Early Gallbladder Carcinoma A Clinicopathologic …...of 11 sections per case was examined. The...
Transcript of Early Gallbladder Carcinoma A Clinicopathologic …...of 11 sections per case was examined. The...

Am J Clin Pathol 2011;135:637-642 637637 DOI: 10.1309/AJCPFRKCFEDLV03Y 637
© American Society for Clinical Pathology
Anatomic Pathology / Early Gallbladder Carcinoma
Early Gallbladder Carcinoma
A Clinicopathologic Study of 13 Cases of Intramucosal Carcinoma
Jorge Albores-Saavedra, MD,1,2 Fredy Chable-Montero, MD,1,2 David Angeles-Albores,2 Arnold Schwartz, MD, PhD,3 David S. Klimstra, MD,4 and Donald E. Henson, MD5
Key Words: Intramucosal carcinoma; Gallbladder; Histologic types; Clinical course
DOI: 10.1309/AJCPFRKCFEDLV03Y
A b s t r a c t
We report the clinicopathologic features of 13 cases of intramucosal carcinoma (IMC) of the gallbladder. All IMCs were incidental findings in cholecystectomy specimens for cholelithiasis. However, one of the patients had a carcinoma of the pancreas, and the gallbladder incidentally removed during the Whipple procedure showed an IMC. Another patient had a small cell carcinoma of the gallbladder, and one of the sections showed an IMC. Of the IMCs, 10 were well-differentiated adenocarcinomas, 1 was a moderately differentiated adenocarcinoma, 1 was an undifferentiated carcinoma, and 1 was a squamous cell carcinoma. Of the patients, 8 were disease-free from 3 to 11 years, and 2 patients died, one as a result of the pancreatic ductal carcinoma and the other with disseminated metastases of the small cell carcinoma. The follow-up of another patient was too short to be significant. Two patients were lost to follow-up. Our findings suggest that a simple cholecystectomy is a curative procedure for IMCs of the gallbladder.
The prognosis of carcinoma of the gallbladder continues to depend primarily on the extent of the tumor and histologic type.1-3 Depth of tumor invasion and the presence of regional or distant metastasis are the most significant prognostic fac-tors.1-3 Invasive papillary carcinomas are characterized by a less aggressive clinical course than conventional adenocarci-nomas and mucinous and adenosquamous carcinomas.1-3 The 5- and 10-year relative survival rates of small cell carcinoma of the gallbladder are 8% and 0%, respectively.4,5 Because early gallbladder carcinomas are usually asymptomatic, they are most commonly detected incidentally in gallbladders removed for cholelithiasis, a procedure now generally per-formed laparoscopically. When incidental carcinomas are detected in laparoscopic cholecystectomy specimens, reop-eration with resection of the gallbladder bed, cystic duct remnant, and portal lymph nodes provides improved survival for patients with carcinomas invading into the muscularis or through the wall into adjacent soft tissues.6
In 1986, we described and illustrated the first example of intramucosal carcinoma (IMC) of the gallbladder, which was an incidental finding in a cholecystectomy specimen for cholelithiasis.7 At that time, the clinical course of this early stage of gallbladder carcinoma, defined as invasive carci-noma confined to the lamina propria (pT1a), was unknown. To our knowledge, there have been no studies of IMC of the gallbladder to determine the clinical behavior of these tumors and the best form of surgical management. Because of the higher rates of laparoscopic cholecystectomies in the treatment of gallstone disease, more cases of early gallblad-der carcinoma are now being detected, and, consequently, we are beginning to better understand the patient outcomes after cholecystectomy alone.

638 Am J Clin Pathol 2011;135:637-642638 DOI: 10.1309/AJCPFRKCFEDLV03Y
© American Society for Clinical Pathology
Albores-Saavedra et al / Early Gallbladder Carcinoma
The purposes of this study were to describe the clinico-pathologic features of 13 cases of IMC of the gallbladder, define morphologic criteria for diagnosis, determine the surviv-al rate of the patients, and make therapeutic recommendations.
Materials and Methods
From the personal consultation files of 2 of us (J.A.-S., 12 cases and D.S.K., 1 case), 13 cases of IMCs were retrieved and evaluated. H&E-stained sections were available for review in all 13 gallbladders, which were submitted in total. An average of 11 sections per case was examined. The histologic grad-ing and subtyping of the carcinomas were defined according to previously described criteria.1 In addition, immunostains for cytokeratin (CK) 7 and CK20 were examined in 7 cases. In 4 of these 7 cases, immunostains for MUC1, MUC2, and CDX2 were also reviewed, as were the corresponding control samples. Follow-up was obtained through the referring physi-cians or the clinical charts.
Results
All IMCs were not recognized grossly and were inciden-tal microscopic findings in cholecystectomy specimens for cholelithiasis; they were pT1a, as defined by the American Joint Committee on Cancer.8 The age of patients at diagnosis ranged from 42 to 79 years (mean age, 63 years) with a female predominance (9 women and 4 men). A diagnosis of carcino-ma of the gallbladder was not suspected preoperatively in any
of the patients. However, one of the patients had a clinically diagnosed carcinoma of the head of the pancreas that proved to be a well-differentiated ductal carcinoma, intestinal type.9 During the Whipple procedure, the gallbladder was removed because of cholelithiasis and contained an undifferentiated IMC. Another patient had a small cell carcinoma of the gall-bladder that was extensively sampled. One of the sections showed an incidental IMC in proximity to but separated from the small cell carcinoma.
Of the patients, 11 underwent laparoscopic cholecystec-tomies, 1 patient was treated with a Whipple procedure and a cholecystectomy, and 1 patient underwent an open cholecys-tectomy. No patient was subjected to surgical reexploration or hepatic resection, and none of the patients with only IMC of the gallbladder received chemotherapy or radiotherapy. Of the 13 patients, 8 were disease-free from 3 to 11 years (mean, 4.8 years) after cholecystectomy; 2 died, one as a result of ductal carcinoma of the pancreas and the other with dissemi-nated metastases of small cell carcinoma of the gallbladder, tumors coincident but unrelated histologically to the IMC of the gallbladder. The follow-up of 1 patient was too short to be significant, and 2 patients were lost to follow-up.
Microscopic PathologyOf the 13 IMCs, 2 were polypoid and 1 arose in a tubular
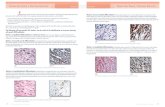
adenoma pyloric type. Classification of the IMCs resulted in 10 classified as well-differentiated adenocarcinomas, 1 as a moderately differentiated adenocarcinoma, 1 as an undifferen-tiated carcinoma, and 1 as a squamous cell carcinoma ❚Image 1❚, ❚Image 2❚, ❚Image 3❚, ❚Image 4❚, and ❚Image 5❚. Of the carcinomas, 7 had an intestinal phenotype (CK20+, MUC2+,
A B
❚Image 1❚ A, A small polypoid ulcerated intramucosal adenocarcinoma (H&E, ×15). B, Higher magnification showing invasive small and medium-sized neoplastic glands confined to the lamina propria (H&E, ×250).

Am J Clin Pathol 2011;135:637-642 639639 DOI: 10.1309/AJCPFRKCFEDLV03Y 639
© American Society for Clinical Pathology
Anatomic Pathology / Original Article
and CDX2+), 5 a biliary phenotype (CK7+ and MUC1+), and 1 a squamous phenotype. A dense inflammatory infiltrate was seen in 2 IMCs (Image 3). The undifferentiated carcinoma arose in a background of cholesterolosis (Image 4).The IMC that arose in a tubular adenoma pyloric type had an intestinal phenotype (Image 5A). All tumors expanded the lamina pro-pria, but the muscle layer was not involved.
The mucosa adjacent to 11 IMCs showed high-grade dysplasia/carcinoma in situ. A desmoplastic stroma was present
in only 1 IMC, and there was no lymphovascular invasion in any of the tumors. The cystic lymph node was examined in 7 cases and was free of tumor. The autopsy of the patient with small cell carcinoma did not reveal adenocarcinoma in any of the metastases. A biopsy of a metastatic deposit from the patient with carcinoma of the pancreas revealed a well-differentiated ductal carcinoma intestinal type similar to the primary pancreatic tumor and unlike the undifferentiated IMC of the gallbladder.
A B
B
❚Image 3❚ A, Well-differentiated intestinal-type adenocarcinoma containing few goblet cells. Small clusters of neoplastic cells including signet ring cells infiltrate the lamina propria between neoplastic glands (arrow and inset) (H&E, ×25). B, Moderately differentiated intramucosal carcinoma containing a dense lymphocytic infiltrate (H&E, ×30).
❚Image 2❚ A, Low-power view of an intramucosal adenocarcinoma that coexisted with but was separated from a small cell carcinoma (arrow) (H&E, ×10). B, Higher magnification of the neoplastic glands lined by columnar cells with an intestinal phenotype. An abnormal mitotic figure is seen (H&E, ×250).
A

640 Am J Clin Pathol 2011;135:637-642640 DOI: 10.1309/AJCPFRKCFEDLV03Y
© American Society for Clinical Pathology
Albores-Saavedra et al / Early Gallbladder Carcinoma
Discussion
IMCs have been described and characterized in the esophagus,10 stomach,11 and colon.12,13 They have been the source of controversy regarding clinical course and surgi-cal management. Depending on the origin of the carcinoma, some authorities have advocated conservative endoscopic treatment, whereas others have recommended a more aggres-sive surgical approach because a small proportion of IMCs may recur or metastasize.13,14 IMCs of the gallbladder are practically unknown, and, therefore, little is known about their
demographics, histologic features, and biologic behavior. Similar to invasive gallbladder carcinomas that extend beyond the lamina propria, IMCs are more common in females. However, the mean age of patients is 8 years younger than patients with invasive carcinomas that extend beyond the lamina propria.1,3
As IMCs of the esophagus, stomach, and colon, those arising in the gallbladder were confined to the expanded lam-ina propria. In contrast with colonic and similar to esophageal and gastric IMCs, the vast majority of IMCs of the gallblad-der developed through the high-grade dysplasia carcinoma
A B
A B
❚Image 4❚ A, An undifferentiated intramucosal carcinoma that coexisted with a ductal carcinoma of the pancreas (H&E, ×20). B, The undifferentiated carcinoma arose in a background of cholesterolosis (H&E, ×300).
❚Image 5❚ A, An intestinal-type intramucosal carcinoma arose in a tubular adenoma of the pyloric type. Clusters of benign pyloric-type glands of the adenoma and the malignant glands are clearly depicted (H&E, ×20). B, Intramucosal, well-differentiated squamous cell carcinoma (H&E, ×30).

Am J Clin Pathol 2011;135:637-642 641641 DOI: 10.1309/AJCPFRKCFEDLV03Y 641
© American Society for Clinical Pathology
Anatomic Pathology / Original Article
sequence. Of the 13 IMCs, 11 were associated with high-grade dysplasia/carcinoma in situ in the gallbladder mucosa adjacent to the tumors. Only 1 of the 13 gallbladder tumors reported herein arose in a tubular adenoma pyloric type, again emphasizing that most gallbladder IMCs arise from flat dysplastic precursors rather than polypoid adenomas. All 13 IMCs expanded the lamina propria, but only 1 showed a desmoplastic stromal response, a finding also typical of IMCs of the tubular gastrointestinal tract. There was no vascular invasion in any of the tumors. Most gallbladder IMCs (11/13) were well- or moderately differentiated adenocarcinomas, one was classified as undifferentiated carcinoma, and another was classified as squamous cell carcinoma. There were no cases of signet ring cell carcinomas, as have been described in the stomach and the colon. However, we have described 2 cases of signet ring cell carcinoma in situ of the gallbladder similar to those reported in the stomach and colon.5
As indicated, 2 patients in our series died with second-primary synchronous carcinomas. These 2 cases serve to emphasize the propensity for multicentric neoplasia with-in the pancreaticobiliary tree, as previously reported.15-17 Furthermore, these examples have led to the concept that a field of carcinogenesis exists in the gallbladder, extrahepatic bile ducts, ampulla, and pancreas.18 Carcinomas have co-occurred in the gallbladder, extrahepatic bile ducts, ampulla, and in the pancreas, all foregut derivatives that have a com-mon embryologic origin.15-17,19,20 Metachronous multiple biliary cancers have been reported in only 12 cases.21,22 In 1 case, metachronous bile duct carcinoma developed 9 years after resection of a primary gallbladder cancer.21 Double pri-mary cancers of the gallbladder and extrahepatic biliary ducts not associated with an anomalous junction of the pancreatico-biliary duct system have also been reported.23,24 In addition, these double primary tumors have often had different genetic and immunohistochemical profiles.21
The fact that none of the 10 patients with IMCs of the gallbladder with adequate follow-up died as a result of the gallbladder neoplasm suggests that a simple cholecystecto-my is a curative surgical procedure. However, the question whether these patients should be subjected to a reexploration remains open. First, the number of patients included in this study is small, and only 1 case of undifferentiated carci-noma (a highly aggressive neoplasm) was included. It is therefore clear that more information is needed based on larger series and, ideally, long-term follow-up. On the other hand, a reexploration with liver resection (segmentectomy) and dissection of the porta hepatis lymph nodes is a radical procedure associated with a risk of morbidity and mortality that has proven to be beneficial only in T2 and T3 gallblad-der carcinomas.25 For patients with T1 disease only, the benefits for additional surgery are less clear.26 Since the general T1 category includes muscle-invasive carcinomas
(pT1b) and IMC (pT1a), the need for reexcision following laparoscopic cholecystectomy for IMC will likely be dif-ficult to demonstrate.
Because of the increasing number of laparoscopic chole-cystectomies for the treatment of gallstone disease, we believe the detection of IMC of the gallbladder will increase (11 of 13 cases in the current series were diagnosed after 1995).27,28 To exclude invasion into the muscularis propria and learn more about the biologic behavior of IMC of the gallbladder, it is prudent to make the following recommendations for surgi-cal pathologists: The entire gallbladder should be submitted for microscopic examination, and at least 3 levels should be obtained from each paraffin block demonstrating carcinoma. For diagnosis, the tumor should be confined to the lamina propria, and the muscle layer (muscularis propria) should not be involved. It is important to remember that the gallbladder lacks a muscularis mucosa and a submucosa. If present, the cystic lymph node should be examined microscopically. A detailed search for lymphovascular invasion should be made. The histologic type of carcinoma should be recorded. By following these recommendations, pathologists can provide useful information to stage the tumor and help to determine the best form of therapy.
We reported 13 cases of IMC of the gallbladder, 11 of which were diagnosed after 1995. Two patients had symp-tomatic synchronous second-primary malignant neoplasms, one a ductal carcinoma of the pancreas and the other a small cell carcinoma of the gallbladder, that were responsible for the death of the patients. Eight patients remained asymptomatic from 3 to 11 years (mean, 4.8 years). The follow-up of another patient was too short to be significant. Two patients were lost to follow-up. Most of the neoplasms were well-differentiated adenocarcinomas with an intestinal or a biliary phenotype. Only 1 undifferentiated carcinoma and 1 squamous cell car-cinoma were included in the series. In contrast with colonic IMCs, only 1 IMC in the current series arose in a tubular adenoma pyloric type. Our results suggest that a simple cholecystectomy is a curative procedure for IMCs of the gall-bladder. Because of the small number of cases, however, we believe that larger series with long-term follow-up are needed to confirm our findings.
From the 1Department of Pathology, Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” and 2Medica Sur Clinic and Foundation, Mexico City, Mexico; 3Department of Pathology, George Washington University Hospital, Washington, DC; 4Memorial Sloan-Kettering Cancer Center, New York, NY; and 5George Washington University Cancer Institute, Washington, DC.
Address reprint requests to Dr Albores-Saavedra: Department of Pathology, Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán,” Vasco de Quiroga 15, Tlalpan, Mexico City, Mexico.

642 Am J Clin Pathol 2011;135:637-642642 DOI: 10.1309/AJCPFRKCFEDLV03Y
© American Society for Clinical Pathology
Albores-Saavedra et al / Early Gallbladder Carcinoma
References 1. Albores-Saavedra J, Henson DE, Klimstra DS. Tumors of
the Gallbladder, Extrahepatic Bile Ducts and Ampulla of Vater. Washington, DC: Armed Forces Institute of Pathology; 2000. Atlas of Tumor Pathology: Third series, Fascicle 27.
2. Albores-Saavedra J, Mathew T, McLaren BK, et al. Papillary carcinomas of the gallbladder: analysis of noninvasive and invasive types. Arch Pathol Lab Med. 2005;129:905-909.
3. Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder: histologic grade, stage of disease, grade and survival. Cancer. 1992;70:1493-1497.
4. Albores-Saavedra J, Batich K, Hossain S, et al. Carcinoid tumors and small cell carcinomas of the gallbladder and extrahepatic bile ducts: a comparative study based on 221 cases from the Surveillance, Epidemiology, and End Results Program. Ann Diagn Pathol. 2009;13:378-383.
5. Albores-Saavedra J, Molberg K, Henson DE. Unusual malignant epithelial tumors of the gallbladder. Semin Diagn Pathol. 1996;13:326-338.
6. Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior non-curative intervention. Ann Surg. 2000;232:557-569.
7. Albores-Saavedra J, Henson DE. Tumors of the Gallbladder and Extrahepatic Bile Ducts. Washington, DC: Armed Forces Institute of Pathology; 1986:108. Atlas of Tumor Pathology; Second series, Fascicle 22.
8. Edge SB, Byrd DR, Compton CC, et al, eds. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010.
9. Albores-Saavedra J, Simpson K, Dancer YJ, et al. Intestinal type adenocarcinoma: a previously unrecognized variant of ductal carcinoma of the pancreas. Ann Diagn Pathol. 2007;11:3-9.
10. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high grade dysplasia and intramucosal carcinoma: an American single center experience. Am J Gastroenterol. 2009;104:2684-2692.
11. Iriyama K, Asakawa T, Koike H, et al. Is extensive lymphadenectomy necessary for surgical treatment of intramucosal carcinoma of the stomach? Arch Surg. 1989;124:309-311.
12. Lewin MR, Fenton H, Burkart AL, et al. Poorly differentiated colorectal carcinoma with invasion restricted to the lamina propria (intramucosa carcinoma): a follow-up study of 15 cases. Am J Surg Pathol. 2007;31:1882-1886.
13. Shia J, Klimstra DS. Intramucosal poorly differentiated colorectal carcinoma: can it be managed conservatively [letter]? Am J Surg Pathol. 2008;32:1586-1588.
14. Okabe S, Shia J, Nash G, et al. Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg. 2004;8:1032-1039.
15. Minami Y, Hasuike Y, Takeda Y, et al. Metachronous double cancer of the gallbladder and pancreas associated with pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 2008;15:330-333.
16. Sato K, Maekawa T, Yabuki K, et al. A case of triple synchronous cancers occurring in the gallbladder, common bile duct, and pancreas. J Gastroenterol. 2003;38:97-100.
17. Ueda N, Nagakawa T, Ohta T, et al. Synchronous cancer of the biliary tract and pancreas associated with anomalous arrangement of the pancreatobiliary ductal system. J Clin Gastroenterol. 1992;15:136-141.
18. Henson DE, Schwartz AM, Nsouli H, et al. Carcinomas of the pancreas, gallbladder, extrahepatic bile ducts, and ampulla of Vater share a field of carcinogenesis: a population-based study. Arch Pathol Lab Med. 2009;133:67-71.
19. Agoff SN, Crispin DA, Bronner MP, et al. Neoplasms of the ampulla of Vater with concurrent pancreatic intraductal neoplasia: a histological and molecular study. Mod Pathol. 2001;14:139-146.
20. Nishihara K, Tsuneyoshi M, Shimura H, et al. Three synchronous carcinomas of the papilla of Vater, common bile duct and pancreas. Pathol Int. 1994;44:325-332.
21. Joo HJ, Kim GH, Jeon WJ, et al. Metachronous bile duct cancer 9 years after resection of gallbladder cancer. World J Gastroenterol. 2009;15:3440-3444.
22. Nordback IH, Hruban RH, Cameron JL. Second-primary lesions in the biliary tree after successful resection of ampullary carcinoma. Surgery. 1992;112:111-115.
23. Hori H, Ajiki T, Fujita T, et al. Double cancer of gallbladder and bile duct not associated with anomalous junction of the pancreaticobiliary duct system. Jpn J Clin Oncol. 2006;36:638-642.
24. Itoh T, Fuji N, Taniguchi H, et al. Double cancer of the cystic duct and gallbladder associated with low junction of the cystic duct. J Hepatobiliary Pancreat Surg. 2008;15:338-343.
25. Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive resection is beneficial. Cancer. 1998;83:423-427.
26. Shoup M, Fong Y. Surgical indications and extent of resection in gallbladder cancer. Surg Oncol Clin N Am. 2002;11:985-994.
27. Osborne DA, Alexander G, Boe B, et al. Laparoscopic cholecystectomy: past, present, and future. Surg Technol Int. 2006;15:81-85.
28. Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205-211.