Diagnosis of Endometrial Biopsies and Curettings · endometrium undergoes a variety of morpho-logic...
Transcript of Diagnosis of Endometrial Biopsies and Curettings · endometrium undergoes a variety of morpho-logic...

Diagnosis of Endometrial Biopsies and Curettings
Second Edition

Michael T. Mazur, MDClinical Professor of Pathology, State University of New York, UpstateMedical University, Syracuse, New York, and ClearPath Diagnostics,Syracuse, New York
Robert J. Kurman, MDRichard W. TeLinde Distinguished Professor, Departments of Gynecology, Obstetrics and Pathology, The Johns Hopkins Hospital,and The Johns Hopkins University School of Medicine, Baltimore,Maryland
Diagnosis of Endometrial Biopsiesand Curettings
A Practical ApproachSecond Edition
With 230 Illustrations, 77 in Full Color

Michael T. Mazur, MDClinical Professor of PathologyState University of New YorkUpstate Medical UniversitySyracuse, NY 13210andClearPath DiagnosticsSyracuse, NY 13202USA
Robert J. Kurman, MDRichard W. TeLinde Distinguished
ProfessorDepartments of Gynecology,
Obstetrics and PathologyThe Johns Hopkins HospitalandThe Johns Hopkins University
School of MedicineBaltimore, MD 21231USA
Library of Congress Cataloging-in-Publication DataMazur, Michael T.
Diagnosis of endometrial biopsies and curettings : a practical approach / Michael Mazur, Robert J. Kurman.
p. ; cm.Includes bibliographical references and index.ISBN 0-387-98615-4 (h/c : alk. paper)1. Endometrium—Biopsy. 2. Endometrium—Diseases—Cytodiagnosis. 3.
Endometrium—Cytopathology. I. Kurman, Robert J. II. Title.[DNLM: 1. Uterine Diseases—diagnosis. 2. Biopsy—methods. 3. Dilatation
and Curettage—methods. 4. Endometrium—pathology. 5. Pregnancy Complications—diagnosis. WP 440 M476d 2004]RG316.M39 2004618.1¢42—dc22 2004046866
ISBN 0-387-98615-4 Printed on acid-free paper.
© 1995, 2005 Springer Science+Business Media, Inc.All rights reserved. This work may not be translated or copied in whole or in part withoutthe written permission of the publisher (Springer Science+Business Media, Inc., 233 SpringStreet, New York, NY 10013, USA), except for brief excerpts in connection with reviewsor scholarly analysis. Use in connection with any form of information storage and retrieval,electronic adaptation, computer software, or by similar or dissimilar methodology nowknown or hereafter developed is forbidden.The use in this publication of trade names, trademarks, service marks, and similar terms,even if they are not identified as such, is not to be taken as an expression of opinion as towhether or not they are subject to proprietary rights.While the advice and information in this book are believed to be true and accurate at thedate of going to press, neither the authors nor the editors nor the publisher can accept anylegal responsibility for any errors or omissions that may be made. The publisher makes nowarranty, express or implied, with respect to the material contained herein.
Printed in the United States of America.
9 8 7 6 5 4 SPIN 10689416
springer.com
ISBN 978-0-387-98615-9 SBN 978-0-387-26321-2e I

v
Preface to the Second Edition
This second edition of Diagnosis of Endometrial Biopsies and Curettings:A Practical Approach follows a number of favorable comments wereceived about the first edition. As before, this book is designed to offera practical reference for the everyday interpretation of endometrialbiopsies. This edition has been extensively updated to reflect theadvances in our understanding of the pathology and pathophysiology ofthe endometrium over the past few years. In addition, a large number ofcolor illustrations have been added to help the reader understand themorphologic changes described in the text.
Although the entire book has been revised, several areas received par-ticular attention. Our knowledge of the utility of immunohistochemistryin the interpretation of these specimens, especially trophoblastic diseaseand endometrial neoplasia, has expanded considerably since the firstedition. Accordingly, in this edition the problems, pitfalls, and utility ofthis valuable diagnostic adjunct have received greater attention.While immunohistochemistry is discussed in all the chapters, it is alsosummarized in the final chapter, which addresses methods of endome-trial evaluation.
Expanded knowledge of newer entities, such as the epithelioid tro-phoblastic tumor, endometrial intraepithelial carcinoma, and the effectsof tamoxifen on the endometrium have received increased emphasis inthis edition. Because hydatidiform mole is now commonly recognized atan earlier stage of gestation, the features of these “early moles” are dis-cussed in greater detail. The chapter on polyps was revised to furtherclarify the terminology of these common lesions, as they demonstrate awide spectrum of morphologic features. Information about the distinc-tion of endometrial carcinoma from endocervical adenocarcinoma alsowas significantly revised.
Most importantly, however, the text continues its focus on thoseaspects of endometrial biopsy interpretation that can be especiallyvexing, such as the diagnosis of atypical hyperplasia, grading of endome-trial carcinoma, and the myriad of benign changes and artifacts that canbe confusing to the pathologist. In addition, a clear understanding of theterminology that the pathologist uses to communicate diagnostic infor-mation to the clinician is critically important. A diagnosis of carcinoma

is straightforward, but a clear and precise diagnosis of the various benign,yet abnormal pattens of endometrial development and bleeding can bea challenge.
Finally, we have tried to not only provide a reference for evaluatingthe morphologic details of a wide variety of lesions but also to conveyto the reader the manner by which we approach the evaluation of theendometrial biopsy. It is not possible to cover every aspect of endome-trial pathology in a single text of this size, but we believe that the bookis a reasonable foundation and starting point for the diagnosis of thesespecimens. We hope it will be useful to pathologists and gynecologists.
Michael T. Mazur, MDRobert J. Kurman, MD
vi Preface to the Second Edition

vii
Preface to the First Edition
The incentive for writing this book came from a short course, “Endome-trial Biopsy Interpretation,” that we presented for five years at theUnited States and Canadian Academy of Pathology. The enthusiasticresponse we received from this endeavor prompted us to considerwriting a practical text on the histologic interpretation of these speci-mens, which are commonly encountered in the surgical pathology labo-ratory but are given short shrift in standard texts. Several gynecologicpathology textbooks, such as Blaustein’s Pathology of the Female GenitalTract, 4th ed. (1994), describe the morphologic features and classificationof benign and malignant endometrial lesions, but little attention is givento the subtle differences between physiologic changes and pathologicconditions and the artifacts of biopsy and processing. In addition, micro-scopic findings that can be safely ignored because they have no clinicalbearing are generally not discussed in standard texts. It is our impressionthat it is precisely these areas that present most of the difficulties in dailypractice, more so, in fact, that the diagnosis of a malignant tumor.
This text is not a reference or atlas that describes pathologic curiosi-ties that one might never encounter in a lifetime of practice. Instead, weattempt to provide a logical approach to formulating a pathologic diag-nosis from the diverse array of fragmented, often scant pieces of tissueand blood received in the laboratory. As such, the material is presentedin a less traditional fashion. Conventional histopathologic classificationsremain an integral part of the text, but the various chapters focus on aclinically oriented approach to the microscopic diagnosis of commonproblems. For example, the individual chapters address the clinical ques-tions and specifics of reporting the findings, aspects that vary accordingto the patient’s age and the clinical circumstances.
One important subject is that of changes in the endometrium inducedby breakdown and bleeding, independently of the underlying pathology.These alterations are highly prevalent in endometrial biopsies and areoften misinterpreted, so they are described in detail. The updated WorldHealth Organization classification of endometrial hyperplasia is basedon the distinction of atypical and non-atypical hyperplasia. This topic isespecially important, since cytologic atypia is the critical prognosticfeature in their behavior, yet the characteristics of what constitutes

atypia are not well appreciated. Metaplasia and other benign changescan mimic hyperplasia and carcinoma, so the text focuses on these lesionsin detail. Clinical management of endometrial carcinoma is greatly influ-enced by the histologic evaluation of the curettings. Accordingly, the dis-cussion of endometrial carcinoma considers not only the differentialdiagnosis, but also the grading of carcinoma and the distinction ofendometrial from endocervical primary tumors.
Trophoblast presents unique problems in diagnosis. This is largelybecause the pathologist lacks experience with the diverse morphologicarray of trophoblastic changes in benign and malignant lesions. Gesta-tional trophoblastic disease is rare in routine practice. Furthermore, tro-phoblast of abortion specimens, including the trophoblast of theimplantation site, usually receives little scrutiny. Two chapters have beenincluded to cover this complex subject, one devoted to physiologic andone to neoplastic conditons.
Almost all the illustrations used in this text are from biopsies, andsome show artifact and distortion, as occurs in routine specimens. Weintentionally use this less-than-perfect material, since it better illustratesthe problems that the pathologist faces in the interpretation of thesespecimens.
Since this monograph is not a reference text or atlas, we suggestreading it in its entirety in order to appreciate the clinically orientedproblem-solving approach that we advocate. We hope that the readerfinds this approach informative, useful, and enjoyable.
Michael T. Mazur, MDRobert J. Kurman, MD
viii Preface to the First Edition

ix
Contents
Preface to the Second Edition . . . . . . . . . . . . . . . . . . . . . . . . . . . vPreface to the First Edition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . vii
Chapter 1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Chapter 2 Normal Endometrium and InfertilityEvaluation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Chapter 3 Pregnancy, Abortion, and Ectopic Pregnancy . . . . 34
Chapter 4 Gestational Trophoblastic Disease . . . . . . . . . . . . . 67
Chapter 5 Dysfunctional Uterine Bleeding . . . . . . . . . . . . . . . 100
Chapter 6 Effects of Hormones . . . . . . . . . . . . . . . . . . . . . . . 121
Chapter 7 Endometritis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 147
Chapter 8 Polyps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 163
Chapter 9 Endometrial Hyperplasia, EndometrialIntraepithelial Carcinoma, and EpithelialCytoplasmic Change . . . . . . . . . . . . . . . . . . . . . . . . 178
Chapter 10 Endometrial Carcinoma . . . . . . . . . . . . . . . . . . . . . 208
Chapter 11 Other Tumors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 249
Chapter 12 Methods of Endometrial Evaluation . . . . . . . . . . . 275
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 289

interpretation requires appropriate fixation,processing, and sectioning of the tissue.
Indications for Biopsy
There are four main indications for endome-trial biopsy or curettage:1–7
1. Determination of the cause of abnormaluterine bleeding.
2. Evaluation of the status of theendometrium in infertile patients, including histologic dating.
3. Evacuation of products of conception,either spontaneous abortions or termination ofpregnancy.
4. Assessment of the response of theendometrium to hormonal therapy, especiallyestrogen replacement in perimenopausal andpostmenopausal women and Tamoxifentherapy for breast cancer.
Other indications for biopsy may arise. Anoccasional patient will have atypical or abnor-mal glandular cells of undetermined signifi-cance (AGUS) in a cervical–vaginal cytologicspecimen that requires endometrial sampling to exclude hyperplasia or carcinoma. Uterinescreening with transvaginal ultrasound canshow a thickened endometrial stripe in post-menopausal patients, and a biopsy can be per-formed to exclude significant pathology.8 Someclinicians sample the endometrium prior to hysterectomy to exclude significant pathology,although this procedure reveals little pathology
1
1Introduction
Endometrial biopsies and curettings are amongthe most common tissue specimens received inthe pathology laboratory. In several respectsthese specimens present a unique challenge for the surgical pathologist. The normalendometrium undergoes a variety of morpho-logic changes, especially during the reproduc-tive years, when cyclical hormonal influencesand pregnancy affect uterine growth. Biopsy-induced artifacts confound this heterogeneousgroup of morphologic changes. Whether thebiopsy is limited or a thorough curettage, theprocedure usually is “blind,” with no visualiza-tion of the tissue sampled. The final specimencontains multiple, irregularly oriented tissuefragments mixed with blood and contaminatingcervical tissue and mucus.
Interpreting the biopsy material demands alogical approach that takes into account manyfactors, including patient history; the specificrequests of the clinician performing the biopsy;and an appreciation of the limitations, potentialpitfalls, and complex array of patterns encoun-tered in the microscopic sections. As in evalua-tion of any pathologic specimen, proper
Indications for Biopsy . . . . . . . . . . . . . . . . 1Clinical History and BiopsyInterpretation . . . . . . . . . . . . . . . . . . . . . . 2
Abnormal Uterine Bleeding . . . . . . . . . 2Infertility Biopsy . . . . . . . . . . . . . . . . . . 3Products of Conception . . . . . . . . . . . . . 4Hormone Therapy . . . . . . . . . . . . . . . . . 4Other Considerations . . . . . . . . . . . . . . 4
Clinical Queries and Reporting . . . . . . . . 4

in the absence of a history of abnormal bleed-ing.9 Likewise, endometrial biopsy for screen-ing of endometrial cancer or precursor lesionsin asymptomatic perimenopausal and post-menopausal patients has a very low yield of sig-nificant abnormalities and is not cost effective.10
At times these indications for endometrialsampling overlap. For example, some complica-tions of pregnancy, such as a missed abortion or trophoblastic disease, are accompanied byabnormal uterine bleeding. Nonetheless, thesebroad categories provide a clinicopathologicframework for approaching the microscopicanalysis of endometrial biopsy specimens. Thetext has therefore been divided into chaptersthat correspond to these clinical indications.
Clinical History and BiopsyInterpretation
Abnormal Uterine Bleeding
The most common reason for performing anendometrial biopsy is abnormal uterine bleed-ing, a term that refers to any nonphysio-logic uterine bleeding. Age and menstrual/ menopausal status are especially importantdata, as causes of abnormal uterine bleedingvary significantly according to the age and men-strual status of the patient, as discussed later.
Abnormal uterine bleeding is a common sign of a number of different uterine disorders ranging from dysfunctional (nonorganic)abnormalities or complications of pregnancy toorganic lesions such as polyps, hyperplasia,or carcinoma.5;6;11–16 Several clinical terms areemployed to describe different patterns ofuterine bleeding (Table 1.1).
The prevalence of the various abnormalitiesthat lead to abnormal bleeding is difficult todetermine precisely, varying with the patientpopulation and the terms used by investiga-tors.2–4 A practical approach to the possiblediagnoses associated with abnormal bleedingtakes age into account (Tables 1.1 to 1.5). Preg-nancy-related and dysfunctional disorders aremore common in younger patients whereasatrophy and organic lesions become more fre-quent in older individuals.6 Polyps in peri-menopausal and postmenopausal patients havebeen found in 2% to 24% of patients.17–24
Hyperplasia is found in up to 16% of post-menopausal patients undergoing biopsy, andendometrial carcinoma in fewer than 10% ofpatients.17;24 One consistent observation instudies of postmenopausal patients is the find-ing that atrophy is a common cause of abnor-mal bleeding, being found in 25% or more ofcases.17;18;20;23;25;26
Even among perimenopausal and post-menopausal patients, the proportion of cases
2 1. Introduction
Table 1.1. Clinical terms for abnormal uterine bleeding.
Amenorrhea Absence of menstruationHypermenorrhea Uterine bleeding occurring at regular intervals but increased in amount. The period of flow is
normalHypomenorrhea Uterine bleeding occurring at regular intervals but decreased in amount. The period of flow
is the same or less than the usual duration.Menorrhagia Excessive uterine bleeding in both amount and duration of flow occurring at regular intervalsMetrorrhagia Uterine bleeding, usually not heavy, occurring at irregular intervalsMenometrorrhagia Excessive uterine bleeding, usually with prolonged period of flow, occurring at frequent and
irregular intervalsOligomenorrhea Infrequent or scanty menstruation. Usually at intervals greater than 40 daysAbnormal uterine A term that describes any bleeding from the uterus. Menorrhagia, metrorrhagia,
bleeding (AUB) menometrorrhagia, and postmenopausal bleeding are all forms of AUB.Dysfunctional uterine Abnormal uterine bleeding with no organic cause. The term implies bleeding caused by
bleeding (AUB) abnormalities in ovulation or follicle development and is a disorder of premenopausalwomen.
Postmenopausal Abnormal uterine bleeding that occurs at least 1 year after menopause (the cessation ofmenses)

A history of anovulation, obesity, hyperten-sion, diabetes, and exogenous estrogen useshould alert the pathologist that the patient isat increased risk for hyperplasia and adenocar-cinoma, but this information is rarely includedon the requisition. Typically, there is littleaccompanying clinical data except the patient’sage and a short history of abnormal bleeding.Consequently, hyperplasia and adenocarci-noma must be diagnostic considerations formost endometrial specimens received in thelaboratory. On rare occasions, hyperplasia oreven adenocarcinoma is found in biopsies per-formed during an infertility workup where theclinical question was histologic dating ratherthan suspicion of these disorders.27
Infertility Biopsy
When a patient undergoes biopsy for evalua-tion of infertility, the clinical information often
Clinical History and Biopsy Interpretation 3
attributable to any of the aforementioned conditions is age dependent. Atrophy and carcinoma occur more frequently in patientsolder than 60 years of age, while polyps andhyperplasia are more common in patients who are perimenopausal or more recently postmenopausal. In addition to these uterinecauses of bleeding, other abnormalities, such asatrophic vaginitis, can cause vaginal bleeding,and this may be difficult to distinguish fromuterine bleeding until the patient undergoesthorough clinical evaluation.
Ideally, the clinical history that accompaniesan endometrial sample should include somedescription of the pattern and amount of bleed-ing. Often in a patient of reproductive age orone who is perimenopausal the history is simplydysfunctional uterine bleeding (DUB). Clini-cally, this term suggests no other causes ofbleeding except ovarian dysfunction. In thisscenario the clinician performs the biopsy torule out an organic lesion.
Table 1.2. Causes of abnormal uterine bleeding inadolescence.
Common Uncommon
Dysfunctional bleeding EndometritisAnovulatory cycles Clotting disorders
Complications of pregnancya
a See Chapter 3, Table 3.1 (Complications of pregnancy).
Table 1.3. Causes of abnormal uterine bleeding inthe reproductive years.
Common Uncommon
Complications of pregnancya HyperplasiaEndometritis NeoplasiaDysfunctional bleeding Endometrial carcinoma
Anovulatory cycles Cervical carcinomaInadequate luteal phase Clotting disordersIrregular shedding
Organic lesionsLeiomyomasPolyps (endometrial, endocervical)Adenomyosis
Exogenous hormonesBirth controlProgestin therapy
a See Chapter 3, Table 3.1 (Complications of pregnancy).
Table 1.4. Causes of abnormal uterine bleeding inperimenopausal years.
Common Uncommon
Dysfunctional bleeding Complications of pregnancya
Anovulatory cycles EndometritisOrganic lesions Adenomyosis
Hyperplasia NeoplasiaPolyps (endometrial, Cervical carcinoma
endocervical) Endometrial carcinomaExogenous hormones Sarcoma
Birth control Clotting disordersEstrogen replacementProgestin therapy
a See Chapter 3, Table 3.1 (Complications of pregnancy).
Table 1.5. Causes of abnormal uterine bleeding inpostmenopausal years.
Common Uncommon
Atrophy EndometritisOrganic lesions Sarcoma
Hyperplasia Clotting disordersPolyps (endometrial)
NeoplasiaEndometrial carcinoma
Exogenous hormonesEstrogen replacementProgestin therapy (e.g., therapy of breast carcinoma)

is limited, but here, too, the history shouldinclude the date of the last menstrual period(LMP) to place an approximate time in themenstrual cycle. This information is useful butnot precise for determining the actual day ofthe cycle, as ovulatory frequency and length ofthe follicular phase are highly variable amongpatients. Usually the main objective of biopsiesfor infertility is to determine whether there ismorphologic evidence of ovulation and, if so,the histologic date (see Chapter 2). The gyne-cologist may seek other specific information,such as response to hormone therapy, so it isimportant that the pathologist be given anyadditional history that may be necessary for theinterpretation.
Products of Conception
When endometrial sampling is performed toremove products of conception, clinical infor-mation often is sparse, as the main goal of theprocedure is simply to remove the placentaland fetal tissue. Significant pathologic changesare rare. Nonetheless, it is helpful to know ifpregnancy is suspected, and, if so, the approxi-mate gestational age of the pregnancy. If thereis a suspicion of trophoblastic disease, thisshould be stated. In such instances the humanchorionic gonadotropin (hCG) titer is relevant.If an ectopic pregnancy is suspected, alertingthe pathologist can ensure rapid processing andinterpretation of the specimen.
Hormone Therapy
Because the endometrium is responsive to hor-mones, the history of hormone use is importantinformation. Clinical uses of steroid hormones(estrogens, progestins, or both) include oralcontraceptive use; postmenopausal replace-ment therapy; and therapy for endometriosis,hyperplasia, DUB, infertility, and breast carci-noma. As with other facets of the clinical data,this information may be absent or, if present,unreadable on the requisition. Consequently,the pathologist must be prepared to recognizehormonal effects in the absence of history indi-cating use of hormones.
Other Considerations
Pregnancy history is useful, especially in pre-menopausal patients, regardless of the indica-tion for biopsy, as recent and remote effects of pregnancy, such as a placental site nodule or gestational trophoblastic disease, may beencountered in biopsy material. The history ofrecent or past pregnancies is expressed as gra-vidity and parity. The letter G (gravidity) fol-lowed by a number (G1, G2, etc.) indicates thenumber of pregnancies, and the letter P (parity)followed by a number indicates the number ofdeliveries. For example, G4, P2 indicates that awoman has had four pregnancies and two deliv-eries. Further information on parity often isdesignated by four numbers indicating full-term pregnancies, premature pregnancies (>20but <37 weeks’ gestation), abortions (<20weeks’ gestation), and living children. Thus apatient who is G5, P3013 is currently pregnantand has had three previous full-term pregnan-cies and one abortion, and the three childrenfrom the term pregnancies are alive.
The type of procedure, that is, biopsy versuscurettage, is important for deciding whetherfocal changes represent significant abnormali-ties and whether or not small specimens areadequate. Although office-based biopsies gen-erally provide a representative sample, theymay not contain sufficient tissue to ensure thebest diagnosis of some changes. For example,the irregular glands of hyperplasia may resem-ble patterns seen in some polyps, low-grade adenocarcinomas, and even artifactually dis-torted normal endometrium. Furthermore,atypia can be focal in hyperplasia; thereforebiopsy specimens may preclude a definitivediagnosis. In these cases, a more thoroughbiopsy or curettage is necessary to permit thebest diagnosis.
Clinical Queries and Reporting
In general, it is best to avoid diagnostic termssuch as “no pathologic diagnosis” or “no signif-icant pathologic findings,” as there is a widerange of normal histology. When the tissue
4 1. Introduction

lacks abnormalities, stating the normal phase ofthe endometrium, for example, proliferative orsecretory, provides more useful information forthe clinician.
In biopsies for abnormal uterine bleeding,the pathologic information sought varies withthe patient’s age and clinical history. The gyne-cologist wishes to know the following:
1. Is there an organic lesion, such as a com-plication of pregnancy, inflammation, or apolyp?
2. Is there evidence of active or old breakdownand bleeding?
3. Is there evidence to suggest dysfunctionalbleeding?
4. Is there evidence of hyperplasia or carcinoma?
For example, in the premenopausal patientthe possibility of pregnancy and related bleed-ing is a frequent question. In a perimenopausalpatient the concern shifts to hyperplasia andcarcinoma, and in postmenopausal patients theimportance of ruling out carcinoma becomesparamount. In any of these conditions, glandu-lar and stromal breakdown may be presenteither focally or diffusely. It is the underlyingdisorder that is most important to report. Thechanges of breakdown and bleeding are sec-ondary and do not indicate a primary disorderby themselves. Nonetheless, when there is ahistory of abnormal bleeding, it can be helpfulto note whether or not there is histologic evidence of glandular and stromal breakdown(see Chapter 5), especially if the tissue lacksevidence of an organic process such as hyper-plasia or carcinoma. This information serves todocument to the gynecologist that bleeding is,in fact, endometrial in origin. Even when thereis no evidence of active bleeding, foci of stromalfoam cells or hemosiderin, sometimes withfibrosis, indicate that abnormal bleeding hastaken place and deserve comment.
Besides reporting the morphologic changespresent, noting significant negative findings can be helpful to the clinician. As an example,the diagnosis of chronic endometritis is morehelpful if it includes a comment regarding thepresence or absence of specific etiologic factors
such as evidence of a recent pregnancy. Like-wise, if an organic lesion such as a polyp ispresent, it is helpful to indicate whether nonin-volved tissue is present and, if so, its appear-ance. In perimenopausal and postmenopausalpatients, if the gynecologist indicates a specificconcern regarding the presence of hyperplasia,atypia, or carcinoma, then a statement notingthe absence of these lesions is reassuring.
For all cases, specimen adequacy is a con-sideration, but this needs to be specificallyaddressed only in limited samples in which thediagnosis is not clear-cut. Scant tissue obtainedby an office-based biopsy may be insufficient toallow thorough assessment of the status of theendometrium. In these cases the pathologistshould indicate in the report that the specimenis scant. For instance, small samples may revealhyperplastic glands, but it may be difficult todetermine whether the abnormality representsa localized polyp with a proliferative/hyper-plastic pattern (see Chapter 8) or a diffusehyperplasia. Some assessment of theendometrium can be done even on very limitedspecimens. For example, histologic dating is pos-sible on small samples. Atrophic endometriumtypically yields a very small amount of tissue,yetthese specimens should not be regarded as inad-equate (see Chapter 5). The subsequent chap-ters consider in greater detail the queries likelyto arise in various circumstances and the infor-mation that the pathologist should incorporatein the final report.
References
(1) Noyes RW, Hertig AT, Rock J. Dating theendometrial biopsy. Fertil Steril 1950; 1:3–25.
(2) Baitlon O, Hadley JO. Endometrial biopsy.Pathologic findings in 3,600 biopsies fromselected patients. Am J Clin Pathol 1975; 63:9–15.
(3) Nickelsen C. Diagnostic and curative value ofuterine curettage. Acta Obstet Gynecol Scand1980; 65:693–697.
(4) Van Bogaert L-J, Maldague P, Staquet J-P.Endometrial biopsy interpretation. Shortcom-ings and problems in current gynecologic prac-tice. Obstet Gynecol 1978; 51:25–28.
References 5

(5) Galle PC, McRae MA. Abnormal uterinebleeding. Finding and treating the cause. Post-grad Med 1993; 93:73–81.
(6) Speroff L, Glass RH, Kase NG. Clinical gyne-cologic endocrinology and infertility. 6th ed.Baltimore: Lippincott Williams & Wilkins; 1999.
(7) Merrill JA. The interpretation of endometrialbiopsies. Clin Obstet Gynecol 1991; 34:211–221.
(8) Fleischer AC, Jones HW, III. Early detection ofovarian and endometrial cancer with transvagi-nal and color Doppler sonography. In: FleischerAC, Manning FA, Jeanty P, Romero R, eds.Sonography in obstetrics and gynecology. Prin-ciples and practice. 6th ed. New York: McGraw-Hill; 2001: 1001–1017.
(9) Stovall TG, Solomon SK, Ling FW. Endometrialsampling prior to hysterectomy. ObstetGynecol 1989; 73:405–409.
(10) Archer DF, McIntyre-Seltman K, Wilborn WW,Dowling EA, Cone F, Creasy GW, et al.Endometrial morphology in asymptomaticpostmenopausal women. Am J Obstet Gynecol1991; 165:317–322.
(11) Goldfarb JM, Little AB. Abnormal vaginalbleeding. N Engl J Med 1980; 302:666–669.
(12) Povey WG. Abnormal uterine bleeding atpuberty and climacteric. Clin Obstet Gynecol1970; 13:474–488.
(13) Stenchever MA. Differential diagnosis of majorgynecologic problems by age groups. In:Stenchever MA, Droegemueller W, Herbst AL,Mishell DR, Jr, eds. Comprehensive gynecol-ogy. 4th ed. St. Louis: Mosby; 2001: 155–177.
(14) Butler WJ. Normal and abnormal uterinebleeding. In: Rock JA, Jones HW, III, eds. TeLinde’s operative gynecology. 9th ed. Philadel-phia: Lippincott Williams & Wilkins; 2003:457–481.
(15) Kilbourn CL, Richards CS. Abnormal uterinebleeding. Diagnostic considerations, manage-ment options. Postgrad Med 2001; 109:137–4,147.
(16) Wren BG. Dysfunctional uterine bleeding.AustFam Phys 1998; 27:371–377.
(17) Rubin SC. Postmenopausal bleeding: Etiology,evaluation, and management. Med Clin N Am1987; 71:59–69.
(18) Schindler AE, Schmidt G. Post-menopausalbleeding: A study of more than 1000 cases.Maturitas 1980; 2:269–274.
(19) Van Bogaert L-J. Clinicopathologic findings inendometrial polyps. Obstet Gynecol 1988;71:771–773.
(20) Choo YC, Mak KC, Hsu C, Wong TS, Ma HK.Postmenopausal uterine bleeding of nonor-ganic cause. Obstet Gynecol 1985; 66:225–228.
(21) Mencaglia L, Perino A, Hamou J. Hysteroscopyin perimenopausal and postmenopausalwomen with abnormal uterine bleeding. JReprod Med 1987; 32:577–582.
(22) Pacheco JC, Kempers RD. Etiology of post-menopausal bleeding. Obstet Gynecol 1968;32:40–46.
(23) Lidor A, Ismajovich B, Confino E, David MP.Histopathological findings in 226 women withpost-menopausal uterine bleeding. Acta ObstetGynecol Scand 1983; 65:41–43.
(24) Moghal N. Diagnostic value of endometrialcurettage in abnormal uterine bleeding—ahistopathological study. J Pak Med Assoc 1997;47:295–299.
(25) Meyer WC, Malkasian GD, Dockerty MB,Decker DG. Postmenopausal bleeding fromatrophic endometrium. Obstet Gynecol 1971;38:731–738.
(26) Gambrell RD. Postmenopausal bleeding. J AmGeriatr Soc 1974; 22:337–343.
(27) Scurry J, Brand A, Sheehan P, Planner R. High-grade endometrial carcinoma in secretoryendometrium in young women. a report of fivecases. Gynecol Oncol 1996; 60:224–227.
6 1. Introduction

biopsy or curettage is part of a comprehensiveworkup of the patient in the operating roomthat includes laparoscopy, hysteroscopy, or hysterosalpingography to assess the presenceor absence of uterine or tubal lesions that con-tribute to infertility. In these cases, the endome-trial sampling may not be timed as precisely forthe mid- to late luteal phase. Nonetheless, his-tologic evaluation provides the gynecologistwith information regarding the response of theendometrium to hormonal stimulation, includ-ing indirect evidence of ovulatory function.
The secretory phase is constant in the normalcycle, lasting 14 days from the time of ovula-tion to the onset of menstruation.1 Variations incycle length occur because the proliferativephase of the cycle varies, both between cyclesand between women. Accordingly, the gynecol-ogist correlates the cycle date by histology withthe woman’s cycle date based on the time ofonset of the upcoming menstrual period, notthe last menstrual period. Ovulation with se-cretory endometrial changes ceases in mostwomen by age 53, although rarely ovulationwith secretory endometrium and a confirmedcorpus luteum of the ovary has been seen up toa least age 56 (personal observation).
The biopsy findings help confirm that ovula-tion occurred, and indicate whether there wassufficient secretory effect, mediated by proges-terone, during the luteal phase. To utilize fullythe morphologic interpretation, the gynecolo-gist compares the histologic date to the clinicaldata, including the date of the rise in the basalbody temperature, the time of the serum
7
2Normal Endometrium and Infertility Evaluation
The histologic features of what constitutes“normal” endometrium change with a woman’sage, through the premenarchal, reproductive,perimenopausal, and postmenopausal years.1–3
During the reproductive years, the cyclical hor-monal changes of the menstrual cycle providea continuously changing morphologic pheno-type that is “normal.” In biopsy specimens, thecombination of these cyclical changes alongwith artifacts and limited sampling can makenormal patterns difficult to interpret. Duringthe reproductive years, deviations from normal,either in histologic pattern or in temporal rela-tionship to ovulation, often indicate underlyingabnormalities that may cause female infertility.
The endometrial biopsy is an important partof the evaluation of the woman with infertil-ity.4;5 Biopsies for the evaluation of infertilityoften are performed in the office using a smallcurette or a Pipelle aspirator and therefore the specimens tend to be small.6 Occasionally,
General Considerations in HistologicEvaluation . . . . . . . . . . . . . . . . . . . . . . . . . 8Histologic Dating of the Normal,Cycling Endometrium . . . . . . . . . . . . . . . . 10
Proliferative Phase Endometrium . . . . . 11Secretory Phase Endometrium . . . . . . . 12Menstrual Endometrium . . . . . . . . . . . . 19
Pitfalls in Dating . . . . . . . . . . . . . . . . . . . . 21Artifacts and Contaminants . . . . . . . . . . . 23Luteal Phase Defect and AbnormalSecretory Phase Patterns . . . . . . . . . . . . . . 26Clinical Queries and Reporting . . . . . . . . 29

luteinizing hormone (LH) surge, transvaginalultrasound evaluation of follicular or corpusluteum development, serum progesteronelevel, or subtraction of 14 days from the onsetof menses.4;7–9 Consequently, the biopsy typi-cally is timed to coincide with the luteal (secre-tory) phase of the cycle. In addition to definingthe precise histologic date, an endometrialbiopsy is part of the infertility workup toexclude other organic uterine abnormalities.
This chapter reviews the morphologic varia-tions caused by ovarian hormonal stimulationthat provide a background for the interpreta-tion of endometrial biopsies in infertilitypatients. These patterns include changes result-ing from normal hormonal fluctuations duringthe menstrual cycle and variations in normaldevelopment that are caused by abnormalitiesin the endogenous ovarian hormonal levelsduring the reproductive years. The latter repre-sent the so-called dysfunctional abnormali-ties that are, for the most part, due to abnor-malities in ovarian follicular development or inhormone production by the corpus luteum.Ovarian dysfunction also can result in abnormalbleeding, and Chapter 5 reviews dysfunctionaluterine bleeding caused by ovulatory abnor-malities. During gestation the endometriumundergoes other “normal,” that is, physiologic,alterations as discussed in Chapter 3.
General Considerations inHistologic Evaluation
Histologic evaluation begins with identificationof surface epithelium, a prerequisite for orient-ing the underlying glands and stroma. The sur-face epithelium is less responsive to sex steroidhormones than the underlying glands, but itoften shows alteration in pathologic conditions,especially when the abnormalities are subtle orfocal. For example, during the proliferativephase, estrogenic stimulation induces develop-ment of ciliated cells along the surface.10 In con-trast, ciliated surface epithelial cells are farmore frequent in pathologic conditions, par-ticularly those associated with unopposedestrogen stimulation, such as hyperplasia andmetaplasia.2;3;11–13
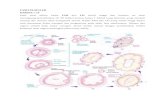
The subsurface endometrium is divided intotwo regions, the functionalis (stratum spongio-sum) and the basalis (stratum basale) (Fig. 2.1).The functionalis, situated between the surfaceepithelium and the basalis, is important to evaluate because it shows the greatest degreeof hormonal responsiveness. The size and distribution of glands as well as the cytologicfeatures of the glandular epithelial cells are important features in the histologic evalua-tion. Under normal conditions, the glandsshould be regularly spaced and have a per-pendicular arrangement from the basalis to the surface epithelium. In the secretory phase,the endometrium also shows a stratum com-pactum, a thin region beneath the surfaceepithelium. In the stratum compactum thestroma is dense and the glands are straight and narrow, even when the glands in the functionalis are tortuous. The basalis adjoinsthe myometrium, serving to regenerate thefunctionalis and surface epithelium followingshedding during menses. The endometrium ofthe basalis is less responsive to steroid hor-mones, and typically shows irregularly shaped,inactive appearing glands, dense stroma, andaggregates of spiral arteries. The spiral arteriesof the basalis (basal arteries) have thicker mus-cular walls than those in the functionalis. Inbiopsies, tissue fragments that contain basalisoften do not have surface epithelium. Theglands and stroma of the basalis cannot bedated, as they are unresponsive to steroid hormones. A specimen consisting solely ofendometrium from the basalis is thereforeinadequate for dating.
Tissue from the lower uterine segment oristhmus is another region of the endometriumthat is less responsive to steroid hormones. Inthe lower uterine segment the endometriumhas shorter, poorly developed, inactive glandsdispersed in a distinctive stroma (Fig. 2.2). Thecolumnar cells lining the glands resemble thoseof the corpus. Some glands near the junctionwith the endocervix show a transition to muci-nous endocervical-type epithelium.The stromalcells in the lower uterine segment are elongateand resemble fibroblasts with more abundanteosinophilic cytoplasm, in contrast to the ovalto rounded stromal cells with minimum cyto-plasm seen in the corpus.
8 2. Normal Endometrium and Infertility Evaluation

The tangential orientation of the functionalisin biopsies and the tortuosity of the glands, par-ticularly in the late proliferative phase, oftenlead to irregular cross sections of the tissue. In
this instance, gland development can be diffi-cult to assess. Furthermore, not all fragments oftissue in a biopsy or curettage include surfaceepithelium, which helps to orient the glands.
General Considerations in Histologic Evaluation 9
Figure 2.1. Normal secretory phase endometrium.Surface epithelium orients the tissue.The midportionof the tissue consists of functionalis where glands,stroma, and blood vessels demonstrate the typicalpatterns of maturation through the menstrual cycle.
The basalis in the lower portion of the illustrationconsists of irregular, closely spaced glands, densestroma, and aggregates of arteries. The stratum com-pactum is composed of the surface epithelium and asubjacent thin layer of dense stroma.

Nonetheless, at least focally, portions of better-oriented glands usually can be traced throughthe functionalis to the surface epithelium, andthese foci are critical for assessing appropriateglandular and stromal development.
Histologic Dating of the Normal,Cycling Endometrium
In the ovulatory patient, normal endometriumhas two phases.The first is the proliferative (fol-licular or preovulatory) phase characterized bygrowth of glands, stroma, and vessels that isinfluenced by estradiol produced mainly bygranulosa cells in the ovarian follicles. Fol-lowing ovulation, the secretory (luteal or postovulatory) phase reflects the effect of the combined production of progesterone andestradiol by luteinized granulosa and theca cells
of the corpus luteum.4 The regular sequence ofmorphologic changes determined by the fluctu-ating levels of ovarian steroid hormones formsthe basis for histologic dating.
Dating uses an arbitrarily defined “normal”cycle of 28 days, with day 1 the first day of men-strual bleeding.1 Histologic dating is mostprecise in the postovulatory secretory phase,as the follicular phase can be highly variable in length. Furthermore, proliferative phasechanges are not as discrete as those in the secre-tory phase. The date of the secretory phase isexpressed either as the specific day of the 28-day menstrual cycle, assuming ovulation occurson day 14, or is stated as the postovulatory day(e.g., secretory day 21 or postovulatory [P.O.]day 7). Local custom often determines the pre-ferred method of stating the histologic date.
There are nine histologic features of theglands and stroma that determine the phase of
10 2. Normal Endometrium and Infertility Evaluation
Figure 2.2. Lower uterine segment. Small, poorly developed glands are seen in nonreactive stroma that iscomposed of widely spaced spindle cells. Tissue from the lower uterine segment cannot be dated.

the cycle and the histologic date (Table 2.1).1
Five of these features affect glands: (1) tortu-osity, (2) gland mitoses, (3) orientation of nuclei(pseudostratified versus basal), (4) basal sub-nuclear cytoplasmic vacuoles, and (5) luminalsecretions with secretory exhaustion. Four features relate to the stromal: (6) edema, (7)mitoses, (8) predecidual change, and (9) infil-tration of granular lymphocytes. Practically, themost important glandular features are orienta-tion of nuclei, subnuclear cytoplasmic vacuoles,and luminal secretions with secretory exhaus-tion (3, 4, and 5), and the most importantstromal features are edema, predecidualchange, and granular lymphocytic infiltration(6, 8, and 9). These salient features are usuallyreadily apparent when present, allowing thepathologist to assign a histologic date.
Proliferative Phase Endometrium
During the proliferative phase, the endo-metrium grows from about 0.5mm up to 4.0 to5.0mm in thickness, so by the late proliferativephase, a biopsy obtains a moderate amount oftissue. Proliferative endometrium has threestages: early, mid, and late (Table 2.2).2 Thesedivisions are seldom used in dating biopsies,however. Usually the diagnosis of proliferativephase alone is sufficient, indicating that theendometrium is growing, shows a normal glan-dular distribution, and evidence of ovulation isnot present.
Growth of endometrium is the main charac-teristic of the proliferative phase (Figs. 2.3 and
2.4). Glands and stroma show brisk mitoticactivity. In early proliferative phase endo-metrium, the functionalis contains small,tubular glands. The glands progressively elon-gate and become tortuous from the mid- to the late proliferative phase because the glandgrowth is disproportionate to the stromalgrowth. Despite the tortuosity, the glands main-tain a relatively regular spacing between eachother. Throughout the proliferative phase, theepithelium lining the glands has pseudostrati-fied, oval nuclei with small nucleoli and densebasophilic cytoplasm. The pseudostratifiednuclei remain oriented to the basement mem-brane, but some nuclei are raised above thebasement membrane, giving a two-dimensionallayering of the nuclei. The pseudostratificationof the nuclei and the presence of mitotic activ-ity in the glands and stroma are two constantfeatures of the proliferative phase.
In the proliferative phase, the stromal cellsare widely separated in the functionalis (Fig.2.4).They are small and oval, with dense nuclei,scant wisps of cytoplasm, and ill-defined cellborders. Some stromal edema is normal at mid-proliferative phase. A few lymphocytes also arescattered throughout the stroma, being mostprominent around the vessels. Small spiralarteries and thin-walled venules are present.
The orientation and outline of proliferativephase glands and their relationship to intactstroma are important features for recognizing
Histologic Dating of the Normal, Cycling Endometrium 11
Table 2.1. Morphologic features used in endome-trial dating.
Glandular changes1. Tortuosity2. Mitoses3. Orientation of nuclei (pseudostratified or basal)a
4. Subnuclear cytoplasmic vacuolesa
5. Secretory exhaustion (luminal secretions)a
Stromal changes1. Edemaa
2. Mitoses3. Predeciduaa
4. Granular lymphocyte infiltratea
a Salient features used in dating the secretory phase.
Table 2.2. Proliferative phase changes.a
Early (4–7 days)Thin regenerating epitheliumShort narrow glands with epithelial mitosesStroma compact with mitoses (cells stellate or spindle
shaped)
Mid (8–10 days)Long, curving glandsColumnar surface epitheliumStroma variably edematous, mitoses frequent
Late (11–14 days)Tortuous glandsPseudostratified nucleiModerately dense, actively growing stroma
a These changes are subtle. They are rarely used for actualdating.

Figure 2.3. Proliferative endometrium. Glands aretubular and regularly spaced in abundant stroma.The stroma contains small vessels with thin walls.Both the glandular and the stromal cells show
this normal pattern, as hyperplastic glands orglands in a polyp can have cytologic featuresidentical to those of glands in the proliferativephase. The regular spacing and uniform shapeof the glands are characteristics of normal proliferative endometrium. Assessing gland orientation can be complicated, however, bybiopsy-induced fragmentation, an especiallycommon artifact in early to mid-proliferativephase biopsies when the mucosa is still thin.Detached and disrupted glands may appearabnormally crowded or irregular. To separatefragmentation artifact from true abnormalities,it is important to assess the integrity of thestroma as well as the glands and to use surfaceepithelium to help orient the tissue fragments.Detached and poorly oriented glands that showpseudostratified nuclei and mitotic activityusually represent proliferative endometriumunless better-oriented tissue suggests another
diagnosis. Also, proliferative phase glands fre-quently show the telescoping artifact (seelater).
Secretory Phase Endometrium
In the secretory phase, the glands and stromadevelop in an orderly sequence and display spe-cific histologic features of secretory activityfrom histologic day 16 through day 28. Theendometrium attains a thickness of up to 7.0 to8.0mm. Unlike in the proliferative phase, thechanges in the glands and stroma are relativelydiscrete, varying sharply from one day to thenext, thus permitting accurate dating. Dating of the first half of the secretory phase is basedprimarily on glandular changes whereas datingof the second half is based mainly on stromalalterations (Table 2.3).
12 2. Normal Endometrium and Infertility Evaluation
mitotic activity. Focal hemorrhage beneath thesurface epithelium is a result of the biopsy and doesnot represent a pathologic change.

Histologic Dating of the Normal, Cycling Endometrium 13
Figure 2.4. Proliferative endometrium. The prolif-erative phase gland shows pseudostratified nucleiwith mitotic activity. The stromal cells have oval
Table 2.3. Endometrial dating, secretory phase.
Interval phase, 14–15d.a No datable changes for 36–48hours after ovulation
Early secretory phase, 16–20d. Glandular changespredominate
16d. Subnuclear vacuoles (Note: Scattered smallirregular vacuoles can be caused byestrogen alone.)
17d. Regular vacuolation—nuclei lined up withsubnuclear vacuoles
18d. Vacuoles decreased in sizeEarly secretions in lumenNucleus approaches base of cell.
19d. Few vacuoles remain.Intraluminal secretionNo pseudostratification, no mitoses
20d. Peak of intraluminal secretions
Mid- to late secretory phase, 21–27d. Stromal changespredominate, variable secretory exhaustion
21d. Marked stromal edema22d. Peak of stromal edema—cells have “naked
nuclei”23d. Periarteriolar predecidual change
Spiral arteries prominent24d. More prominent predecidual change
Stromal mitoses recur.25d. Predecidual differentiation begins under
surface epithelium.Increased numbers of granular lymphocytes
26d. Predecidua starts to become confluent.27d. Granular lymphocytes more numerous
Confluent sheets of predeciduaFocal necrosis
24–27d. Secretory exhaustion of glands—tortuous withintraluminal tufts (saw-toothed), raggedluminal borders, variable cytoplasmicvacuolization, and lumenal secretions
nuclei and indistinct cytoplasm. Scattered lympho-cytes are normally present.
a d. = day of ideal 28-day menstrual cycle. To state as postovulatory day, subtract 14

The morphologic changes of the secretoryphase begin 36 to 48 hours after ovulation.There is an interval phase of 36 to 48 hoursbetween ovulation and the first recognizablehistologic changes of the endometrium attrib-utable to ovulation. During the interval phase,the glands become more tortuous and begin toshow subnuclear vacuoles (Fig. 2.5). The firstdiagnostic evidence of ovulation, however, isthe presence of abundant subnuclear glycogenvacuoles in the undulating, tortuous glands(Fig. 2.6). At this time the stroma is indistin-guishable from that of the late proliferativephase. Because focal subnuclear vacuolizationmay occur in the proliferative phase, at least50% of the glands should contain vacuoles toconfirm ovulation. In addition, at least 50% ofthe cells in a gland should contain vacuoles. Ifthe 50% rule is not fully met, but the clinicalhistory and morphology suggest recent ovula-tion, the endometrium may be in the interval
phase. Special stains for glycogen add little toroutine histologic evaluation for establishingthe presence of secretory changes.
Subnuclear vacuoles are abundant by day 17,and by day 18 the vacuoles begin to move fromthe basal to the supranuclear cytoplasm (Fig.2.7). Concurrently, the nuclei become basallyoriented and line up in a single layer perpen-dicular to the basement membrane. The cyto-plasmic contents then form mucin that isexpelled into the gland lumen. Luminal secre-tions peak at day 20 (Fig. 2.8).
After day 20, the stromal changes are moreimportant for dating than the glandularchanges. Nonetheless, the glands continue toshow increasing tortuosity, and variableamounts of luminal secretions persist until justbefore menses. From days 20 to 22 the glandsin the functionalis begin to show secretoryexhaustion, a change that becomes more promi-nent by days 24 to 25 (Fig. 2.9). Secretory
14 2. Normal Endometrium and Infertility Evaluation
Figure 2.5. Interval endometrium. The glands main-tain proliferative phase characteristics and showscattered subnuclear vacuoles. The extent of cyto-
plasmic vacuolization is not sufficient to be certainovulation has occurred.

Figure 2.6. Early secretory endometrium, days16–17. Postovulatory changes are clearly presentwith a regular distribution of subnuclear vacuoles inthe serpiginous glands.The stroma shows no changes
Figure 2.7. Early secretory endometrium, days 17–18. Glandular cell vacuoles remain prominent but beginto migrate to the supranuclear cytoplasm. A portion of the stroma shows mild edema.
compared to the late proliferative phase. Inset: Everygland cell contains a vacuole, resulting in a uniformalignment of nuclei away from the basement mem-brane.

Figure 2.8. Mid-secretory endometrium, days 20–21. Glands are distended with secretions.The stroma showsedema and there is no predecidual change.
Figure 2.9. Late secretory endometrium, days 23–24. Predecidual stromal change is evident around spiralarteries with intervening zones showing edema. The glands are tortuous and show secretory exhaustion.

Histologic Dating of the Normal, Cycling Endometrium 17
exhaustion is characterized by the presence ofa single layer of cells that lie in disarray withloss of orientation. The cytoplasmic borderalong the luminal surface becomes ragged, andluminal secretions are usually, although notinvariably, present. By days 24 to 25 the glandsoften develop a serrated, “saw-toothed”luminal border (Fig. 2.10). The glandular cellsmay continue to show a variable degree of vac-uolization throughout the remainder of thesecretory phase. Cytoplasmic vacuolization is aphysiologic change as long as the glands other-wise have appropriate tortuosity; the cytoplas-mic changes from vacuolization to completesecretory exhaustion with no vacuoles repre-sent a continuum of normal development. Byday 27, cellular necrosis (apoptosis) becomesevident with accumulation of nuclear debris inthe basal cytoplasm of the glandular epithelialcells. Throughout the secretory phase, the
glands in the stratum compactum immediatelybeneath the surface epithelium remain smalland tubular despite their increasing tortuosityin the functionalis.
As the glandular cells develop cytoplasmicvacuoles and produce luminal secretions,edema, the first stromal change, begins andpeaks quickly at days 21 to 22 (Table 2.3). Oncestromal changes begin, the glandular changesare less important for dating. Because of theedema, the stromal cells take on the so-callednaked nucleus appearance at days 21 to 22.With this change the stromal cells are widelydispersed and have small nuclei with scant,imperceptible cytoplasm (Fig. 2.8). This phaseof pure stromal edema is brief, and the subse-quent predecidual transformation of the stromabecomes the main feature in dating the latesecretory phase. Although stromal edema ismaximal at days 21 to 22, edema begins in a
Figure 2.10. Late secretory endometrium. Stromal cells around spiral arteries show predecidual change withincreased cytoplasm. The gland shows secretory exhaustion with patchy cytoplasmic vacuolization.

patchy distribution in the early secretory phaseat day 17 to 18. Therefore, some edema in theearlier portion of the secretory phase does notrepresent an irregularity of maturation.
Predecidual change characterizes the latesecretory phase (days 23 to 28). With theappearance of predecidua (not “pseudode-cidua”), the cells gain identifiable cytoplasm(Fig. 2.9). These cells become oval to polygonalshaped in the functionalis and show a moder-ate amount of eosinophilic to amphophiliccytoplasm (Figs. 2.10 and 2.11). Just below thesurface epithelium they can be spindle shaped.Cell borders of predecidual cells often areindistinct in formalin-fixed specimens. Prede-cidual transformation begins on day 23 aroundspiral arteries, making the walls appear thicker
and leading to prominence of the vessels (Fig.2.9). In the predecidua there is a resurgence ofstromal mitotic activity at day 24, while theglandular epithelium lacks mitoses. Predecidualchange expands, extending to the subsurfacestroma on day 25. The predecidual changearound vessels and beneath the surface epithe-lium becomes confluent, forming larger sheetsby day 26 (Fig. 2.12). Predecidua is easy to rec-ognize when advanced, but this change can besubtle when it is early and not confluent. Inter-vening stroma often shows some edema, anddating remains based on the most advancedchanges. By day 27, predecidual change isextensive.
With predecidual transformation, the stromashows a gradually increasing number of smaller
18 2. Normal Endometrium and Infertility Evaluation
Figure 2.11. Predecidua and granular lymphocytesin late secretory endometrium. Predecidualizedstromal cells in the late secretory phase appear ovalto polygonal with a moderate amount of pale cyto-
plasm. At this time in the secretory phase, mitoticactivity recurs in the stromal cells. Stromal granularlymphocytes are scattered throughout the stroma.These cells have dark, often lobulated nuclei.

mononuclear and bilobate cells with faintlygranular cytoplasm (Fig. 2.11). Others havesmall round and dense nuclei. Variably termed“stromal granulocytes,” “granulated lympho-cytes,” “K cells,” “leukocytes,” or “neutrophils,”these cells are not polymorphonuclear leuko-cytes (neutrophils).2;3;14;15 The latter occur nor-mally only in menstrual endometrium. Recentimmunohistochemical studies reveal that mostof these cells are T lymphocytes.16–18 We there-fore prefer the designation “granular lympho-cytes.” They are normally present in smallnumbers earlier in the cycle but become promi-nent by the late secretory phase.
At day 27 the endometrium is premenstrual.Predecidua is present in sheets with many inter-spersed granular lymphocytes. The glands arehighly convoluted and saw-toothed. The glandsbegin to show apoptosis with nuclear dust at
their base. On day 28 fibrin thrombi begin toform in small vessels, and hemorrhage followswith extravasation of erythrocytes into thestroma.
Menstrual Endometrium
Menstrual endometrium shows glandular andstromal breakdown that rapidly affects all thefunctionalis by the end of day 28. This stageshows fibrin thrombi in small vessels, con-densed and collapsed stroma, and necroticdebris (Figs. 2.13 and 2.14). With this necrosis,a true neutrophilic infiltrate becomes a part ofthe physiologic process.19 When the bleeding isextensive, it may not be possible to assess thedevelopment of the glands or stroma or the“normality” of the tissue. Once breakdownstarts, the stromal cells coalesce into aggregates
Histologic Dating of the Normal, Cycling Endometrium 19
Figure 2.12. Late secretory endometrium, days26–27. The stroma consists of sheets of predecidual-ized cells with a heavy infiltrate of granular lympho-cytes. The glands in the functionalis remain tortuous
and show a variable amount of intraluminal secre-tion. In the stratum compactum beneath the surfaceepithelium the glands are small and tubular.

Figure 2.13. Menstrual endometrium. Hemorrhage into the stroma forms lakes of erythrocytes. The hemorrhage disrupts the glands and stroma, although the tortuosity of the glands persists.
Figure 2.14. Menstrual endometrium.With stromal hemorrhage, the predecidual cells collapse and they losetheir abundant cytoplasm.

and clusters that often show little cytoplasm.With extensive stromal collapse during men-struation, the predecidual change in the stromalcells becomes indistinct (Figs. 2.14 and 2.15).The extensive breakdown also can result instriking morphologic alterations with artifac-tual glandular crowding. As a result, menstrualendometrium can be confused with hyperplasiaor even carcinoma if the background bleedingpattern is not recognized. Conversely, hyper-plasia and carcinoma are proliferative pro-cesses that rarely show extensive breakdown of the type displayed by menstrual endo-metrium. Because of the artifacts induced by the breakdown and bleeding of the men-strual phase, this tissue is not suitable for eval-uation of glandular and stromal development.Some advocate biopsy at the onset of bleedingto be certain that the procedure does not inter-rupt an early pregnancy, but this tissue is not optimal unless obtained very early in the
menstrual phase before breakdown becomesextensive.4
Pitfalls in Dating
The preceding description summarizes thebasic histologic changes of endometrial devel-opment. In addition to understanding thenormal morphology in ideal situations, oneneeds to consider a number of practical pointswhen interpreting the endometrial biopsy.There are several caveats and potential pitfalls,knowledge of which assists in accurate diagno-sis of normal endometrium and helps avoiderrors in dating. The following, in our opinion,are especially important aspects to consider inevaluating this biopsy material:
1. Endometrium with surface epithelium isbest for interpretation. Absence of surfaceepithelium compromises the interpretation.
Pitfalls in Dating 21
Figure 2.15. Menstrual endometrium. Glands andstroma near the basalis undergo collapse as thesuperficial tissue sloughs. The glands retain tortuous
shapes but show nuclear dust accumulating in thesubnuclear cytoplasm (arrowheads). Predecidualchange in the stroma has become indistinct.

2. Tissue from the lower uterine segment or basalis is not satisfactory for dating.Endometrium from these regions does notrespond fully to hormones.
3. Straight, tubular glands beneath the sur-face are normal and not a sign of irregularity inmaturation in the late secretory phase.
4. Scattered subnuclear vacuoles in glandsare not sufficient evidence of ovulation. To becertain that ovulation has occurred, more than50% of the glands must show subnuclear vacuoles.
5. The presence of secretions in the glandu-lar lumen does not indicate secretory endome-trium. Proliferative, hyperplastic, and neoplasticglands can contain luminal secretions. It is theglandular cytoplasm and nuclear changes thatare most important for determining the pres-ence or absence of secretory changes.
6. Focal glandular crowding caused by tan-gential sectioning can occur in proliferative orsecretory endometrium (Figs. 2.16 and 2.17).
This artifact can result in back-to-back glandsthat do not represent hyperplasia.
7. Focal cystic glands or nonreactive glandscan occur in normal endometrium and have nosignificance by themselves.
8. Patchy stromal edema is normal by days17 to 18 of the secretory phase and does notsignify irregular maturation.
9. Identifying very early pregnancy based onendometrial changes alone is very difficult.Apparent “hypersecretory” late secretoryphase glands with vacuolated cytoplasm usuallyare a variation of normal development and donot, by themselves, indicate early pregnancy(see Chapter 3).
10. Compact predecidua with spindle-shaped stromal cells may not be appreciated asa true predecidual reaction. Directing attentionto stromal changes around spiral arteries assistsin the identification of predecidua.
11. Lymphocytes and granular lymphocytesnormally become prominent in the stroma of
22 2. Normal Endometrium and Infertility Evaluation
Figure 2.16. Artifactual crowding of late secretoryendometrium. Tangential sectioning of normal latesecretory endometrium near the basalis yields a
pattern of focal glandular crowding. This artifact hasno significance and should not be misinterpreted ashyperplasia.