increasing whirling, banging,hitting self, rocking, and toe walking (McDougle et al., 1996).
Other scientists focused on the relationship between devel-opmental disorders and metabolic disturbances. Researchersexamined 55 children ages 5–16 years with ASD and 44 healthycontrols of similar age, gender, and geographical distribution.The study was aimed at comparing the metabolic and nutri-tional status of ASD children with that of control children andinvestigated autism severity to the Trp-related biomarkers.Results showed significantly decreased Trp in the children withASD. This might be due to reduced protein intake, and dys-function in synthesizing protein into amino acids in the diges-tive tract. Decreased Trp could further impair serotoninsynthesis. A deficiency of Trp and thus serotonin lead to a sig-nificant worsening in behavior in ASD participants (Adamset al., 2011).
Similar results were also found in other studies. For exam-ple, Naushad et al. (2013) examined 138 autistic children, 120males and 18 females, and 138 non-autistic controls, 120 malesand 18 females. Children were matched for age, gender, ethnic-ity and geographical area. Researchers observed markedly lowerlevels of Trp in the ASD children (Trp levels decreased by anaverage of 48%), compared to healthy controls (Naushad et al.,2013).
5. Parkinson and alzheimer diseases
The pathogenesis of neurodegenerative disorders such as Par-kinson’s (PD) and Alzheimer’s diseases (AD) are not entirelyknown. However, it is believed that in this pathogenic processare involved immunologic mechanisms. In the developmentand progression of PD and AD, a cause has been ascribed tostimulate immunocompetent cells and a significant number of(proinflammatory) cytokines (Widner et al., 2002). Parkinson’sdisease categorically belongs to chronic, progressive, and irre-versible neurodegenerative diseases, which are caused mainlyby the progressive degeneration of the dopaminergic pathway.The second most common neurodegenerative disease amongolder adults is PD. So far, the disease has long been considereda disease of old age (over 60 years of age), but it also occursincreasingly in younger people. When the damage of dopami-nergic neurons reaches 50–60%, and the striatum does notreach adequate dopaminergic input, there arise characteristicmotor symptoms and behaviors (Andersen et al., 2017).
Symptoms and signs of PD are resting tremor, curved pos-ture, bradykinesia, rigidity, depression, and postural instability,shuffling gait. For severe disability progressively lead to long–term complications of dopaminergic treatment, which focuses
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 11
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

on minimizing the symptoms like motor blocks and dyskinesia.The close relationship might observe between neurodegenera-tive diseases and nutritional status (Barichella, Cereda, and Pez-zoli, 2009). PD is very difficult, if not impossible, to diagnosebefore motor symptoms have begun developing. Other symp-toms associated with early stage disease are very unspecificincluding obstipation, olfactory deficiency, depression, andsleep disorders (Andersen et al., 2017).
Increased risk of PD can relate to many factors, such asconsumption of (processed) dairy products, past traumaticbrain injury, heavy metal toxicity, certain food additives,polypharmacy, certain parasites, and exposure to pesticidesand even history of melanoma (although this latter factorappears strictly correlational). In contrast, the factors thatreduce the risk of occurring PD are associated with caffeineconsumption, smoking, physical activity, and higher serumurate concentrations of NSAIDs. Caffeine, nicotine, andurate may be neuroprotective and give benefits in patientswith early PD. Whether this mild evidence is offset by theother more serious detriments implicated by caffeine, nico-tine, and NSAIDs is another matter. Researchers are lookingfor possible ways to identify this disease in its early stagesand possibly using (healthy) neuroprotective interventionsbefore the presentation of motor symptoms (Ascherio andSchwarzschild, 2016).
Tryptophan is the precursor not only of serotonin, but alsois degraded to the kynurenic acid, 3-hydroxykynurenine, andquinolinic acid. Kynurenine pathway that regulates the synthe-sis of these neuroactive metabolites. The human immune sys-tem controls the kynurenine pathway. Hyperfunction orhypofunction of neuroactive metabolites is caused by dysregu-lation of the kynurenine pathway, which relates closely to neu-rological and neurodegenerative disorders. The concentrationof 3-hydroxykynurenine (3-HK) is increased in the basal gan-glia of PD patients, whereas kynurenic acid (KYNA) andkynurenine levels are slightly reduced (Ogawa et al., 1992;Schwarcz et al., 2012). The strongest quinolinic acid (QUIN) isfound in glial cells. This fact suggests that QUIN might partici-pate in the pathogenic process in Alzheimer’s disease (Guille-min et al., 2005; Schwarcz et al., 2012). Interferon g (INF- g)product large amounts of neopterin, wherein IFN-g inducesindoleamine 2,3-dioxygenase (IDO), which causes degradationL-tryptophan to kynurenine.
In the literature, there are reports on high concentrations ofneopterin, Trp, and kynurenine found in serum and CSF sam-ples. Widner,Leblhuber, and Fuchs (2002) examined 22patients with PD (15 females and seven males) and 11 age-matched controls group, without obvious neuropsychiatricsymptoms (6 females and five males). From eight patients withPD were collected cerebrospinal fluid specimens. The resultsshowed significantly higher concentrations of neopterin andkynurenine/Trp ratio (kyn/trp ratio) and lower Trp concentra-tions in serum samples of PD patients compared to healthycontrols. Similar relationships were found in CSF from eightPD patients. Comparing the two body fluids, serum neopterinconcentrations were higher than in CSF. It can be assumed thatreduced dietary intake of Trp could significantly contribute toTrp depletion in PD patients (Widner et al., 2002). Similarresults were obtained by Ogawa et al. (1992) who observed in
PD patients increased the level of 3-hydroxykynurenine (3-HA), while the level of KYNA decreased.
The search for new biomarkers is always scientific interest.Lewitt et al. (2013) employed targeted metabolomics, usingCSF from PD patients and controls. They observed changes inthe ratio of 3-hydroxykynurenine (3-HK)/kynurenic acid(KYNA). This variation in the ratio 3-HK/KYNA is significantbecause 3-HK is a precursor of the quinolinic acid and by gen-eral hydroxyl radicals might cause oxidative damage. WhereasKYNA has neuroprotective potential. Promote neurodegenera-tion in the brain might cause an increased ratio of 3-HK/KYNA (Lewitt et al., 2013). In another study, Mollenhauer andZhang (2013) tried to unveil the candidate metabolic pathwayrelated to PD. They examined 35 patients with PD withoutdementia, and as a control group, 15 healthy age-matched par-ticipants without PD. The results showed that metabolomicprofiles of patients with PD were substantially different fromcontrol groups. PD profiles had significantly lower levels ofTrp. Decreased serum Trp levels appear to be significantlyrelated to psychiatric problems in patients with PD (Mollenha-uer and Zhang, 2013).
Alzheimer’s disease was first described more than a centuryago. This disease affects approximately 35.6 million peopleworldwide in 2010 and by the year 2050 estimated 115 millionpeople (Van Wijngaarden et al., 2017). One of the major causesof dementia is AD. So far, the pathogenesis of this disease isnot completely understood. It is, however, well known that thekynurenine pathway is the principal route for the metabolismof the Trp. Among other metabolic pathways, Trp is the kynur-enine pathway involved in AD pathogenesis (Kincses, Toldi,and V�ecsei, 2010). Hence, changes in AD behaviors have beenobserved in the kynurenine pathway. These changes are basedon a reduction in the serum concentration of KYNA and Trpand in increased concentrations of 3- hydroxykynurenine (3-HA) and kynurenine (O’Farrell and Harkin, 2017).
The mechanism of AD is similar to other neurodegenerativediseases such PD. Mechanisms of AD are associated with thekynurenine pathway (KP). Many proinflammatory cytokinesactivate kynurenine pathway, and then they create metabolitesassociated with the pathogenesis of AD. For limiting kynure-nine pathway responsible is indoleamine-2-3 dioxygenase(IDO). The expression of IDO is markedly increased with theproliferation of proinflammatory cytokines INF-g. Overexpres-sion of IDO is induced by INF-g in the presence of amyloidplaques, which leads to dysregulation of KP. In dysregulationof KP is also involved interleukin-18 (IL-18), which inducesstrong inflammatory reactions. IL-18 appears to be responsiblefor the production of neurotoxic QUIN, wherein is promotedneurodegeneration. Furthermore, QUIN may cause anincreased level of lipid peroxidation in oxidative stress and mayprovoke neuronal death by cytotoxicity. In AD patients,unchanged levels of QUIN in cerebral and CSF have beenobserved, whereas the level of KYNA was increased in the stria-tum, but in CSF and plasma, the level of KYNA was decreased.At this time, there is still no clear explanation concerning tohow decrease KYNA levels that contribute to Alzheimer’s dis-ease (Tan, Yu, and Tan, 2012).
(Kincses, Toldi, and V�ecsei, 2010) presented evidence ofthe participation of the kynurenine pathway in the
12 J. KAºU _ZNA-CZAPLI�NSKA ET AL.
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

pathogenesis of AD. They measured the kynurenine, Trpand KYN/Trp ratio in the plasma. Examined were tenpatients with AD (six females and four males) and 15healthy controls (11 females and four males). The resultsshowed that Trp concentration was significantly lower inAD patients than controls, while KYN showed no signifi-cant differences between AD patients compared to controls,and the KYN/Trp ratio was considerably higher in patientswith AD (Kincses, Toldi, and V�ecsei, 2010).
In the pathogenesis of AD, inflammation and immune acti-vation are factors related to increased blood concentration ofcertain biomarkers, such as the kynurenine to Trp ratio (KYN/Trp) and neopterin. Wissmann et al. (2013) examined 43 ADpatients (26 females and 17 males, range aged 57–99 years),they measured neopterin, Trp, and kynurenine concentration.They observed lower Trp levels, higher kynurenine levels, and ahigher KYN/Trp ratio, which is correlated with the higher con-centration of neopterin. (Wissmann et al., 2013). Similar resultswere obtained by Widner et al. (1999), which examined 24patients with AD and observed lower Trp levels, higher kynure-nine levels, and KYN/Trp ratio.
Other scientists examined the concentrations of the com-pound in CSF of patients with AD. They explored correlationsbetween KYNA levels, well-established AD, cognitive declineand proinflammatory markers. Then they measured KYNA lev-els in 19 AD patients, aged 72–79 years, and 20 healthy con-trols, age matched. The results showed that AD patients havesignificantly KYNA levels versus the healthy controls. Addi-tionally, they observed that female AD patients had signifi-cantly higher KYNA levels compared to male AD patients,wherein this result was not observed in the healthy controlgroup (Wennstr€om et al., 2014).
6. Tryptophan and sleep disorders
Sleep disorders are a serious problem in industrialized soci-eties and concerns not only adults but also children andyoung people. It is estimated that sleep disorders affect atleast 20–40% of adults, and half of them consider it to beimportant. Likewise, various types of sleep difficulties con-cern 25–62% of the population of children, depending ontheir stage of development (Blader et al., 1997; Kaczor andSkalski, 2016).
In recent years, experts were interested in the relation-ship between sleep and diet. The basis for the discussion ofthe problem is the enzyme pathway of melatonin synthesis,which precursor for melatonin is serotonin. This, in turn, issynthesized by enzymatic transformation of Trp (Kaczorand Skalski, 2016).
Hormones produced in the brain, such as serotonin andmelatonin, control sleep and circadian rhythms in humans.Melatonin is an active biological compound that is respon-sible for regulating diurnal rhythms and influences theimmune and reproductive system, and gastrointestinalmotility and other digestive processes. The pineal glandsecretes melatonin during periods of darkness. Its task is toregulate circadian rhythms and sleep patterns (Richardet al., 2009; Szczepanik, 2007). Tryptophan is often used forthe treatment of sleep disorders. In the diet Trp produces
therapeutic effects through melatonin. A crucial feature ofTrp treatment is that it does not directly reduce cognitiveability (Richard et al., 2009).
One hypothesis is that sunlight accelerates serotonin synthe-sis. Studies in Japan suggest that combined intervention of abreakfast rich in Trp, regular morning sun exposure, and even-ing lighting combine to improve higher melatonin secretion atnight. Associated with this was found improved sleep qualityand reduced time required to fall asleep (Nakade et al., 2012;Wada et al., 2013).
Infant sleep problems constitute a serious disorder andmight affect brain development (and be implicated in othermore serious health problems, as well). In the literature, thereis a report about the impact of diet on improving the conditionsof sleep. Cubero et al. (2009) examined 30 children with sleepproblems, aged 8–16 months old. In the evening meal, theyadministrated to infants were cereals with varying content ofTrp over a five-week period. Feeding of enriched cereals led tothe maintenance of calmer children and restored sleep. Theyconcluded that regulation of circadian cycle can be influencedby diet (Cubero et al., 2009).
One of many sleep disorders is night terrors. Sharp wakingfrom sleep characterizes night terrors, accompanied by persis-tent terror and fear or increased heart rate, sweating, andscreaming. Promising results have been demonstrated that Trpsupplementation for night terrors. Bruni et al. (2004) examinedthe influence L-5-hydroxytryptophan on sleep terrors. Theystudied 45 children (34 males and 11 females, aged 3.2–10.6 years) safer from sleep terrors. The studied group was sup-plementation by L-5-hydroxytryptophan. Within a month,they observed a reduction of more than half night terror epi-sodes in over 93% of children. These results confirm thatarousal level might be positively influenced by treatment withL-5-hydroxytryptophan, resulting in reduced sleep terrorbehaviors in children (Bruni et al., 2004).
7. Conclusion
The interest in Trp is growing throughout research and healthcommunity worldwide (Figure 2). The role of Trp in the
Figure 2. Diagram of the frequency of scientific reports on the use of tryptophansupplementation in the study of different diseases in 2007–2016. The literaturereview was based on PubMed sources, sorted by best match, for the phrase: tryp-tophan supplementation or Trp supplementation and diseases.
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 13
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

relationship serotonin-Trp uptake with diet is particularlyintriguing and deserves much more insightful data to achieve aforthright and clearer conclusion. Certainly, research in thenutritional fields must be further investigated and implemented,to elucidate the role of supplemented Trp in foods and mealsthat improve human health and prevent many serotonin-relatedpathologies. We believe that an optimized and personalized dietcan help to minimize the symptoms of illness, which will resultin improved health.
ORCID
Salvatore Chirumbolo http://orcid.org/0000-0003-1789-8307Geir Bjørklund http://orcid.org/0000-0003-2632-3935
References
Adams, J. B., and C. Holloway. 2004. Pilot study of a moderate dose multi-vitamin/mineral supplement for children with autistic spectrum disor-der. J Altern Complement Med. 10:1033–39.
Adams, J. B., T. Audhya, S. McDonough-Means, R. A. Rubin, D. Quig, E.Geis, E. Gehn, M. Loresto, J. Mitchell, S. Atwood, S. Barnhouse, andW. Lee. 2011. Nutritional and metabolic status of children with autismvs. neurotypical children, and the association with autism severity.Nutr Metab (Lond). 8:34.
Andersen, A. D., M. Binzer, E. Stenager, and J. B. Gramsbergen. 2017.Cerebrospinal fluid biomarkers for Parkinson’s disease – a systematicreview. Acta Neurol Scand. 135:34–56.
Andr�e, C., A. L. Dinel, G. Ferreira, S. Lay�e, and N. Castanon. 2014. Diet-induced obesity progressively alters cognition, anxiety-like behaviorand lipopolysaccharide-induced depressive-like behavior: focus onbrain indoleamine 2,3-dioxygenase activation. Brain Behav Immun.41:10–21.
Ascherio, A., and M. A. Schwarzschild. 2016. The epidemiology of Parkin-son’s disease: risk factors and prevention. Lancet Neurol. 15:1257–72.
Askenazy, F., M. Candito, H. Caci, M. Myquel, P. Chambon, G. Darcourt,and A. J. Puech. 1998. Whole blood serotonin content, tryptophan con-centrations, and impulsivity in anorexia nervosa. Biol Psychiatry.43:188–195.
Atkinson, W., S. Lockhart, P. J. Whorwell, B. Keevil, and L. A. Houghton.2006. Altered 5-hydroxytryptamine signaling in patients with constipa-tion- and diarrhea-predominant irritable bowel syndrome. Gastroen-terology. 130:34–43.
Bara, B. G., M. Bucciarelli, and L. Colle. 2001. Communicative abilities inautism: evidence for attentional deficits. Brain Lang. 77:216–40.
Barichella, M., E. Cereda, and G. Pezzoli. 2009. Major nutritional issues inthe management of Parkinson’s disease.Mov Disord. 24:1881–92.
Becker, A. E., R. A. Burwell, K. Navara, and S. E. Gilman. 2003. Binge eat-ing and binge eating disorder in a small-scale, indigenous society: theview from Fiji. Int J Eat Disord. 34:423–31.
Beretich, G. R.. 2009. Reversal of autistic symptoms by removal of low-rel-ative tryptophan foods: case report.Med Hypotheses. 73:856–57.
Blader, J. C., H. S. Koplewicz, H. Abikoff, and C. Foley. 1997. Sleep prob-lems of elementary school children. A community survey. Arch PediatrAdolesc Med. 151:473–80.
Blundell, J. E., and C. L. Lawton. 1995. Serotonin and Dietary Fat Intake:Effects of Dexfenfluramine.Metabolism. 44:33–37.
Brandacher, G., E. Hoeller, D. Fuchs, and H. G. Weiss. 2007. Chronicimmune activation underlies morbid obesity: is IDO a key player? CurrDrug Metab. 8:289–95.
Bray, G. A.. 2001. Drug Treatment of Obesity. Rev Endocr Metab Disord.2:403–18.
Bruni, O., R. Ferri, S. Miano, and E. Verrillo. 2004. L -5-Hydroxytrypto-phan treatment of sleep terrors in children. Eur J Pediatr. 163:402–07.
Caballero, B., N. Finer, and R. J. Wurtman. 1988. Plasma amino acids andinsulin levels in obesity: response to carbohydrate intake and trypto-phan supplements. Metabolism. 37:672–76.
Catanzaro, R., S. Occhipinti, F. Calabrese, M. G. Anzalone, M. Milazzo, A.Italia, and F. Marotta. 2014. Irritable bowel syndrome: new findings inpathophysiological and therapeutic field. Minerva Gastroenterol Dietol.60:151–63.
Catrysse, L., and G. Van Loo. 2017. Inflammation and the Metabolic Syn-drome: The Tissue-Specific Functions of NF-kB. Trends Cell Biol.8924:30024–27.
Christensen, D. L., J. Baio, K. V. Braun, D. Bilder, J. Charles, J. N. Constan-tino, J. Daniels, M. S. Durkin, R. T. Fitzgerald, M. Kurzius-Spencer, L.C. Lee, S. Pettygrove, C. Robinson, E. Schulz, C. Wells, M. S. Wingate,W. Zahorodny, and M. Yeargin-Allsopp. 2016. Prevalence and charac-teristics of autism spectrum disorder among children aged 8 years –Autism and Developmental Disabilities Monitoring Network, 11 sites,United States, 2012.MMWR Surveillance Summaries. 65:1–23.
Comai, S., A. Bertazzo, N. Carretti, A. Podfigurna-Stopa, S. Luisi, and C. V.Costa. 2010. Serum levels of tryptophan, 5-hydroxytryptophan andserotonin in patients affected with different forms of amenorrhea. Int JTryptophan Res. 3:69–75.
Comai, S., A. Bertazzo, J. Vachon, M. Daigle, J. Toupin, G. Cot�e, G. Tur-ecki, and G. Gobbi. 2016. Tryptophan via serotonin/kynurenine path-ways abnormalities in a large cohort of aggressive inmates: markers foraggression. Prog Neuropsychopharmacol Biol Psychiatry. 70:8–16.
Cubero, J., B. Chancl�on, S. S�anchez, M. Rivero, A. B. Rodr�ıguez, and C.Barriga. 2009. Improving the quality of infant sleep through the inclu-sion at supper of cereals enriched with tryptophan, adenosine-50-phos-phate, and uridine-50-phosphate. Nutr Neurosci. 12:272–80.
Davies, S. K., J. E. Ang, V. J. Revell, D. J. Skene, A. Manna, F. P. Rob-ertsona, N. Cuia, B. Middletona, K. Ackermannc, M. Kayserc, A. E.Thumsera, F. I. Raynaudb, and D. J. Skenea. 2014. Effect of sleepdeprivation on the human metabolome. Proc Natl Acad Sci U S A.111 (29).
de Figueiredo, L. F., T. I. Gossmann, M. Ziegler, and S. Schuster. 2011.Pathway analysis of NADCmetabolism. Biochem J. 439:341–48.
Drossman, D. A., M. Camilleri, E. A. Mayer, and W. E. Whitehead. 2002.AGA technical review on irritable bowel syndrome. Gastroenterology.123:2108–131.
Dunlop, S. P., N. S. Coleman, E. Blackshaw, A. C. Perkins, G. Singh, C. A.Marsden, and R. C. Spiller. 2005. Abnormalities of 5-hydroxytrypta-mine metabolism in irritable bowel syndrome. Clin Gastroenterol Hep-atol. 3:349–57.
Ehrlich, S., L. Franke, N. Schneider, H. Salbach-Andrae, R. Schott, E. Cra-ciun Pfeiffer, R. Uebelhack, and U. Lehmkuhl. 2009. Aromatic aminoacids in weight-recovered females with anorexia nervosa. Int J Eat Dis-ord. 42:166–72.
Fernstrom, J. D., R. J. Wurtman, B. Hammarstrom-Wiklund, W. M. Rand,H. N. Munro, and C. S. Davidson. 1979. Diurnal variations in plasmaconcentrations of tryptophan, tryosine, and other neutral amino acids:effect of dietary protein intake. Am J Clin Nutr. 32:1912–22.
Fitzgerald, P., M. Cassidy Eugene, G. Clarke, P. Scully, S. Barry, M. M.Quigley Eamonn, F. Shanahan, J. Cryan, and T. G. Dinan. 2008. Tryp-tophan catabolism in females with irritable bowel syndrome: relation-ship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol Motil. 20:1291–97.
Gauthier, C., C. Hassler, L. Mattar, J. M. Launay, J. Callebert, H. Steiger, J.C. Melchior, B. Falissard, S. Berthoz, V. Mourier-Soleillant, F. Lang, M.Delorme, X. Pommereau, P. Gerardin, S. Bioulac, M. Bouvard, EVHANGroup, and N. Godart. 2014. Symptoms of depression and anxiety inanorexia nervosa: links with plasma tryptophan and serotonin metabo-lism. Psychoneuroendocrinology. 39:170–78.
Grundmann, O., S. L. Yoon, and B. Moshiree. 2010. Current developmentsfor the diagnosis and treatment of irritable bowel syndrome. CurrPharm Des. 16:3638–45.
Guillemin, G. J., B. J. Brew, C. E. Noonan, O. Takikawa, and K. M. Cullen.2005. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreac-tivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neuro-biol. 31:395–404.
Haleem, D. J.. 2012. Serotonin neurotransmission in anorexia nervosa.Behav Pharmacol. 23:478–95.
Halford, J. C., and J. E. Blundell. 2000. Separate systems for serotonin andleptin in appetite control. Ann Med. 32:222–32.
14 J. KAºU _ZNA-CZAPLI�NSKA ET AL.
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

Heitkemper, M. M., C. J. Han, M. E. Jarrett, H. Gu, D. Djukovic, R. J. Shul-man, D. Raftery, W. A. Henderson, and K. C. Cain. 2016. Serum Tryp-tophan Metabolite Levels During Sleep in Patients With and WithoutIrritable Bowel Syndrome (IBS). Biol Res Nurs. 18:193–98.
Hoshino, Y., T. Yamamoto, M. Kaneko, and H. Kumashiro. 1986. Plasmafree tryptophan concentration in autistic children. Brain Dev. 8:424–27.
Houghton, L. A., W. Atkinson, R. P. Whitaker, P. J. Whorwell, and M. J.Rimmer. 2003. Increased platelet depleted plasma 5-hydroxytrypta-mine concentration following meal ingestion in symptomatic femalesubjects with diarrhoea predominant irritable bowel syndrome. Gut.52:663–70.
Hulsken, S., A. M€artin, M. H. Mohajeri, and J. R. Homberg. 2013. Food-derived serotonergic modulators: effects on mood and cognition. NutrRes Rev. 26:223–34.
Hundal, R. S., M. Krssak, S. Dufour, D. Laurent, V. Lebon, V. Chandra-mouli, S. E. Inzucchi, W. C. Schumann, K. F. Petersen, B. R. Landau,and G. I. Shulman. 2000. Mechanism by which metformin reduces glu-cose production in type 2 diabetes. Diabetes. 49:2063–69.
Kaczor, M., andM. Skalski. 2016. Treatment of behavioral sleep problems inchildren and adolescents – literature review. Psychiatr Pol. 50:517–84.
Ka»u_zna-Czapli�nska, J., and S. B»aszczyk. 2012. The level of arabinitol inautistic children after probiotic therapy. Nutrition. 28:124–26.
Ka»u _zna-Czapli�nska, J., M. Michalska, and J. Rynkowski. 2010. Deter-mination of tryptophan in urine of autistic and healthy childrenby gas chromatography/mass spectrometry. Med Sci Monit.16:488–92.
Ka»u _zna-Czapli�nska, J., E. _Zurawicz, W. Struck, and M. Markuszewski.2014. Identification of organic acids as potential biomarkersin the urine of autistic children using gas chromatography/massspectrometry. J Chromatogr B Analyt Technol Biomed Life Sci.966:70–6.
Karu, N., C. McKercher, D. S. Nichols, N. Davies, R. A. Shellie, E. F. Hilder,and M. D. Jose. 2016. Tryptophan metabolism, its relation to inflam-mation and stress markers and association with psychological and cog-nitive functioning: Tasmanian Chronic Kidney Disease pilot study.BMC Nephrol. 17:171.
Kaye, W.. 2008. Neurobiology of anorexia and bulimia nervosa. PhysiolBehav. 94:121–35.
Kaye, W. H., G. K. Frank, U. F. Bailer, S. E. Henry, C. C. Meltzer, J. C.Price, C. A. Mathis, and A. Wagner. 2005. Serotonin alterations inanorexia and bulimia nervosa: new insights from imaging studies. Phys-iol Behav. 85:73–81.
Kaye, W. H., K. A. Gendall, M. H. Fernstrom, J. D. Fernstrom, C. W.McConaha, and T. E. Weltzin. 2000. Effects of acute tryptophan deple-tion on mood in bulimia nervosa. Biol Psychiatry. 47:151–57.
Kaye, W. H., H. E. Gwirtsman, D. T. George, D. C. Jimerson, and M. H.Ebert. 1988. CSF 5-HIAA concentrations in anorexia nervosa: reducedvalues in underweight subjects normalize after weight gain. Biol Psychi-atry. 23:102–05.
Kaye, W., K. Gendall, and M. Strober. 2001. Nutrition, serotonin andbehavior in anorexia and bulimia nervosa. Nestle Nutr Workshop SerClin Perform Programme. 5:153–68.
Keszthelyi, D., F. J. Troost, D. M. Jonkers, E. L. Van Donkelaar, J. Dekker,W. A. Buurman, and A. A. Masclee. 2012. Does acute tryptophandepletion affect peripheral serotonin metabolism in the intestine? Am JClin Nutr. 95:603–08.
Kilkens, T. O., A. Honig, M. A. van Nieuwenhoven, W. J. Riedel, and R. J.Brummer. 2004. Acute tryptophan depletion affects brain-gutresponses in irritable bowel syndrome patients and controls. Gut.53:1794–800.
Kincses, Z. T., J. Toldi, and L. V�ecsei. 2010. Kynurenines, neurodegenera-tion and Alzheimer’s disease. J Cell Mol Med. 14:2045–54.
Klempel, M. C., and K. A. Varady. 2011. Reliability of leptin, but not adi-ponectin, as a biomarker for diet-induced weight loss in humans. NutrRev. 69:145–54.
Lewitt, P. A., J Li, M Lu, TG Beach, CH Adler, L Guo, and Arizona Parkin-son’s Disease Consortium. 2013. 3-hydroxykynurenine and other Par-kinson’s disease biomarkers discovered by metabolomic analysis. MovDisord. 28:1653–60.
Liu, W., S. Mi, Z. Ruan, J. Li, X. Shu, K. Yao, M. Jiang, and Z. Deng. 2017.Dietary Tryptophan Enhanced the Expression of Tight Junction Pro-tein ZO-1 in Intestine. J Food Sci. 82:562–567.
Longstreth, G. F., W. G. Thompson, W. D. Chey, L. A. Houghton, F.Mearin, and R. C. Spiller. 2006. Functional bowel disorders. Gastroen-terology. 130:1480–91.
L€onnqvist, F., P. Arner, L. Nordfors, and M. Schalling. 1995. Overexpres-sion of the obese (ob) gene in adipose tissue of human obese subjects.Nat Med. 1:950–53.
Luan, H., L. F. Liu, Z. Tang, M. Zhang, K. K. Chua, J. X. Song, V. C. Mok,M. Li, and Z. Cai. 2015. Comprehensive urinary metabolomic profilingand identification of potential noninvasive marker for idiopathic Par-kinson’s disease. Sci Rep. 5:13888.
Madara, J. L., and S. Carlson. 1991. Supraphysiologic L-tryptophan elicitscytoskeletal and macromolecular permeability alterations in hamstersmall intestinal epithelium in vitro. J Clin Invest. 87:454–62.
Mangge, H., K. L. Summers, A. Meinitzer, S. Zelzer, G. Almer, R. Prassl, W.J. Schnedl, E. Reininghaus, K. Paulmichl, D. Weghuber, and D. Fuchs.2014. Obesity-related dysregulation of the tryptophan-kynureninemetabolism: role of age and parameters of the metabolic syndrome.Obesity (Silver Spring). 22:195–201.
Manousopoulou, A., Y. Koutmani, S. Karaliota, C. H. Woelk, E. S. Manola-kos, K. Karalis, and S. D. Garbis. 2016. Hypothalamus proteomics frommouse models with obesity and anorexia reveals therapeutic targets ofappetite regulation. Nutr Diabetes. 6:e204.
McDougle, C. J., S. T. Naylor, D. J. Cohen, G. K. Aghajanian, G. R.Heninger, and L. H. Price. 1996. Effects of tryptophan depletion indrug-free adults with autistic disorder. Arch Gen Psychiatry. 53:993–1000.
McDougle, C. J., S. T. Naylor, W. K. Goodman, F. R. Volkmar, D. J. Cohen,and L. H. Price. 1993. Acute tryptophan depletion in autistic disorder: acontrolled case study. Biol Psychiatry. 33:547–50.
Ming, X., T. P. Stein, V. Barnes, N. Rhodes, and L. Guo. 2012. Metabolicperturbance in autism spectrum disorders: a metabolomics study. JProteome Res. 11:5856–62.
Mollenhauer, B., and J. Zhang. 2013. Biochemical Pre-motor Biomarkersfor Parkinson Disease.Mov Disord. 27:644–50.
Moskwa, A., M. Wi�sniewska – Jarosi�nska, K. Stec-Michalska, K. Szadkow-ski, E. Felicka, J. �Smigielski, and C. Chojnacki. 2007. Serum serotoninconcentration and urine 5-hydroxyindole acetic acid excretion inpatients with irritable bowel syndrome. Pol Merkur Lek. 22:366–8.
Musil, F., V. Blaha, A. Ticha, R. Hyspler, M. Haluzik, J. Lesna, A. Smahe-lova, and L. Sobotka. 2015. Effects of body weight reduction on plasmaleptin and adiponectin/leptin ratio in obese patients with type 1 diabe-tes mellitus. Physiol Res. 64:221–8.
Nakade, M., O. Akimitsu, K. Wada, M. Krejci, T. Noji, N. Taniwaki, H.Takeuchi, and T. Harada. 2012. Can breakfast tryptophan and vitaminB6 intake and morning exposure to sunlight promote morning-typol-ogy in young children aged 2 to 6 years? J Physiol Anthropol. 31:11.
Namkung, J., H. Kim, and S. Park. 2015. Peripheral Serotonin: a NewPlayer in Systemic Energy Homeostasis.Mol Cells. 38:1023–28.
Naureen, F., Masroor, K., Khatoon, F., Ayub, S., Ahmed, M. I., Hasnat, A.,and Samad, N. 2014. Zinc and tryptophan levels in anorexia nervosa; aco-relational study. International Journal of Biomedical Engineeringand Science (IJBES). 1:27–33.
Naushad, S. M., J. M. Jain, C. K. Prasad, U. Naik, and R. R. Akella. 2013.Autistic children exhibit distinct plasma amino acid profile. Indian JBiochem Biophys. 50:474–8.
O’Farrell, K., and A. Harkin. 2017. Stress-related regulation of the kynure-nine pathway: Relevance to neuropsychiatric and degenerative disor-ders. Neuropharmacology. 112:307–23.
Ogawa, T., W. R. Matson, M. F. Beal, R. H. Myers, E. D. Bird, P. Milbury,and S. Saso. 1992. Kynurenine pathway abnormalities in Parkinson’sdisease. Neurology. 42:1702–6.
Oh, C. M., S. Park, and H. Kim. 2016. Serotonin as a New Therapeutic Tar-get for Diabetes Mellitus and Obesity. Diabetes Metab J. 40:89–98.
Oxenkrug, G.. 2013. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide meta-bolic pathways.Mol Neurobiol. 48:294–301.
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 15
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

Oxenkrug, G. F.. 2015. Increased Plasma Levels of Xanthurenic andKynurenic Acids in Type 2 Diabetes.Mol Neurobiol. 52:805–10.
Palego, L., L. Betti, A. Rossi, and G. Giannaccini. 2016. Tryptophan Bio-chemistry: Structural, Nutritional, Metabolic, and Medical Aspects inHumans. Journal of Amino Acids. 2016:13.
Patrick, R. P., and B. N. Ames. 2014. Vitamin D hormone regulatesserotonin synthesis. Part 1: relevance for autism. FASEB Journal.28:2398–13.
Porter, R. J., E. F. Marshall, and J. T. O’Brien. 2002. Effects of rapid trypto-phan depletion on salivary and plasma cortisol in Alzheimer’s diseaseand the healthy elderly. J Psychopharmacol. 16:73–78.
Raheja, U. K., D. Fuchs, I. Giegling, L. A. Brenner, S. F. Rovner, I. Mohyud-din, D. Weghuber, H. Mangge, D. Rujescu, and T. T. Postolache. 2015.In psychiatrically healthy individuals, overweight women but not menhave lower tryptophan levels. Pteridines. 26:79–84.
Rahman, S. A., Kayumov, L., Tchmoutina, E. A., and Shapiro, C. M. 2009.Clinical efficacy of dim light melatonin onset testing in diagnosingdelayed sleep phase syndrome. Sleep Med. 10:549–555.
Rambali, B., I. Van Andel, E. Schenk, G. Wolterink, G. van der Werken, H.Stevenson, and W. Vleeming. 2002. The contribution of cocoa additiveto cigarette smoking addiction. RIVM Report 650270002/2002, Minis-terie van Volksgexondheid, Welzijn en Sport, Rijksinstituut voorVolksgezondheid en Milieu, Bilthoven, The Netherlands.
Reininghaus, E. Z., R. S. McIntyre, B. Reininghaus, S. Geisler, S. A. Ben-gesser, N. Lackner, K. Hecht, A. Birner, F. Kattnig, R. Unterweger, H.P. Kapfhammer, S. Zelzer, D. Fuchs, and H. Mangge. 2014. Tryptophanbreakdown is increased in euthymic overweight individuals with bipo-lar disorder: a preliminary report. Bipolar Disord. 16:432–40.
Richard, D. M., M. A. Dawes, C. W. Mathias, A. Acheson, N. Hill-Kapturc-zak, and D. M. Dougherty. 2009. L-Tryptophan: Basic metabolic func-tions, behavioral research and therapeutic indications. Int J TryptophanRes. 2:45–60.
Ritze, Y., C. Hengelhaupt, G. B�ardos, B. Ernst, M. Thurnheer, J. G. D’Ha-ese, S. C. Bischoff, and B. Schultes. 2015. Altered intestinal neuroendo-crine gene expression in humans with obesity. Obesity (Silver Spring).23:227885.
Ritze, Y., A. Schollenberger, M. Hamze Sinno, N. B€uhler, M. B€ohle, G.B�ardos, H. Sauer, I. Mack, P. Enck, S. Zipfel, T. Meile, A. K€onigsrainer,M. Kramer, and S. C. Bischoff. 2016. Gastric ghrelin, GOAT, leptin,and leptin expression as well as peripheral serotonin are dysregulatedin humans with obesity. Neurogastroenterol Motil. 28:806–15.
Russo, S., I. P. Kema, F. Bosker, J. Haavik, and J. Korf. 2009. Tryptophan asan evolutionarily conserved signal to brain serotonin: molecular evi-dence and psychiatric implications.World J Biol Psychiatry. 10:258–68.
Sainio, E. L., K. Pulkki, and S. N. Young. 1996. L-Tryptophan: Biochemical,nutritional and pharmacological aspects. Amino Acids. 10:21–47.
Savino, F., S. A. Liguori, S. Benetti, M. Sorrenti, M. F. Fissore, and L. Cor-dero di Montezemolo. 2013. High serum leptin levels in infancy canpotentially predict obesity in childhood, especially in formula-fedinfants. Acta Pædiatr. 102:455–9.
Schreiber, W., U. Schweiger, D. Werner, G. Brunner, R. J. Tuschl, R. G.Laessle, J. C. Krieg, M. M. Fichter, and K. M. Pirke. 1991. Circadianpattern of large neutral amino acids, glucose, insulin, and food intakein anorexia nervosa and bulimia nervosa.Metabolism. 40:503–07.
Schwarcz, R., J. P. Bruno, P. J. Muchowski, and H. Q. Wu. 2012. Kynure-nines in the mammalian brain: when physiology meets pathology. NatRev Neurosci. 13:465–77.
Schweiger, U., M. Warnhoff, J. Pahl, and K. M. Pirke. 1986. Effects of car-bohydrate and protein meals on plasma large neutral amino acids, glu-cose, and insulin plasma levels of anorectic patients. Metabolism.35:938–43.
Shi, H. L., C. H. Liu, L. L. Ding, Y. Zheng, X. Y. Fei, L. Lu, X. M. Zhou, J. Y.Yuan, and J. Q. Xie. 2015. Alterations in serotonin, transient receptorpotential channels and protease-activated receptors in rats with irrita-ble bowel syndrome attenuated by Shugan decoction. World J Gastro-enterol. 21:4852–63.
Shufflebotham, J., S. Hood, J. Hendry, D. A. Hince, K. Morris, D. Nutt, C.Probert, J. Potokar. 2006. Acute Tryptophan Depletion Alters Gastro-intestinal and Anxiety Symptoms in Irritable Bowel Syndrome. Am JGastroenterol. 101:2582–87.
Silva, L. C., M. B. Viana, J. S. Andrade, M. A. Souza, I. C. C�espedes, and V.D’Almeida. 2017. Tryptophan overloading activates brain regionsinvolved with cognition, mood and anxiety. An Acad Bras Cienc.89:273–83.
Strasser, B., K. Berger, and D. Fuchs. 2015. Effects of a caloric restrictionweight loss diet on tryptophan metabolism and inflammatory bio-markers in overweight adults. Eur J Nutr. 54:33–42.
Szczepanik, M.. 2007. Melatonin and its influence on immune system. JPhysiol Pharmacol. 58S6:115–24.
Tan, L., J. T. Yu, and L. Tan. 2012. The kynurenine pathway in neurode-generative diseases: mechanistic and therapeutic considerations. J Neu-rol Sci. 323:1–8.
Trupp, M., P. Jonsson, A. Ohrfelt, H. Zetterberg, O. Obudulu, L. Malm, A.Wuolikainen, J. Linder, T. Moritz, K. Blennow, H. Antti, and L. For-sgren. 2014. Metabolite and peptide levels in plasma and CSF differen-tiating healthy controls from patients with newly diagnosedParkinson’s disease. J Parkinsons Dis. 4:549–60.
USDA Food Composition Databases. 2017. https://ndb.nal.usda.gov/ndb/Van Doorn, C., V. A. Macht, C. A. Grillo, and L. P. Reagan. 2017. Leptin
resistance and hippocampal behavioral deficits. Physiol Behav.176:207–13.
Van Wijngaarden, P., X. Hadoux, M. Alwan, S. Keel, and M. Dirani. 2017.Emerging ocular biomarkers of Alzheimer disease. Clin Exp Ophthal-mol. 45:54–61.
Wada, K., S. Yata, O. Akimitsu, M. Krejci, T. Noji, M. Nakade, H. Takeu-chi, and T. Harada. 2013. A tryptophan-rich breakfast and exposure tolight with low color temperature at night improve sleep and salivarymelatonin level in Japanese students. J Circadian Rhythms. 11:4.
Wennstr€om, M., H. M. Nielsen, F. Orhan, E. Londos, L. Minthon, and S.Erhardt. 2014. Kynurenic Acid levels in cerebrospinal fluid frompatients with Alzheimer’s disease or dementia with lewy bodies. Int JTryptophan Res. 7:1–7.
Widner, B., F. Leblhuber, and D. Fuchs. 2002. Increased neopterin produc-tion and tryptophan degradation in advanced Parkinson’s disease. JNeural Transm (Vienna). 109:181–89.
Widner, B., F. Leblhuber, J. Walli, G. P. Tilz, U. Demel, and D. Fuchs.1999. Degradation of tryptophan in neurodegenerative disorders. AdvExp Med Biol. 467:133–38.
Widner, B., F. Leblhuber, J. Walli, G. P. Tilz, U. Demel, and D. Fuchs.2000. Tryptophan degradation and immune activation in Alzheimer’sdisease. J Neural Transm (Vienna). 107:343–53.
Winkler, L. A., J. S. Frølich, C. Gudex, K. Hørder, N. Bilenberg, and R. K.Støving. 2016. Patient- and clinician- reported outcome in eating disor-ders. Psychiatry Res. 30:230–35.
Wissmann, P., S. Geisler, F. Leblhuber, and D. Fuchs. 2013. Immune acti-vation in patients with Alzheimer’s disease is associated with highserum phenylalanine concentrations. J Neurol Sci. 329:29–33.
Wollny, T., G. Rydzewska, D. Pawlak, E. Turecka-Kulesza, W. Buczko, andW. ºaszewicz. 2006. Kynurenic pathway metabolites in serum ofpatients with irritable bowel syndrome – possible role in the mecha-nism of visceral pain. Gastroenterologia Polska. 13:159–62.
Wurtman, R. J., and J. J. Wurtman. 1995. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 3:477–80.
Youssef, M.. 2015. The effect of dietary intervention by low carbohydratediet, and low fat diet, on weight loss, leptin and adiponectin. Life Sci J.12:33–42.
Yu, E., M. Ruiz-Canela, M. Guasch-Ferr�e, Y. Zheng, E. Toledo, C. B. Clish,J. Salas-Salvad�o, L. Liang, D. D. Wang, D. Corella, M. Fit�o, E. G�omez-Gracia, J. Lapetra, R. Estruch, E. Ros, M. Cof�an, F. Ar�os, D. Romaguera,L. Serra-Majem, J. V. Sorl�ı, F. B. Hu, and M. A. Martinez-Gonzalez.2017. Increases in plasma tryptophan are inversely associated with inci-dent cardiovascular disease in the Prevenci�on con dieta Mediterr�anea(PREDIMED) Study. J Nutr. 147:314–22.
Zablotsky, B., L. I. Black, M. J. Maenner, L. A. Schieve, and S. J. Blumberg.2015. Estimated prevalence of autism and other developmental disabil-ities following questionnaire changes in the 2014 National HealthInterview Survey. Natl Health Stat Report. 87:1–20.
_Zelowski, A., S. Wojtu�n, J. Gil, and P. Dyrla. 2013. Irritable bowel syn-drome – diagnostics and treatment principles. Pediatria i MedycynaRodzinna. 9:250–55.
16 J. KAºU _ZNA-CZAPLI�NSKA ET AL.
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017

Zhang, C., A. Yin, H. Li, R. Wang, G. Wu, J. Shen, M. Zhang, L. Wang, Y.Hou, H. Ouyang, Y. Zhang, Y. Zheng, J. Wang, X. Lv, Y. Wang, F.Zhang, B. Zeng, W. Li, F. Yan, Y. Zhao, X. Pang, X. Zhang, H. Fu, F.Chen, N. Zhao, B. R. Hamaker, L. C. Bridgewater, D. Weinkove, K.Clement, J. Dore, E. Holmes, H. Xiao, G. Zhao, S. Yang, P. Bork, J. K.Nicholson, H. Wei, H. Tang, X. Zhang, and L. Zhao. 2015. Dietary
modulation of gut microbiota contributes to alleviation of both geneticand simple obesity in children. EbioMedicine. 2:968–84.
Zhou, T., M. Shimabukuro, K. Koyama, Y. Lee, M. Y. Wang, F. Trieu, C. B.Newgard, and R. H. Unger. 1997. Induction by leptin of uncouplingprotein-2 and enzymes of fatty acid oxidation. Proc Natl Acad Sci U SA. 94:6386–90.
CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 17
Dow
nloa
ded
by [
Um
eå U
nive
rsity
Lib
rary
] at
09:
39 0
1 Se
ptem
ber
2017
View publication statsView publication stats



![Title: The Positron Emission Tomography ligand [11C]5 ......2014/05/19 · dynamic Positron Emission Tomography with the radiolabeled serotonin precursor [11C]5 Hydroxy-Tryptophan](https://static.fdocuments.net/doc/165x107/611dae361e650223bf6e3c0b/title-the-positron-emission-tomography-ligand-11c5-20140519-dynamic.jpg)