Developmental abnormalities in the trunk and limbs of the talpicT … · (neural tube, notochord,...
Transcript of Developmental abnormalities in the trunk and limbs of the talpicT … · (neural tube, notochord,...
/ . Embryol. cxp. Morph., Vol. 12, Part 2, pp. 339-356, June 1964Printed in Great Britain
Developmental abnormalities in the trunk andlimbs of the talpicT mutant of the fowl
by D. A. E D E 1 and w. A. KELLY2
From the Department of Biological Sciences, Wye College, University of London, and theAgricultural Research Council's Poultry Research Centre, Edinburgh
WITH TWO PLATES
THREE talpid mutants have been described in the fowl, all of which are character-ized by lethal embryonic abnormalities. As explained in a previous paper (Ede &Kelly, 1964), Cole's talpid and talpid3 are similar in all essential respects and showstriking abnormalities in the head region, whereas talpid2 has a relatively normalhead. In all three, embryos surviving to 11 days and over show the followingabnormalities in the trunk region: (1) The vertebral column is shortened. (2) Thelimbs are extremely short and the number of digits on each is greater than normal.(3) The viscera are ectopic, i.e. they protrude through the ventral body wall,which remains open. (4) Feather development is incomplete. (5) There is muchsubcutaneous oedema and haemorrhage. Instances of inherited polydactylyhave always excited interest on account of the light they may throw on the originsand production of the pentadactyl limb pattern in typical vertebrate embryos. Thetalpid mutants are particularly interesting in this respect since the effect on limbdevelopment is much more drastic than in other cases of inherited polydactylyin the fowl, producing a totally different pattern rather than simply duplicatingone of the digits as in polydactyly (Po), duplicate (Pod) and diplopodia (dp) (re-viewed by Hutt, 1949). Also, unlike these other mutants in which other structuresare affected only to a very minor degree, the effect of the talpid genes is extremelypleiotropic, producing widespread developmental disturbances. The unravellingof complex pleiotropic patterns of damage has long been established as a power-ful technique of embryological investigation, especially by Griineberg in manystudies on mouse mutants (reviewed by Griineberg, 1963). In these studies it ispostulated that gene action is unitary, and that by establishing a 'pedigree ofcauses' the primary action of the gene, producing the primary defect from whichall the others ultimately arise, is revealed. In this paper the development of the
1 Author's address: Poultry Research Centre, West Mains Road, Edinburgh 9, U.K.2 Author's address: Department of Biological Chemistry (Agricultural Biochemistry),
University of Aberdeen, Aberdeen, U.K.
340 D. A. EDE andw. A. KELLY
abnormalities in the trunk and limbs of talpid3 is analysed with a view to dis-covering the connexions between them and relating them to the abnormalities ofthe head region. The basic defect appears to lie in some disturbance of themesenchyme, affecting its segregation into mesenchymal condensations, andlater the formation within it of cartilage and bone. It is hoped that further workwill test this hypothesis, and give more precise information about the fundamentaldevelopmental disturbances.
MATERIALS AND METHODS
These have been described (Ede & Kelly, 1964). In addition, serial transverseand longitudinal sections were made of the fore and hind-limbs of normal andtalpid3 embryos at 4^, 5, 5^, 6 and 11 days of incubation.
RESULTS
In the following account the external appearance of normal and talpid3 embryosis compared at 3,4,6 and 11 days of incubation. This is followed by descriptionsof the development in each case of the limbs and girdles, the axial structures(neural tube, notochord, vertebral column and ribs) and the myotomes. Finallythere is a comment on the viscera in talpid3.
External appearance of the embryos3 days
The normal embryo (Text-fig. 1 A) lies on its left side. The cervical flexure is sopronounced that the dorsal outline of the embryo forms a U-shaped curve. Thelimb buds are simple in form, and the wing buds are considerably smaller than theleg buds.
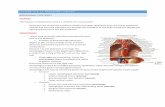
EXPLANATION OF PLATES
Abbreviations: AER, apical ectodermal ridge; CP, costal process; D, dermatome; DM,dermal mesenchyme; EPI, epidermis; LVC, lateral vertebral cartilages; MVS, marginalvenous sinus; MY, myotome; MYC, myocoele; NA, neural arch; NC, neural canal; NO,notochord; NT, neural tube; NTV, neural tube vesicle; OE, oedema; SCL, sclerotome;SG, spinal ganglion; SNB, subnotochordal bar; VB, vertebral body; VBC, vertebral bodycomponent.
PLATE 1
FIG. A. Lateral view of normal embryo at 6 days.FIG. B. Dorsal view of the same normal embryo.FIG. C. Lateral view of talpid3 embryo at 6 days.FIG. D. Dorsal view of the same talpid3 embryo.FIG. E. Transverse section through the wing-bud region of a 6-day normal embryo.FIG. F. Transverse section through the wing-bud region of a 6-day talpid3 embryo.FIG. G. Section (transverse to body axis) through wing bud of a 6-day normal embryo.FIG. H. Section (transverse to body axis) through wing bud of a 6-day talpid3 embryo.FIG. I. Transverse section through the wing-bud region of a 2^-day normal embryo.FIG. J. Transverse section through the wing-bud region of a 2^-day talpid3 embryo.
The talpid3 mutant of the fowl 341
The head of the talpid3 embryo (Text-fig. IB) is completely turned on to its leftside, but the trunk is not. The cervical flexure is reduced, so that the dorsal out-line of the embryo is only gently curved. The limb buds are again simple in form,but the difference in size between wing and leg buds is not so pronounced. Fromthe embryos examined at this stage it appears that the wing bud is more extensivethan in the normal.
4 daysIn the normal embryo (Text-fig. 1C) the limb buds are slightly elongated, in-
clined somewhat backward and growing in a ventral direction, so that right andleft buds of each pair are almost parallel. The leg bud is distinctly larger than thewing bud. There is an epidermal fold above each limb bud which is continuedfaintly along the trunk between the limbs, along the ventral edge of the somites.The divisions between successive somites are marked by faint transverse epidermalcorrugations. The dorsal ridge of the trunk is smoothly domed in transversesection.
In the talpid3 embryo (Text-fig. ID) the trunk is still not completely turnedon to its side. The lateral walls of the body diverge from each other, as do theright and left members of each pair of limb buds. The limb buds are still simplecurved outgrowths; in sharp contrast to the normal the wing bud is considerablylarger than the leg bud and at this stage it is beginning to show its characteristicshape. The fold above the limb buds is continued as a deep epidermal groovealong the trunk between the limbs, following the ventral edge of the somites. Thesomites themselves are clearly outlined by deep transverse corrugations in theepidermis, and there is a median longitudinal groove along the dorsal ridge of thetrunk.
6 days
In the normal embryo (Text-fig. 2E; Plate 1, Figs. A, B) the limb rudiments areelongated and asymmetrical, with distinct elbow and knee joints and the contoursof the digits visible on the foot plates. The dorsal outline of the body is curved(Plate 2, Fig. K). The body wall has closed over the viscera ventrally.
PLATE 2
(Note: In Figs. K-R the anterior end of the section is to the right.)FIG. K. Median longitudinal section through trunk of a 6-day normal embryo.FIG. L. Median longitudinal section through trunk of a 6-day talpid3 embryo.FIG. M. Median longitudinal section through trunk of a 9-day normal embryo.FIG. N. Longitudinal section, slightly oblique to the median line, through a 9-day talpid*
embryo.FIG. O. Vertical longitudinal section, lateral to neural tube, of a 4^-day normal embryo.FIG. P. Vertical longitudinal section, lateral to neural tube, of a 4^-day talpid3 embryo.FIG. Q. Detail from Fig. O.FIG. R. Detail from Fig. P.
342 D. A. EDE andW. A. KELLY
1 mmB
1 mm D
TEXT-FIG. 1. A. normal embryo at 3 days. B. talpid1 embryo at 3 days. C. normal embryo at4 days. D. talpid3 embryo at 4 days. Camera lucida drawings from whole mounts (A, B)and fixed embryos (C, D).
The talpid3 mutant of the fowl 343
3 mm
10 mm
H
TEXT-FIG. 2. E. normal embryo at 6 days. F. talpid1 embryo at 6 days. G. normal embryo at11 days. H. talpid* embryo at 11 days. Drawings from photographs of fixed embryos.
23
344 D. A. EDE and w. A. KELLY
In the talpid* embryo (Text-fig. 2F; Plate 1, Figs. C, D) the lateral body wallshave at this stage turned ventrally, but have not grown together in the mid-line.The limb rudiments remain spread-eagled rather than growing down parallel totheir opposite partners. The wing bud is very broad and its outline is mushroom-shaped, terminating in an extensive curved paddle. The leg rudiment is elongatedand more like the normal in form, but again the outer edge presents a broadregular curve rather than coming to show the shape of the foot. The dorsal out-line of the body is straight over most of its length (Plate 2, Fig. L). The longi-tudinal trunk folds, the transverse somite divisions and the dorsal groove are stillobvious (Plate 1, Fig. D).
11 daysIn the normal embryo (Text-fig. 2G) the definitive form of the chick is estab-
lished, with the essential structure and shape of the wings and legs completed.The neck is now elongated, and the head is set on it at right angles. Featherdevelopment is well-advanced.
The talpid3 embryo (Text-fig. 2H) is shorter than the normal, chiefly due to anextreme reduction of the neck. The head (as determined by the line of thebrain) is set on the neck in line with it. The body wall is not closed ventrally, andthe heart and parts of the gut protrude through it. Feather germs are present,protruding slightly above the surface, but are not developed as feather papillae.There is considerable subcutaneous oedema, particularly in the neck and trunkregions, and haemorrhage. The wings are now smaller than the legs, forming shortspatulate outgrowths with slightly concave outer edges. Up to eight digits arepresent in each wing, but they are not visible externally and the wing may there-fore be called syndactylous. The legs are extremely short and are polydactylous;there are up to eight digits which are clearly visible externally, though they arepartly or completely connected by a web (Text-figs. 2H, 3H).
The skeleton of the limbs and girdles6 days
In the normal embryo the mesenchymal condensations representing the longbones of the limbs and the metacarpal and metatarsal elements are clearly dis-tinguishable (Text-fig. 3 A, E). Around the distal edge of the limb rudiments theectoderm projects as the apical ectodermal ridge (AER), and in the mesenchymebehind the ridge there is a vascular channel, the marginal venous sinus (Plate 1,Fig. G.) his sinus extends round the whole length of the limb rudiment, andbranches from it extend along the margins of the metacarpal and metatarsalcondensations.
In the talpid3 embryo mesenchymal condensations form within the limb rudi-ment in the normal proximo-distal sequence, but there is no segregation intodistinct longitudinal condensations within each transverse band. Thus in thewing rudiment (Text-fig. 3C) there is a proximal condensation representing the
The talpid3 mutant of the fowl 345
MCRA
HU
PH
UL
MT
MVS
MC
HU
RA-UL
MC-CA
MT TI-FI
FE
MVS
MTTI-FI
H
TEXT-FIG. 3. Wing rudiments from: A. normal embryo at 6 days. B. normal embryo at 11 days.C. talpid1 embryo at 6 days. D. talpid3 embryo at 11 days. Leg rudiments from: E. normalembryo at 6 days. F. normal embryo at 11 days. G. talpid3 embryo at 6 days. H. talpid3
embryo at 11 days. Drawings from reconstructions from sectioned limb rudiments (A, C, E,G) and from photographs of Lundvall cartilage preparations (B, D, F, H). In A, C, E, Gstippling represents mesenchymal condensations; black represents blood capillaries. InB, D, F, H black represents cartilage. CA, carpal; DI, digit; FE, femur; FI, fibula; HU,humerus; MC, metacarpal; MT, metatarsal; MVS, marginal venous sinus; PH, phalanges;RA, radius; TA, tarsal; TI, tibia; UL, ulna.
346 D. A. EDE and w. A. KELLY
humerus, then a broader condensation representing both radius and ulna, anddistal to that a very broad band representing the metacarpal tissue. At the veryedge of the rudiment, between the AER and the marginal venous sinus there is aconcentration of mesenchyme in which the digits appear to form. The sinus itselfis excessively developed (Plate 1, Fig. H) and large branches from it extend intothe mesenchyme between the metacarpal and the forearm condensations. Theformation of the mesenchymal condensations in the hind-limb is essentiallysimilar (Text-fig. 3G).
11 days
In the normal embryo all the skeletal elements of the wings and legs are laiddown as cartilage at this stage (Text-fig. 3B, F). In the pectoral girdle the coracoidsare represented by rod-shaped cartilages, and the scapulas by a sabre-shapedcartilage on each side (Text-fig. 4A, B); the clavicles are referred to below. Inthe pelvic girdle the most conspicuous element is the ilium, present as a slightlybowed cartilage, and there is a rod-like cartilage representing the ischium on eachside (Text-fig. 4A); the pubic element is inconspicuous.
In the talpid3 embryo the mesenchymal condensations in the wing havebecome chondrified (Text-fig. 3D) but there is scarcely more segregation oflongitudinal elements within each transverse band than at 6 days. The humerus isextremely short, the radius and ulna are short and may be fused, the metacarpals(and presumably, included with them, the carpals) form a single eartilage which isbroader than long. Along the extreme edge of the wing there are up to eight digits,each represented by a single short cartilage. Between the digits and the meta-carpal cartilage is a broad region occupied by mesenchyme with blood capillaries,presumably corresponding to the area occupied earlier by the marginal venoussinus. Between the metacarpal band and the forearm there is another regionwhich is devoid of cartilage, corresponding to the area filled by branches from thesinus in the early rudiment. Development of the leg and foot is similar, but lessdrastically abnormal. The digits, of which there may be up to eight, are betterdeveloped than in the wing, and they are sometimes bifurcated (Text-fig. 3H).
The coracoids in talpid3 are represented by a short cartilaginous rod on eachside (Text-fig. 4C). The scapulas are the most obvious elements in the pectoralgirdle; each is abnormally curved, bowed rather like a boomerang, and has anumber of rounded projections from its dorsal edge (Text-fig. 4C, D). Anteriorlythere are cartilaginous connexions with the neck vertebrae. In the pelvis the onlyconspicuous element is the ilium, which is abnormally curved.
In the normal embryo the development of the bony skeleton is well advancedby 11 days (Text-fig. 5A). In the skull the most obvious bones are the membranebones laid down around the jaws, and other membrane bones cover the roof andsides of the skull. In the trunk there is another pair of membrane bones, theclavicles, which fuse to form the V-shaped furcula of the pectoral girdle, with itsmedian projection. The remaining bones are all formed in cartilage. Those
The talpid3 mutant of the fowl 347
DTEXT-FIG. 4. Cartilage formation in 11-day embryos, limbs removed. A. normal, lateral view.
B. Normal, dorsal view. C. talpid2, lateral view. D. talpid*, dorsal view. Drawings fromphotographs of Lundvall cartilage preparations.
348 D. A. EDE and W. A. KELLY
visible at this stage are the long bones of the limbs, bones of the limb girdles, theribs and the vertebrae.
In talpid3 embryos at 11 days there are no cartilage replacement bones (Text-fig.5B). In the head the membrane bones laid down around the jaws are clearly pre-sent, and the squamosal is very heavily ossified. There are traces of ossificationin the nasal and frontal regions. In the remainder of the body the only ossified
B
TEXT-FIG. 5. Bone formation in 11-day embryos. A. normal, ventral view. B. talpid1, ventralview. Drawings from photographs of Alizarin bone preparations.
structure is the furcula, and this is abnormal in form. The two clavicles are widelyseparated, and are connected by a slender bridge of bone with a projection ateach end. Other parts of the skeleton are represented only by their cartilaginousprecursors.
The axial structures: neural tube, notochord, vertebral column and ribs
6 days
This stage in the development of these structures in the normal embryo is shownin a transverse section of the upper part of the trunk in the wing bud region (Plate 1,Fig. E). The neural tube is bell-shaped in cross-section, and its central canal is avertical slit. The wall of the neural tube forms a thin roof plate dorsal to thecentral canal, and a thin floor plate ventral to it. On each side of the slit are thecells of the ependymal layer. Beneath the floor plate is the ventral fissure, a
The talpid3 mutant of the fowl 349
groove between the slightly protruding ventral columns of neuroblasts and nervefibres. The spinal ganglia are arranged segmentally on either side, against theventral half of the neural tube. Ventral to the neural tube is the notochord,surrounded at this stage by the mesenchymal condensations of the vertebral body.These consist of right and left condensations which have fused ventral to theneural tube around the notochord, their anterior portions forming the sub-notochordal bar (Plate 2, Fig. K). Laterally the condensations are extended toform the costal processes, and dorsally they extend to form the components of theneural arch, which at this stage have not united dorsally. Cartilage formationhas begun in some parts of these mesenchymal condensations, especially in thesub-notochordal centre.
In the talpid2 embryo several striking differences are apparent in a correspond-ing transverse section (Plate 1, Fig. F). The neural tube is not bell-shaped butovoid in cross-section. Its central canal is again a vertical slit, but it does notextend to the floor plate except at intervals (which are probably segmental) wherenarrow extensions from the central canal lead ventrally into small vesicles em-bedded in the lower part of the neural tube (Plate 2, Fig. L). It appears that, exceptin these isolated vesicles, the floor plate has rounded up and the ependymallayers on opposite sides of the central canal have united along the lower part of theneural tube. This is part of a more general deformation of shape which has takenplace in the neural tube. There is no ventral fissure, or it is very shallow, since theventral columns of neuroblasts and nerve fibres are not prominent. The spinalganglia are situated high up against the dorsal half of the neural tube. All thesethings suggest that the neural tissue has been forced upward on each side.
A corresponding deformation is seen in the mesenchymal condensations of thevertebrae. Thus, the lateral condensations which normally fuse around thenotochord to give the vertebral body and the subnotochordal bar are displacedupward, and with them the costal processes, leaving the notochord unenclosed(Plate 1, Fig. F; Plate 2, Fig. L). The lateral condensations giving rise to theneural arch are similarly displaced, so that they appear closer together dorsally.The ectoderm above and between them is slightly pinched in, forming thelongitudinal groove referred to among the external features. At 6 days cartilageformation has begun in the ventral mesenchyme condensations. The notochordis normal.
9-11 days
In the normal embryo at this stage (Text-fig. 4A, B) the vertebrae are fullyformed in cartilage, and some ossification has occurred. Each vertebra consistsof the following elements (Plate 2, Fig. M), all having formed from the mesen-chymal condensations on each side of the notochord and neural tube which havebeen described above: (1) The vertebral body, which encloses the notochord and isextended as a ventral projection anteriorly, the subnotochordal bar. (2) Theneural arches, extending up from the sides of the vertebral body and uniting above
350 D. A. EDE and w. A. KELLY
the neural tube. (3) The costal processes, forming the vertebral ribs, whichextend from the vertebral body on each side but are not fused with it. The sternalribs arise from condensations in the mesenchyme of the lateral plate, and aredistinctly formed at this stage. Late in embryonic development there is consider-able fusion of vertebrae, but at this stage they are quite distinct.
In talpid3 embryos which survive to this stage (Text-fig. 4C, D) cartilaginousvertebrae are present but they are extremely malformed, and there is much fusionof adjacent vertebrae (Plate 2, Fig. N). They are closer together than in thenormal and the cartilages of the right and left sides have not fused, so that thenotochord is not enclosed. These abnormal characteristics are shown especiallyclearly in the cervical region, where the posterior vertebrae are fused not only toeach other but also to projections from the scapula on each side. Sternal ribs arenot present.
The myotomes
The development of the myotomes, giving rise to the muscle fibres connectingadjacent vertebrae, is best seen in longitudinal section. In the normal embryo atA\ days (Plate 2, Figs. O, Q) the mesodermal cells of the myotome have becomealigned strictly longitudinally, interspersed with parallel strands of collagen. Theouter face of the myotome is clearly distinct from the dermal mesenchymeexternal to it, forming a curved boundary. The ectoderm over the myotome isnot corrugated.
In talpid1 embryos the developing muscle cells and the strands of collagenbetween them do not show the strictly longitudinal arrangement that is foundnormally; instead there is a tendency to random orientation (Plate 2, Figs. P, R).The cells at the outer boundary tend to mingle with those of the dermal mesen-chyme which covers them, so that the division between the two is blurred and theouter boundary of the myotomes is not regularly curved. The thickness of thedermal mesenchyme is reduced and the ectoderm over the myotomes is indentedat the boundaries of the somites, forming the transverse corrugations referred toamong the external features.
Division of the paraxial mesoderm into somites is normal in talpid3, but thedevelopment of the somites is already abnormal by 2\ days, the earliest stage atwhich sections were made. In the normal embryo a transverse section of the trunkin the wing bud region (Plate 1, Fig. I) shows that the myotome material hasdoubled back and grown along the internal face of the dermatome, obliteratingthe myocoele cavity. In talpid} a corresponding section (Plate 1, Fig. J) shows thatthe myotome cells are disorientated, mingling with the sclerotome material, andthat the myocoele is not obliterated but, on the contrary, abnormally large.
The viscera
Apart from minor displacements and the protrusion of some of them throughthe gut wall ventrally the viscera appear to be normal in talpid3. The lungs and
The talpid3 mutant of the fowl 351
kidneys were particularly studied for histological abnormalities, but none werefound.
DISCUSSION
The characteristic which has attracted most attention in the talpid mutants isthe development of more than the normal number of digits on both fore and hindlimbs. There has been a particular interest in the causal analysis of polydactylysince Saunders (1948) demonstrated that the apical ectodermal ridge (AER)played an important part in controlling the growth and pattern of the developinglimb of the fowl. Zwilling & Hansborough (1956) and Zwilling (1956) have sinceshown that an AER maintenance factor located in the mesoderm is also involved,and some doubts have arisen as to the complete validity of the concept of the AERas an inductor (Bell, Gasseling, Saunders & Zwilling, 1962). Nevertheless, thehypothesis that the formation of the limbs is controlled by reciprocal interactionsbetween ectodermal and mesodermal factors in the limb buds remains the basisfor discussion of abnormalities in their development.
Thus, Carter (1954) discovered that in the luxate mutant of the mouse, wherethere is a pre-axial polydactyly of the hind-limb, there is a cranial shift of thehind-limb region. He postulated that the abnormality could be accounted for bythe cranial displacement of a limb-inductor relative to limb-potent tissue, and thatthese might well be the precursors of the AER and the lateral mesenchymerespectively. Zwilling & Hansborough (1956) were able to separate the AER fromthe underlying mesoderm in normal and polydactylous {duplicate, Pod) chickembryos, and make various ectoderm-mesoderm exchanges in order to explorethe parts played by each in producing the extra digits. They concluded thatalthough outgrowth of the limb is dependent upon the AER, the ridge itself ismaintained in an active condition by a mesodermal factor. The duplicate genedoes not affect the size of the limb bud at 3 days, but later there is an excessive out-growth of the limb, caused by the development of an abnormally extensive AER.The exchanges showed that the gene acts primarily on the mesoderm, and it ispostulated that although the limb buds at 3 days are no larger than normal, thedistribution of the maintenance factor in the mesoderm is more extensive.
In an earlier study on polydactylism in the guinea-pig, Scott (1937) concludedthat the action of the Pollex gene was to cause an excessive growth at a particulardevelopmental period, and noted that the limb buds were enlarged. Zwilling &Hansborough are inclined to discount this observation, but it may be pointed outthat Pollex has much more widespread developmental effects than duplicate, andthe underlying causal mechanisms may be very different.
Talpid mutants resemble Pollex in the guinea-pig more closely than they dopolydactyly and duplicate in the fowl. In the two latter mutants there is simply aduplication or triplication of the hallux, and there are no other major effects. InPollex there is a more basic alteration of the foot plate, with the production oftenor eleven digits rather than the normal three, there are major abnormalities in
352 D. A. EDE and w. A. KELLY
other organs and the total effect is lethal. Even so, the resemblances betweentalpids and Pollex are superficial, for the talpid limbs are even more drasticallyreorganized, and there is no correspondence between the abnormalities in theother organs.
Abbott, Taylor & Abplanalp (1960) examined talpid2 in the light of the find-ings of Zwilling & Hansborough and have reported that in this mutant the AERis increased in area, and that 'the ridge remains thick and "functional" over thedigit-forming area oftalpid2 embryos long after its regression in normal siblings'.In talpid3 the AER is certainly much more extensive than normal in the mush-room-shaped wing bud since it is developed along the whole length of its veryextensive external edge (Text-fig. 3 C), and preliminary observations by J. R.Hinchliffe suggest that this is also the case in the leg bud at the correspondingstage. In other respects the appearance of the ridge in talpid3 and normal embryosis similar up to the last stage in which it has been followed in the present study, i.e.6 days (Plate 1, Figs. K, L). It may be concluded that an extended AER doesplay some part in producing the polydactylous condition in talpid3 and that themutant mesoderm has the ability to maintain this extended ridge, but it is notpossible to decide on the basis of the present data whether the elongated AER isthe cause of the enlarged limb paddle or a consequence of it.
The wingless gene in the fowl is also pleiotropic, affecting the development ofthe lungs and metanephric kidneys as well as the limbs, and Zwilling (1949) haspointed out that in each case there has been a failure of an inductive actioninvolving the effect of an epithelial component upon a mesenchymal one. Intalpid3 the development of the lung and metanephros is normal, and there isevidently no such disturbance of epithelio-mesenchymal interactions in theseorgans.
Apart from the limbs the most striking effect of the talpid3 gene in the trunkregion is upon the vertebral column and, to a lesser extent, upon the spinal cord,and the possibility of a disturbance of an inductive process must be consideredhere. A number of workers (reviewed Holtzer, 1959) have shown that in the chickembryo differentiation of the sclerotome cells to form cartilage depends uponinductive influences exerted by the neural tube and the notochord. In a numberof mouse mutants, e.g. Short-tail (Chesley, 1935) and Danforth's Short-tail(Gluecksohn-Schoenheimer, 1945), there is a disturbance of this inductivemechanism, so that the formation of vertebrae, along the whole of the body or inthe tail region only, is defective. In these cases the notochord appears to beprimarily affected, and there are irregularities and degenerative changes in theneural tube.
In talpid3 the notochord is normal, and although abnormalities of the nervecord occur they are minor in character, and involve no degenerative changes.The abnormal form of the floor plate somewhat resembles the condition found inembryos of certain mouse mutants (reviewed by Theiler, 1959) in which absenceof the notochord, or its separation from the developing neural tube, results in the
The talpid3 mutant of the fowl 353
replacement of the floor plate by a thick 'basal mass'. It appears that in talpid3
the notochord is sometimes slightly displaced away from the neural tube (Plate 1,compare Figs. E and F), and this might result in a comparable failure of theinductive stimulus normally exerted by the notochord. But, in general, theposition of the notochord relative to the neural tube is not markedly affected(Plate 2, compare Figs. K and L) and the abnormal form of the floor plate may bea direct result of mechanical pressures exerted by the surrounding mesoderm.It therefore seems unlikely that the abnormalities of the vertebral column areproduced by failure of the neural tube or notochord as inductors, and the basicdefect should be looked for in the somitic mesenchyme itself. This basic defectappears to be an incapacity of the pre-cartilage mesenchyme cells to segregateproperly in the formation of the vertebral condensations, resulting in mal-formation of the cartilages arising from them and in fusion of the cartilages ofadjacent vertebrae. This abnormality in vertebral cartilage formation in talpid3
corresponds to the defective mesenchymal segregation in the limb buds, shownmost clearly in the failure of separate digital condensations to arise in themetacarpal and metatarsal regions.
This hypothesis is supported by a consideration of the general effects of thetalpid3 gene. Thus in the head (Ede & Kelly, 1964) there is a fusion rather than asegregation of certain of the normally bilateral mesenchymal condensations, re-sulting in mesial displacement of the facial structures, which leads to otherdevelopmental abnormalities. In the trunk region the tendency is not to fusion ofstructures in the mid-line but rather the reverse. For example, the ventral bodywall is not closed, the limbs at an early stage are spread-eagled, and the claviclesare widely separated, though a bridge of bone develops between them. This inno way conflicts with the situation in the head, since in the head the pre-chordalmesoderm in the early embryo is continuous across the mid-line, and a failure orpartial failure of segregation will result in the formation of medial rather thanbilateral structures. The trunk mesoderm on the contrary is paraxially arranged,forming two separate strips along each side of the notochord, and failure ofmesenchymal segregation here will result in tissues being pulled away from themid-line rather than the reverse (Text-fig. 6). This traction away from the mid-line is probably the cause of the dorsal deflection of the vertebral cartilages, andof the regions of the nerve cord.
Although it is chiefly the precartilage mesenchymal areas which are affected,there is some evidence that this is not always the case. Thus the myotome cellsbetween adjacent vertebrae are partially disorientated, and the boundary betweenthem and the overlying dermal cells is blurred. This has the effect of reducing thethickness of the dermis considerably, and this may account for the presence ofectodermal corrugations corresponding to the somitic divisions. Initial formationof the somites is not affected, but the disorientated migration of the sclerotomecells at 2\ days represents another example of defective mesenchymal movement.
Mesenchymal condensation involves the orientated movement of individual
354 D. A. EDE andw. A. KELLY
mesenchyme cells, and it has often been suggested (e.g. Baitsell, 1925; Weiss,1933) that the most important factor in directing the movements of these cells isthe character of the ground substance in which they are embedded. Evidence thatthis is the case has come from studies on the reaggregation of dissociated tissuecells (Moscona, 1960). In these the behaviour of the dissociated cells was foundto be largely determined by the extracellular matrix material produced by the
NTPCM
TEXT-FIG. 6. Diagram to show the effect of the disturbance of pre-cartilage mesenchymalcondensation in the head region, where the prechordal mesoderm is continuous across themid-line (causing mesial fusion of lateral structures in talpid3), and in the trunk region,wherethe paraxial strips of mesoderm are separated by the neural tube and notochord (causingventral structures to be pulled away from the mid-line in talpid3). A. normal, head. B.normal, trunk. C. talpid3, head. D. talpid3, trunk. FG, fore-gut; LHM, lateral headmesoderm; PAM, paraxial mesoderm; PCM, prechordal mesoderm; NO, notochord;NT, neural tube.
cells, thought to be comparable to the intercellular ground substance of theembryo. Walker (1961) has shown that the sulphated mucopolysaccharideswhich make up an important part of the ground substance are associated with themesenchyme of the palatine shelves in the mouse embryo at the time whenpalatal closure is occurring, suggesting that the force responsible for this closuremay reside in the ground substance. Runner (1959), in studies of teratogenicagents on mouse embryos, has shown that interference with carbohydratemetabolism, which will be involved in mucopolysaccharide synthesis, affectscondensation of the precartilage mesenchyme.
The talpid3 mutant of the fowl 355
Two other characteristics of the talpid3 embryo support this line of reasoning:the extensive subcutaneous haemorrhage and the widespread oedema of thedermal mesenchyme. Gersch and Catchpole (1949) have shown that changes incapillary permeability and oedema may result from change in the ground sub-stance, especially in the basement membrane, surrounding the capillaries. Thepresent evidence suggests, therefore, that the primary effect of the talpid3 gene is adisturbance of the metabolism of the mesenchymal cells, producing an alterationof the normal character of the ground substance which leads to abnormal con-densation of the precartilage mesenchyme and through this to other morpho-genetic consequences which have been described.
SUMMARY
1. The talpid3 mutant in the fowl is characterized by a number of morpho-logical abnormalities in the trunk region, notably shortening and polydactyly ofboth pairs of limbs, and malformations of the vertebral column and other parts ofthe cartilaginous skeleton.
2. No cartilage bone is present in the oldest surviving embryos, but themembrane bones forming the furcula are developed.
3. Developmental analysis of the abnormalities indicates that the condensa-tion of the precartilage mesenchyme is disturbed, and it is suggested that the basicdefect may lie in the character of the intercellular ground substance.
RESUME
Des anomalies du tronc et des membres pendant le developpement dupoulet talpid3
1. L'anomalie hereditaire talpid3 du poulet est caracterisee par un nombred'anomalies morphologiques dans la region du tronc, notamment par unraccourcissement et la polydactylie des ailes et des jambes. La colonne vertebraleet d'autres parties du squelette cartilagineaux sont malformees.
2. Les embryons les plus ages n'ont pas d'os enchondraux mais les os desrecouvrement qui forment les clavicules sont presents.
3. Une analyse du developpement de ces anomalies montre que la condensa-tion du mesenchyme pre-cartilagineux est derange, et on suggere que la defautqui est a l'origine des anomalies peut reposer dans le caractere de la substanceintercellulaire.
ACKNOWLEDGEMENTS
We wish to thank Mr Eric Maddison for providing facilities in the Poultry Research Sectionat Wye College, and to the Flock Manager, Mr C. Day, for maintaining the stock. Thanks arealso due to Miss P. Lewis and Miss K. Smith for valuable technical assistance.
356 D. A. EDE andw. A. KELLY
REFERENCES
ABBOTT, U. K., TAYLOR, L. W. & ABPLANALP, H. (1960). Studies with talpid1, an embryoniclethal of the fowl. J. Hered. 51,195-202.
BAITSELL, G. A. (1925). On the origin of the connective-tissue ground-substance in the chickembryo. Quart. J. micr. Sci. 69, 571-89.
BELL, E., GASSELING, T. A., SAUNDERS, J. W. Jr. & ZWILLING, E. (1962). The role of the ecto-derm in limb development. Devel. Biol. 4,177-96.
CARTER, T. C. (1954). The genetics of luxate mice. IV. Embryology. / . Genet. 52,1-35.CHESLEY, P. (1935). Development of the short-tailed mutant in the house mouse. / . exp.
Zool 70,429-59.EDE, D. A. & KELLY, W. A. (1964). Developmental abnormalities in the head region of the
talpid} mutant of the fowl. / . Embryol. exp. Morph. 12,161-182.GERSCH, I. & CATCHPOLE, H. R. (1949). The organization of ground substance and basement
membrane and its significance in tissue injury, disease and growth. Amer. J. Anat. 85,457-521.
GLUECKSOHN-SCHOENHEIMER, S. (1945). The embryonic development of mutants of the Sd-strain in mice. Genetics, 30, 29-38.
GRUNEBERG, H. (1963). The pathology of development. Oxford: Blackwell.HOLTZER, H. (1959). The development of mesodermal axial structures in regeneration and
embryogenesis. In Regeneration in Vertebrates (ed. C. Thornton), pp. 15-33. Chicago:University of Chicago Press.
HUTT, F. B. (1949). Genetics of the Fowl. New York: McGraw-Hill.MOSCONA, A. A. (1960). Patterns and mechanisms of tissue reconstruction from dissociated
cells. In Developing Cell Systems and their Control (ed. D. Rudnick). New York: RonaldPress.
RUNNER, M. N. (1959). Inheritance of susceptibility to congenital deformity, metabolic cluesprovided by experiments with teratogenic agents. Pediatrics, N. Y. 23,245-51.
SAUNDERS, J. W. Jr. (1948). The proximo-distal sequence of origin of the chick wing and therole of the ectoderm. / . exp. Zool. 108, 363^04.
SCOTT, J. P. (1937). The embryology of the guinea-pig. III. The development of the poly-dactylous monster. A case of growth accelerated at a particular period by a semi-dominantlethal gene. / . exp. Zool. 77,123-57.
THEILER, K. (1959). Schwanzmutanten bei Mausen. Ein Beitrag zur Entstehung von Wir-belfehlern. Z. Anat. Entw. Gesch. Ill, 155-64.
WALKER, B. E. (1961). The association of mucopolysaccharides with morphogenesis of thepalate and other structures in mouse embryos. / . Embryol. exp. Morph. 9,22-31.
WEISS, P. (1933). Functional adaptation and the role of ground substances in development.Amer. Nat. 67, 322-340.
ZWILLING, E. (1949). The role of epithelial components in the developmental origin of the'wingless' syndrome of chick embryos. / . exp. Zool. I l l , 175-87.
ZWILLING, E. (1956). Interaction between limb bud ectoderm and mesoderm in the chickembryo. IV. Experiments with a wingless mutant. / . exp. Zool. 132,241-54.
ZWILLING, E. & HANSBOROUGH, L. A. (1956). Interaction between limb bud ectoderm andmesoderm in the chick embryo. III. Experiments with polydactylous limbs. J. exp. Zool.132, 219^0.
{Manuscript received 20th January 1964)