Hepatitis C Antibody / Hepatitis C Confirmatory Test (Anti-HCV)
Hepatitis C Update 2017 - Education · Hepatitis C Update 2017 Shobha Joshi, MD . Natural History...
Transcript of Hepatitis C Update 2017 - Education · Hepatitis C Update 2017 Shobha Joshi, MD . Natural History...
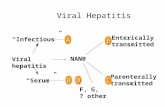
Natural History of Hepatitis C
• HCV RNA virus transmitted parenterally, >5.2 million infected in the US
• Acute phase often not diagnosed
• Approx. 75% develop chronic infection
• Cirrhosis develops in ~30%, leading to liver failure
• HCC develops in ~5% of HCV cirrhotics, 5th most common cancer
• Screening most important to diagnose and confirm active infection
CDC HCV Screening Guidelines 2012
• Offer at least one-time screening for HCV antibody in persons born between 1945 and 1965. 75% exposed to HCV are from this birth cohort.
– Anticipated additional 800,000 identified, save more than 120,000 lives
• High risk individuals
– IVDU, HIV-infected, MSM, patients on HD, accidental needle-stick, tattoos, body piercing
When and in Whom to Initiate anti-HCV Therapy? Factors Associated with Accelerated Fibrosis Progression and HCC
• HOST Factors
– Non-modifiable
• Fibrosis stage, Inflammation grade
• Older age at time of infection*
• Male gender*
• Organ transplant
– Modifiable
• Alcohol consumption*
• NAFLD, Obesity*, Insulin resistance
• VIRAL Factors
– Genotype 3*
– Co-infection with hepatitis B or HIV*
Risk Factors for HCC
Duration of infection
Race
* Marked on left
Untreated HCV
Extrahepatic Manifestations of Hepatitis C
• Hematologic: Cryoglobulinemia, Aplastic anemia, Thrombocytopenia, B-cell lymphoma
• Renal: Glomerulonephritis, Nephrotic syndrome
• Dermatologic: Porphyria cutanea tarda, lichen planus, cutaneous necrotizing vasculitis
• Endocrine: Diabetes mellitus, Antithyroid antibodies
• Salivary: sialadenitis
• Ocular: Uveiitis, Corneal ulcer
What Do You Need Before Starting Treatment of Hepatitis C?
• Confirm infection: HCV RNA quant, genotype
• Fibrosis assessment: Elastography (u/s or MR), Non-invasive markers (Fibrosure, APRI). Stage 0-4 (cirrhosis y/n)
• Treatment-naïve or treatment-failed
• NS5A drug resistance, NS3A drug resistance if treatment failed
• Hepatitis serologies: HAVAb total, HBsAg, HBsAb, HBcAb total. Vaccinate if negative.
• Ultrasound, AFP: evaluate for HCC
• Other meds: DDI evaluation
• Compliance
Lee M-H, et al. J Infect Dis. 2012;206:469-477.
REVEAL HCV: Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer (1991-2008). Anti-HCV seronegative (n=18,541); anti-HCV seropositive (n=1095; detectable HCV RNA: 69.4%). Average follow-up: 16.2 years. Among extrahepatic causes of death, 68.5% and 69.3% were noncancer deaths for HCV seronegative and seropositive, respectively. *P<.001 for comparison among all 3 groups and P<.001 for HCV RNA detectable vs undetectable.
Follow-Up (Years)
20
18
16
14
12
10
2
0 0 2 4 6 8 10 12 14 16 18 20
8
6
4
Follow-Up (Years)
12
10
8
6
4
2
0 0 2 4 6 8 10 12 14 16 18 20
All Causes (n=2394)
Liver Cancer (n=115)
Extrahepatic Diseases (n=2199)
Cu
mu
lati
ve M
ort
alit
y (%
)
Anti-HCV+, HCV RNA detectable Anti-HCV+, HCV RNA undetectable Anti-HCV–
Follow-Up (Years)
35
30
25
20
15
10
5
0 0 2 4 6 8 10 12 14 16 18 20
30.1%*
12.8% 12.4%
10.4%*
1.6%
0.3%
19.8%*
12.2%
11.0%
HCV Viremia Was Associated With Increased Mortality in a Prospective Taiwanese Cohort Study
Lee M-H, et al. J Infect Dis. 2012;206:469-477.
REVEAL HCV: Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer (1991-2008).Anti-HCV seronegative (n=18,541); anti-HCV seropositive (n=1095; detectable HCV RNA: 69.4%). Average follow-up: 16.2 years.Among extrahepatic causes of death, 68.5% and 69.3% were noncancer deaths for HCV seronegative and seropositive, respectively.*P<.001 for comparison among all 3 groups and P<.001 for HCV RNA detectable vs undetectable.
Follow-Up (Years)
20
18
16
14
12
10
2
00 2 4 6 8 10 12 14 16 18 20
8
6
4
Follow-Up (Years)
12
10
8
6
4
2
00 2 4 6 8 10 12 14 16 18 20
All Causes(n=2394)
Liver Cancer(n=115)
Extrahepatic Diseases(n=2199)
Cu
mu
lati
ve
Mo
rta
lity
(%
)
Anti-HCV+,HCVRNAdetectable Anti-HCV+,HCVRNAundetectable Anti-HCV–
Follow-Up (Years)
35
30
25
20
15
10
5
00 2 4 6 8 10 12 14 16 18 20
30.1%*
12.8%12.4%
10.4%*
1.6%
0.3%
19.8%*
12.2%
11.0%
HCVViremiaWasAssociatedWithIncreasedMortalityinaProspectiveTaiwaneseCohortStudy
3’UTR 5’UTR Core E1 E2 NS2 NS3 NS5A NS5B P7
Ribavirin
(RBV)
Polymerase
Daclatasvir (DCV)
Elbasvir (EBR)
Ledipasvir (LDV)
Ombitasvir (OBV)
Velpatasvir (VEL)
Sofosbuvir
(SOF)
Dasabuvir
(DSV)
NS5B
NUC
Inhibitors
NS5A
Replication
Complex
Inhibitors
NS5B
Non-NUC
Inhibitors
(NNI)
Grazoprevir (GZR)
Paritaprevir/Ritonavir
(PTV/RTV)
Simeprevir (SMV)
NS3
Protease
Inhibitors
Protease
Approved DAAs From Multiple Classes: Basis of 2017 Combination HCV Regimens Structural Domain
4A NS4B
Nonstructural Domain
Integrated Efficacy of Sof/Vel for 12 wks: SVR12
Agarwal, EASL 2016, Poster SAT-195
10
ASTRAL-1, -2, -3
SV
R12 (
%)
98 99 95 100 97 100
0
20
40
60
80
100 98
Total
1015
1035
323
328
264
277
116
116
34
35
41
41
237
238
GT 1 GT 2 GT 3 GT 4 GT 5 GT 6
‡
2 relapse
2 LTFU
1 D/C 1 D/C 11 relapse
2 D/C 1 death
Integrated Safety Analysis of SOF/VEL for 12 Weeks
11 Jacobson, EASL 2016, Poster SAT-168
ASTRAL-1, -2, -3
Retrospective integrated analysis of data from 1,035 SOF/VEL patients and
control/placebo patients in ASTRAL-1, -2, and -3
Patients, n (%) SOF/VEL 12 Week N=1035
Placebo 12 Week
N=116
SOF+RBV 12 Week
N=132
SOF+RBV 24 Week
N=275
AE 821 (79) 89 (77) 101 (77) 260 (95)
Grade 3 or 4 AE 33 (3) 1 (<1) 3 (2) 24 (9)
SAE 23 (2)* 0 2 (2) 15 (5)
AE leading to treatment D/C 2 (<1)˄ 2 (2) 0 9 (3)
Death 3 (<1)** 0 0 3 (1)*
*No SAE was assessed as related to SOF/VEL
**None were assessed as related to study treatment ˄Two subjects D/C SOF/VEL for AEs; (1 D/C day 1 due to difficulty concentrating, headache, and anxiety and 1 D/C day 13 of
due to anxiety)
‡
AEs in >10% of Patients
12 Jacobson, EASL 2016, Poster SAT-168
ASTRAL-1, -2, -3
Treatment with SOF/VEL for 12 weeks was well tolerated and had a safety
profile similar to that of placebo treatment
Patients, n (%) SOF/VEL 12 Week N=1035
Placebo 12 Week
N=116
SOF+RBV 12 Week
N=132
SOF+RBV 24 Week
N=275
Headache 296 (29) 33 (28) 29 (22) 89 (32)
Fatigue 217 (21) 23 (20) 47 (36) 105 (38)
Nausea 135 (13) 13 (11) 19 (14) 58 (21)
Insomnia 87 (8) 11 (9) 18 (14) 74 (27)
Nasopharyngitis 121 (12) 12 (10) 2 (2) 33 (12)
Cough 57 (6) 4 (3) 6 (5) 35 (13)
Irritability 49 (5) 4 (3) 9 (7) 40 (15)
Pruritus 33 (3) 5 (4) 7 (5) 35 (13)
Dyspepsia 33 (2) 4 (3) 5 (4) 30 (11)
Severe AEs were rare in SOF/VEL-treated patients, with headache, anxiety, and acute myocardial
infarction occurring >1 patient (both cases of acute myocardial infarction were assessed as not
related to SOF/VEL treatment by the investigators)
‡
Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interactiona
Concomitant Drug Class: Drug Name Effect on Concentrationb Clinical Comment
Acid Reducing Agents:
velpatasvir
Velpatasvir solubility decreases as pH increases. Drugs that increase gastric pH are expected to decrease concentration of velpatasvir.
Antacids (e.g., aluminum and magnesium hydroxide)
Separate antacid and EPCLUSA administration by 4 hours.
H2-receptor antagonistsc (e.g., famotidine)
H2-receptor antagonists may be administered simultaneously with or 12 hours apart from EPCLUSA at a dose that does not exceed doses comparable to famotidine 40 mg twice daily.
Proton-pump inhibitorsc (e.g., omeprazole)
Coadministration of omeprazole or other proton pump inhibitors is not recommended. If it is considered medically necessary to coadminister, EPCLUSA should be administered with food and taken 4 hours before omeprazole 20 mg. Use with other proton pump inhibitors has not been studied.
aThis table is not all inclusive. b = decrease, ↑ = increase cThese interactions have been studied in healthy adults.
Epclusa® [PI]. Gilead Sciences, Inc. Foster City, CA June 2016
Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interactiona
Concomitant Drug Class: Drug Name Effect on Concentrationb Clinical Comment
Antiarrhythmics:
amiodarone
Effect on amiodarone, sofosbuvir, and
velpatasvir concentrations unknown
Coadministration of amiodarone with EPCLUSA may result in serious symptomatic bradycardia. The mechanism of this effect is unknown. Coadministration of amiodarone with EPCLUSA is not recommended; if coadministration is required, cardiac monitoring is recommended [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)].
digoxinc ↑ digoxin
Therapeutic concentration monitoring of digoxin is recommended when coadministered with EPCLUSA. Refer to digoxin prescribing information for monitoring and dose modification recommendations for concentration increases of less than 50%.
Anticancers:
topotecan ↑ topotecan Coadministration is not recommended.
Anticonvulsants:
carbamazepine
phenytoin
phenobarbital
oxcarbazepine
sofosbuvir
velpatasvir Coadministration is not recommended.
Antimycobacterials:
rifabutin
rifampinc
rifapentine
sofosbuvir
velpatasvir Coadministration is not recommended.
aThis table is not all inclusive. b = decrease, ↑ = increase cThese interactions have been studied in healthy adults. Epclusa® [PI]. Gilead Sciences, Inc. Foster City, CA June 2016
Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interactiona
Concomitant Drug Class: Drug Name Effect on Concentrationb Clinical Comment
HIV Antiretrovirals:
efavirenzc velpatasvir Coadministration of EPCLUSA with efavirenz-containing regimens is not recommended.
Regimens containing tenofovir DF ↑ tenofovir
Monitor for tenofovir-associated adverse reactions in patients receiving EPCLUSA concomitantly with a regimen containing tenofovir DF. Refer to VIREAD or TRUVADA prescribing information for recommendations on renal monitoring.
tipranavir/ritonavir sofosbuvir
velpatasvir Coadministration is not recommended.
aThis table is not all inclusive. b = decrease, ↑ = increase cThese interactions have been studied in healthy adults.
Epclusa® [PI]. Gilead Sciences, Inc. Foster City, CA June 2016
Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interactiona
Concomitant Drug Class: Drug Name Effect on Concentrationb Clinical Comment
Herbal Supplements:
St. John’s wort (Hypericum perforatum)
sofosbuvir
velpatasvir Coadministration is not recommended.
HMG-CoA Reductase Inhibitors:
rosuvastatinc
rosuvastatin
Coadministration of EPCLUSA with rosuvastatin may significantly increase the concentration of rosuvastatin which is associated with increased risk of myopathy, including rhabdomyolysis. Rosuvastatin may be administered with EPCLUSA at a dose that does not exceed 10 mg.
atorvastatin atorvastatin
Coadministration of EPCLUSA with atorvastatin is expected to increase the concentrations of atorvastatin, which is associated with increased risk of myopathy, including rhabdomyolysis. Monitor closely for HMG-CoA reductase inhibitor-associated adverse events, such as myopathy and rhabdomyolysis.
aThis table is not all inclusive. b = decrease, ↑ = increase cThese interactions have been studied in healthy adults.
Epclusa® [PI]. Gilead Sciences, Inc. Foster City, CA June 2016
GT1 GT4 8 wks 12 wks
Analysis of HCV treatment in VA healthcare system (N = 107,079)[2]
– Dramatic increases in HCV treatment in 2014-2015 vs 1999-2013 (1999-2011, 1989 to 7196 treatments/yr; 2014, 9180 treatments; 2015, 31,028 treatments)
– Related to improved antiviral efficacy and availability of funding
Real-World HCV Treatment in the US VA Healthcare System
• Analysis of real-world SVR for pts with GT1-4 HCV treated with SOF + RBV ± pegIFN, SOF/LDV, or OBV/PTV/RTV + DSV (N = 17,487)[1]
Slide credit: clinicaloptions.com 1. Ioannou GN, et al. AASLD 2016. Abstract 21. Reproduced with permission. 2. Moon AM, et al. AASLD 2016. Abstract 227.
SVR
(%
)
100
80
60
40
20
0
93 90 95 96
GT1, SOF/LDV
13,974 135 1975 1556 n =
O/P/R + D
93 95
GT1
SOF/LDV
HBV Reactivation in Patients Receiving HCV Therapy
• Case reports of HBV reactivation in pts treated with SMV + SOF ± RBV,
DCV + ASV, and LDV/SOF
• Observational study of Chinese pts treated with DAAs (N = 327 screened)
– 3/10 HBsAg+ pts experienced hepatitis due to HBV reactivation
– Of 124 HBsAg-/HBcAb+ pts, none experienced hepatitis due to HBV reactivation
• Analysis of open-label phase IIIb trial
– No evidence of HBV reactivation in HBsAg-/HBcAb+ pts receiving LDV/SOF (n = 103)
• FDA to require boxed warning for certain DAAs regarding the risk of HBV reactivation and need for HBV screening/monitoring for pts being treated with DAAs
AASLD Guidance on HBV Reactivation in Patients Receiving HCV DAA Therapy
• HBV vaccination recommended for all susceptible individuals (eg., no previous immunization, no evidence of immunization response)
• All pts starting HCV DAA therapy should be assessed for HBV infection (HBsAg, anti-HBs, and anti-HBc total testing)
– If HBsAg+, assess HBV DNA prior to, during, and immediately after HCV DAA therapy
• For active HBV infection, initiate HBV therapy before or simultaneously with HCV DAA therapy
• For low or undetectable HBV DNA, monitor for HBV reactivation during HCV DAA therapy
– Insufficient data to provide recommendations for patients who are HBsAg- and anti-HBc+ or anti-HBs+/anti-HBc+
AASLD/IDSA HCV Guidelines. September 2016.
Recent Changes in Recommendations for Hep B Vaccination
• Expanded recommendation on HBV vaccination for adults with chronic liver disease
- Cirrhosis
- HCV infection
- Fatty liver disease
- Alcoholic liver disease
- Autoimmune hepatitis
- Cholestatic liver diseases
- AST or ALT > 2 x normal
Future Regimens: What Is Needed
• Remaining HCV treatment gaps
– DAA-experienced pts with resistance associated variants (RAVs) need effective options
– Pts with decompensated cirrhosis would benefit from more effective, RBV-free options
– Pts with renal insufficiency and GT 2, 3, 5, or 6 HCV infection still lack IFN- and RBV-free options
• In some cases, it may still be better not to treat until newer, more effective options are available
– e.g., DAA failures without cirrhosis
NS5A Resistance Associated Variants (RAVs)
• Failure rates higher with genotype 1a than 1b
• Approx. 10%-15% of genotype 1-infected patients without prior exposure to NS5A inhibitors have baseline NS5A RAVs
• In patients with genotype 1a infection, the presence of baseline NS5A RAVs that cause a large reduction in the activity of NS5A inhibitors (>5 fold) adversely impact response to NS5A-containing regimens.
NS5A Resistance Associated Variants (RAVs) Contd.
• RAVs include variants at positions M28, Q30, L31, and Y93 in genotype 1a and are found by population sequencing in roughly 5%-10% of patients.
• Given that baseline NS5A RAVs are one of the strongest pre-treatment predictors of treatment outcome with certain regimens in patients with genotype 1a infection, testing for these RAVs prior to deciding on a therapeutic course is recommended in select situations.
Investigational Direct-Acting Antivirals For Hepatitis C Treatment
Drug Abbreviation Class
Glecaprevir (formerly ABT-493)
GLE NS3/4A protease inhibitor
Voxilaprevir VOX NS3/4A protease inhibitor
Pibrentasvir (formerly ABT-530)
PIB NS5A inhibitor
Ruzasvir (formerly MK-8408)
RZR NS5A inhibitor
MK-3682 -- NS5B polymerase nucleotide
inhibitor
Late-Phase Investigational HCV Regimens: What They Offer
Regimen Key Features
Sofosbuvir/velpatasvir/voxilaprevir
(GS-9857) Once-daily FDCs
Pangenotypic
High SVR rates in DAA-experienced
pts (and other populations)
Glecaprevir (ABT-493)/pibrentasvir
(ABT-530)
Grazoprevir/ruzasvir (MK-8408)/
MK-3682
AL-335 + odalasvir + simeprevir Once daily
Pangenotypic
POLARIS-1: SOF/VEL/VOX for 12 Wks After NS5A Failure in GT1-6 HCV
• Randomized, double-blind, placebo-controlled phase III trial
Bourlière M, et al. AASLD 2016. Abstract 194.
SOF/VEL/VOX
400/100/100 mg PO QD
(n = 263)
Placebo PO QD
(n = 152)
DAA-experienced pts with GT1-6 HCV and
NS5A inhibitor experience*
(N = 415)
Stratified by cirrhotic vs noncirrhotic
*Pts with GT1 HCV at screening equally randomized between arms; pts with GT2-6 HCV assigned to active treatment arm.
Wk 12
Previous NS5A treatment in SOF/VEL/VOX group (n = 263)
– LDV, 51%; DCV, 27%; OBV, 11%; other, 13%
Cirrhosis definition for POLARIS studies: METAVIR F4 or Ishak 5-6 on biopsy, or FibroTest > 0.75 + APRI > 2, or FibroScan > 12.5 kPa
Subsequently received deferred
SOF/VEL/VOX
POLARIS-1: SVR12 Rates With 12-Wk SOF/VEL/VOX in Previous NS5A Failure
Bourlière M, et al. AASLD 2016. Abstract 194.
• 7 virologic failures; all cirrhotic pts (GT1a, n = 2; GT3, n = 4; GT4, n = 1)
SVR12, % (n/N) SOF/VEL/VOX
Overall 96 (253/263)
Cirrhosis status
No cirrhosis 99 (140/142)
Cirrhosis 93 (113/121)
Baseline RAVs
None 98 (42/43)
Any 96 (199/208)
SVR12, % (n/N) SOF/VEL/VOX
Genotype
1a 96 (97/101)
1b 100 (45/45)
2 100 (5/5)
3 95 (74/78)
4 91 (20/22)
5 100 (1/1)
6 100 (6/6)
POLARIS-3: 8-Wk SOF/VEL/VOX vs 12-Wk SOF/VEL for Cirrhotic, DAA Naive GT3
• Randomized, open-label, active-controlled phase III trial
SOF/VEL/VOX 400/100/100 mg PO QD
(n = 110)
SOF/VEL 400/100 mg PO QD
(n = 109)
Stratified by prior IFN (experienced vs naive)
Patients with GT3 HCV infection
and cirrhosis with or without prior
IFN experience (N = 219)
Wk 8 Wk 12
IFN experience in 29% to 32% of pts
Foster GR, et al. AASLD 2016. Abstract 258.
POLARIS-3: SVR12 Rates With 8-Wk SOF/VEL/VOX for Cirrhotic GT3 Pts
• Both regimens: P < .001 for superiority vs prespecified 83% goal • Overall VF: SOF/VEL/VOX, n = 2 relapses; SOF/VEL, n = 1 each for relapse and on-treatment failure • No treatment-emergent RAVs in SOF/VEL/VOX arm; Y93H in both virologic failures in SOF/VEL arm
Foster GR, et al. AASLD 2016. Abstract 258.
Treatment Naive Treatment Experienced
No BL RAVs Any BL RAVs Overall
100
80
60
40
20
0
SVR
12
(%
)
SOF/VEL/VOX 8 wks SOF/VEL 12 wks
106/
110
105/
109
72/
75
76/
77
34/
35
29/
32
80/
84
76/
80
23/
23
23/
23
96 96 96 99 97 91 95 95 100 100
n/N =
POLARIS-4: SOF/VEL/VOX for DAA-Exp’d, NS5A Inhibitor-Naive GT1-6 HCV
• Randomized, open-label, active-controlled phase III trial
Zeuzem S, et al. AASLD 2016. Abstract 109.
SOF/VEL/VOX
400/100/100 mg PO QD
(n = 182)
SOF/VEL 400/100 mg PO QD
(n = 151)
DAA-experienced pts with GT1-6 HCV* and
no NS5A inhibitor experience
with or without cirrhosis (N = 333)
Stratified by HCV genotype, cirrhosis (yes vs no)
*Pts with GT1-3 HCV randomized 1:1 between arms. Pts with GT4-6 HCV assigned to SOF/VEL/VOX.
Wk 12
Prior HCV treatment
– SOF, 69%; other NS5B inhibitor, 4%
– SOF + SMV, 11%; other NS5B/NS3 inhibitor combinations, 14%
– Other, 2%
POLARIS-4: Efficacy of SOF/VEL/VOX for DAA-Exp’d, NS5A Inhibitor Naive HCV Geno 1-6 Pts
Zeuzem S, et al. AASLD 2016. Abstract 109.
Cirrhotic
SOF/VEL/VOX: P < .001 for superiority vs prespecified 85% goal; SOF/VEL: P = .092
Overall, reduced SVR12 rate for SOF/VEL driven by increased number of relapses
– SOF/VEL/VOX (n = 182): 1 relapse, 1 death, 3 LTFU
– SOF/VEL (n = 151): 14 relapses, 1 breakthrough
n/N =
Overall Noncirrhotic
SVR
12
(%
)
100
80
60
40
20
0
97 90
98 94 96
86
177/
182
136/
151
96/
98
77/
82
81/
84
59/
69
94 89
84/
89
67/
75
100 90
83/
83
63/
70
No RAVs Any RAVs
SOF/VEL/VOX 12 wks SOF/VEL 12 wks
SURVEYOR-II, Part 3: GLE/PIB for Pts With GT3 HCV ± Cirrhosis
• Partially randomized, open-label phase II trial (N = 131)
Wyles DL, et al. AASLD 2016. Abstract 113.
*Dosing: GLE/PIB given as 3 coformulated 100/40 mg tablets QD for a total dose of 300/120 mg.
GLE/PIB*
(n = 22)
GLE/PIB*
(n = 22)
Treatment-experienced, noncirrhotic
pts with GT3 HCV
GLE/PIB*
(n = 40)
GLE/PIB*
(n = 47)
Treatment-naive pts with GT3 HCV and compensated cirrhosis
Treatment-experienced pts with GT3 HCV and compensated cirrhosis
Wk 12 Wk 16
Prior treatment experience consisted of IFN or pegIFN ± RBV or SOF + RBV ± pegIFN
SURVEYOR-II, Part 3: SVR12 Rates With GLE/PIB for Pts With GT3 HCV ± Cirrhosis
Slide credit: clinicaloptions.com Wyles DL, et al. AASLD 2016. Abstract 113. Reproduced with permission.
Tx Wks Cirrhosis
Tx Experienced
Breakthrough Relapse
LTFU
100
80
60
40
20
0
SVR
12
(%
)
91 96 98 96
12 - +
0 2 0
16 - +
0 1 0
12 + -
0 0 1
16 + +
1 1 0
20/22 21/22 39/40 45/47 n/N =
Slide credit: clinicaloptions.com
EXPEDITION-IV: GLE/PIB for Pts With GT1-6 HCV and Renal Impairment
Gane EJ, et al. AASLD 2016. Abstract LB11.
*Dosing: GLE/PIB given as 3 coformulated 100/40-mg tablets QD for a total dose of 300/120 mg. †Prior treatment experience consisted of IFN or pegIFN ± RBV or SOF + RBV ± pegIFN.
‡1 pt d/c, 1 pt LTFU in ITT analysis of SVR12.
• At baseline, 82% on hemodialysis; 19% cirrhotic; 42% treatment experienced
• SVR12 rate of 98% (ITT; n/N = 102‡/104)
Open-label, single-arm phase III trial
GLE/PIB*
(N = 104)
GT1-6 HCV pts with stage 4 or 5 CKD
with compensated cirrhosis or without cirrhosis and with or without
treatment experience† (N = 104)
Wk 12
Slide credit: clinicaloptions.com
C-CREST 1 & 2: MK-3682/GZR/RZR ± RBV for Treating Pts With GT1-3 HCV
MK-3682/GZR/RZR
(n = 173: GT1, n = 88; GT2, n = 32; GT3, n = 53)
MK-3682/GZR/RZR + RBV
(n = 81: GT2, n = 31; GT3, n = 50)
MK-3682/GZR/RZR
(n = 213: GT1, n = 88; GT2, n = 46; GT3, n = 79)
MK-3682/GZR/RZR + RBV
(n = 96: GT2, n = 16; GT3, n = 80)
MK-3682/GZR/RZR
(n = 76: GT2, n = 26; GT3, n = 50)
MK-3682/GZR/RZR + RBV
(GT3, n = 25)
Dosing: MK-3682/GZR/RZR dosed as two 225/50/30-mg tablets QD. Pts with GT3 HCV could be treatment naive or have failed on pegIFN/RBV; all others treatment naive. Cirrhosis definition in notes.
Patients with GT1-3 HCV, HCV RNA
≥ 10,000 IU/mL, with or without compensated
cirrhosis (N = 664)
Wk 8 Wk 12 Wk 16 • Part B: randomized, open-label phase II trials
Lawitz E, et al. AASLD 2016. Abstract 110.
Baseline: 35% to 43% cirrhotic; 44% of GT3 pts had prior pegIFN/RBV
C-CREST 1 & 2: Efficacy of MK-3682/ GZR/RZR ± RBV for Pts With GT1-3 HCV
Slide credit: clinicaloptions.com Lawitz E, et al. AASLD 2016. Abstract 110. Reproduced with permission.
• Presence of cirrhosis, use of ribavirin, prior tx experience did not impact SVR12 rates
Full Analysis Set
8 wks 12 wks 16 wks
100
80
60
40
20
0
SVR
12
(%
)
GT1a GT1b GT2 GT3
93 98 98 100 86
97 100 95 97 96
39/ 42
47/ 48 n/N =
45/ 46
40/ 40
54/ 63
60/ 62
26/ 26
98/ 103
155/ 159
72/ 75
SVR12 by Baseline RAV
Presence, % (n/N)
GT2 HCV GT3 HCV
No L31M L31M No Y93H Y93H
8 wks 94 (31/33) 80 (20/25) 98 (95/97) 50 (2/4)
12 wks 100 (23/23) 100 (28/28) 99 (147/148) 71 (5/7)
Relapse (n) 2 0 1 0 7 0 4 3 2 0
Slide credit: clinicaloptions.com
C-SURGE: MK-3682/GZR/RZR for GT1 HCV Pts Who Relapsed on DAA Therapy • Randomized, open-label phase II trial (interim analysis)
MK-3682/GZR/RZR + RBV
(n = 45)
MK-3682/GZR/RZR
(n = 49)
Dosing: MK-3682/GZR/RZR two 225/50/30-mg tablets once daily; weight-based RBV (800-1400 mg/day). Trial included compensated cirrhotic and noncirrhotic pts; cirrhosis definition in slidenotes.
Stratified by GT1 subtype (1a vs 1b) and cirrhosis (yes vs no)
Pts with GT1 HCV (HCV RNA ≥ 10,000 IU/mL)
and relapse after SOF/LDV ± RBV or
GZR/EBR ± RBV (N = 94)
Wk 16 Wk 24
Baseline characteristics:
– Previous failing regimen: LDV/SOF 12-24 wks, 61%; LDV/SOF 8 wks, 15%; GZR/EBR 12 wks, 24%
– NS5A RAVs, 84%; NS3 RAVs, 65%
Wyles DL, et al. AASLD 2016. Abstract 193.
Slide credit: clinicaloptions.com
C-SURGE: SVR8 Rates With MK-3682/GZR/RZR for DAA Relapses
Wyles DL, et al. AASLD 2016. Abstract 193. Reproduced with permission.
100
80
60
40
20
0
Perc
ent
of
Pts
Wit
h H
CV
RN
A
< 1
5 IU
/mL
n/N =
16 wks + RBV 24 wks without RBV
98 100
43/44 30/30
98 100
43/44 38/38
91 92
40/44 45/49
TW4 SVR4 SVR8
No impact of NS5A or NS3 RAVs on SVR4, including Y93 RAVs
– 4% of pts had ≥ 3 NS5A RAVs; 55% had dual NS5A and NS3 RAVs
Future of Hepatitis C Treatment
• Treat NS5A inhibitor failed patients
• Better outcomes for Genotype 3 patients, especially those with cirrhosis
• Shorter duration 8 weeks in treatment-naïve and 12-16 weeks in treatment-experienced patients
• Optional treatment in Renal impairment patients
Approved Drug Combinations for Hepatitis C Treatment
• Elbasvir 50 mg + Grazoprevir 100 mg, one tablet daily for Genotypes 1, 4. Duration 12 wk G1b, G1a w/o NS5A polymorphism; 16 weeks for G1a w poly, G4 Peg-I,riba failed. Treatment-naïve, treatment-failed, well-compensated cirrhosis, renal failure
• Ledipasvir 90 mg + Sovaldi 400 mg, one tablet daily for Genotypes 1,4,5,6. Duration 8 weeks for HCV RNA <6 million IU/mL, 12 weeks for all others including decompensated cirrhosis except 24 weeks for treatment-experienced cirrhotics
Approved Drug Combinations for Hepatitis C Treatment, Contd.
• Paritaprevir 150 + Ritonavir 50 + Ombitasvir 25 mg + Dasabuvir 250 mg (PROD), three tablets daily + wt based ribavirin 1000-1200 mg daily for genotypes 1a, 4. PRO alone for genotype 1b. Treatment naïve, experienced, with or without compensated cirrhosis. Contraindicated in decompensated cirrhosis. Duration 12 weeks
• Simeprevir 150 mg + Sovaldi 400 mg for genotypes 1b and 1a without Q80K mutation, treatment-naïve, treatment experienced with or without cirrhosis. Contraindicated in decompensated cirrhotics. Duration 12-24 weeks
Approved Drug Combinations for Hepatitis C Treatment, Contd.
• Sovaldi 400 mg + Velpatasvir 400 mg, one tablet daily for all genotypes 1-6, only pangenotypic, Treatment- naïve, -experienced, with or without cirrhosis. Duration 12 weeks.
• Sovaldi 400 mg + wt-based Ribavirin for genotype 2, treatment-naïve, -experienced, with or without cirrhosis. Duration 24 weeks.
• Sovaldi 400 mg + Daclatasvir 60 mg, two tablets daily for genotype 1 with or without cirrhosis, and for genotype 3 without cirrhosis. Duration 12 weeks for genotype 1, 24 weeks for genotype 3