Depth-enhanced 2-D optical coherence tomography using ... · Optical coherence tomography (OCT) is...
Transcript of Depth-enhanced 2-D optical coherence tomography using ... · Optical coherence tomography (OCT) is...

Depth-enhanced 2-D optical coherence tomography using complex wavefront shaping
Hyeonseung Yu,1,4 Jaeduck Jang,2,4,5 Jaeguyn Lim,3,4 Jung-Hoon Park,1 Wooyoung Jang,3 Ji-Yeun Kim,2 and YongKeun Park1,*
1Dept. of Physics, Korea Advanced Institute of Science and Technology, Daejeon 305-701, South Korea 2Samsung Advanced Institute of Technology, Yongin-si, Gyeonggi-do, South Korea
3Saumsung Electronics, Suwon-si, Gyeonggi-do, South Korea 4Contributed equally to this work
[email protected] *[email protected]
Abstract: We report the enhancement in the obtained signal and penetration depth of 2-D depth-resolved images that were taken by shaping the incident wavefront in optical coherence tomography (OCT). Limitations in the penetration depth and signal to noise ratio (SNR) in OCT are mainly due to multiple scattering, which have been effectively suppressed by controlling the incident wavefront using a digital mirror device (DMD) in combination with spectral-domain OCT. The successful enhancements in the penetration depth and SNR are demonstrated in a wide-range of tissue phantoms, reaching depth enhancement of up to 92%. The hidden structures inside a tissue phantom that could not be seen in conventional OCT are clearly revealed through our proposed system. Its 2-D imaging capability, assisted by further optimization of the system for real-time acquisition speed will boost wide-spread use of OCT for in-vivo tissue diagnosis.
©2014 Optical Society of America
OCIS codes: (170.4500) Optical coherence tomography; (110.0113) Imaging through turbid media; (110.1080) Active or adaptive optics.
References and links 1. P. Sebbah, Waves and Imaging through Complex Media (Kluwer Academic Publishers, 2001). 2. D. Huang, E. A. Swanson, C. P. Lin, J. S. Schuman, W. G. Stinson, W. Chang, M. R. Hee, T. Flotte, K. Gregory,
C. A. Puliafito, and J. G. Fujimoto, “Optical coherence tomography,” Science 254(5035), 1178–1181 (1991). 3. N. Nassif, B. Cense, B. H. Park, S. H. Yun, T. C. Chen, B. E. Bouma, G. J. Tearney, and J. F. de Boer, “In vivo
human retinal imaging by ultrahigh-speed spectral domain optical coherence tomography,” Opt. Lett. 29(5), 480–482 (2004).
4. B. Karamata, M. Laubscher, M. Leutenegger, S. Bourquin, T. Lasser, and P. Lambelet, “Multiple scattering in optical coherence tomography. I. Investigation and modeling,” J. Opt. Soc. Am. A 22(7), 1369–1379 (2005).
5. J. G. Fujimoto, C. Pitris, S. A. Boppart, and M. E. Brezinski, “Optical coherence tomography: An emerging technology for biomedical imaging and optical biopsy,” Neoplasia 2(1-2), 9–25 (2000).
6. M. Bashkansky and J. Reintjes, “Statistics and reduction of speckle in optical coherence tomography,” Opt. Lett. 25(8), 545–547 (2000).
7. B. Hermann, E. J. Fernndez, A. Unterhuber, H. Sattmann, A. F. Fercher, W. Drexler, P. M. Prieto, and P. Artal, “Adaptive-optics ultrahigh-resolution optical coherencetomography,” Opt. Lett. 29, 2142–2144 (2004).
8. R. J. Zawadzki, S. M. Jones, S. S. Olivier, M. Zhao, B. A. Bower, J. A. Izatt, S. Choi, S. Laut, and J. S. Werner, “Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging,” Opt. Express 13(21), 8532–8546 (2005).
9. M. Rueckel, J. A. Mack-Bucher, and W. Denk, “Adaptive wavefront correction in two-photon microscopy using coherence-gated wavefront sensing,” Proc. Natl. Acad. Sci. U.S.A. 103(46), 17137–17142 (2006).
10. K. Kurokawa, K. Sasaki, S. Makita, M. Yamanari, B. Cense, and Y. Yasuno, “Simultaneous high-resolution retinal imaging and high-penetration choroidal imaging by one-micrometer adaptive optics optical coherence tomography,” Opt. Express 18(8), 8515–8527 (2010).
11. N. Iftimia, B. E. Bouma, and G. J. Tearney, “Speckle reduction in optical coherence tomography by “path length encoded” angular compounding,” J. Biomed. Opt. 8(2), 260–263 (2003).
12. M. Pircher, E. Gotzinger, R. Leitgeb, A. F. Fercher, and C. K. Hitzenberger, “Speckle reduction in optical coherence tomography by frequency compounding,” J. Biomed. Opt. 8(3), 565–569 (2003).
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7514

13. R. Fiolka, K. Si, and M. Cui, “Complex wavefront corrections for deep tissue focusing using low coherence backscattered light,” Opt. Express 20(15), 16532–16543 (2012).
14. J. Jang, J. Lim, H. Yu, H. Choi, J. Ha, J. H. Park, W. Y. Oh, W. Jang, S. Lee, and Y. Park, “Complex wavefront shaping for optimal depth-selective focusing in optical coherence tomography,” Opt. Express 21(3), 2890–2902 (2013).
15. Y. Choi, T. R. Hillman, W. Choi, N. Lue, R. R. Dasari, P. T. C. So, W. Choi, and Z. Yaqoob, “Measurement of the time-resolved reflection matrix for enhancing light energy delivery into a scattering medium,” Phys. Rev. Lett. 111(24), 243901 (2013).
16. A. F. Fercher, W. Drexler, C. K. Hitzenberger, and T. Lasser, “Optical coherence tomography-principles and applications,” Rep. Prog. Phys. 66(2), 239–303 (2003).
17. D. B. Conkey, A. M. Caravaca-Aguirre, and R. Piestun, “High-speed scattering medium characterization with application to focusing light through turbid media,” Opt. Express 20(2), 1733–1740 (2012).
18. D. Akbulut, T. J. Huisman, E. G. van Putten, W. L. Vos, and A. P. Mosk, “Focusing light through random photonic media by binary amplitude modulation,” Opt. Express 19(5), 4017–4029 (2011).
19. B. F. Kennedy, S. Loitsch, R. A. McLaughlin, L. Scolaro, P. Rigby, and D. D. Sampson, “Fibrin phantom for use in optical coherence tomography,” J. Biomed. Opt. 15(3), 030507 (2010).
20. B. W. Pogue and M. S. Patterson, “Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry,” J. Biomed. Opt. 11(4), 041102 (2006).
21. S. A. Prahl, M. J. C. van Gemert, and A. J. Welch, “Determining the optical properties of turbid mediaby using the adding-doubling method,” Appl. Opt. 32(4), 559–568 (1993).
22. D. A. Boas, C. Pitris, and N. Ramanujam, Handbook of Biomedical Optics (CRC, 2011). 23. W. Wieser, B. R. Biedermann, T. Klein, C. M. Eigenwillig, and R. Huber, “Multi-megahertz OCT: High quality
3D imaging at 20 million A-scans and 4.5 GVoxels per second,” Opt. Express 18(14), 14685–14704 (2010). 24. A. P. Mosk, A. Lagendijk, G. Lerosey, and M. Fink, “Controlling waves in space and time for imaging and
focusing in complex media,” Nat. Photonics 6(5), 283–292 (2012). 25. J. H. Park, C. Park, H. Yu, Y. H. Cho, and Y. K. Park, “Dynamic active wave plate using random nanoparticles,”
Opt. Express 20(15), 17010–17016 (2012). 26. J. H. Park, C. H. Park, H. Yu, Y. H. Cho, and Y. K. Park, “Active spectral filtering through turbid media,” Opt.
Lett. 37(15), 3261–3263 (2012). 27. Y.-K. Park, W. Choi, Z. Yaqoob, R. Dasari, K. Badizadegan, and M. S. Feld, “Speckle-field digital holographic
microscopy,” Opt. Express 17(15), 12285–12292 (2009). 28. I. M. Vellekoop, A. Lagendijk, and A. P. Mosk, “Exploiting disorder for perfect focusing,” Nat. Photonics 4(5),
320–322 (2010). 29. J.-H. Park, C. Park, H. Yu, J. Park, S. Han, J. Shin, S. H. Ko, K. T. Nam, Y.-H. Cho, and Y. Park,
“Subwavelength light focusing using random nanoparticles,” Nat. Photonics 7(6), 454–458 (2013).
1. Introduction
Imaging through turbid tissue is essential for the diagnosis and treatment of several diseases. However, due to the multiple scattering of light in tissues, this is difficult to achieve using visible light for which the refractive index contrast between the oily membranes and water content of cells results in a high scattering cross section. To overcome this issue, the most powerful imaging techniques to date that work in an in vivo environment for human disease diagnosis utilize either radio frequencies or ultrasound as an imaging modality because they undergo much less scattering through tissues [1].
Optical coherence tomography (OCT) is an optical version of ultrasound tomography that utilizes coherence gating to target and image specific depths inside a medium [2]. It is unique in the fact that it uses near infrared wavelengths which allows up to micrometer level resolution while still allowing imaging depths of up to 1~2 millimeters. However, although OCT has shown great impact in the field of ophthalmology [3], it lacks the same degree of impact in other areas of interest such as imaging through skin. When imaging through highly scattering layers, the OCT signals deteriorate in the sense that the different optical path lengths that are used for coherence gating gets mixed as light from other planes also interfere and contaminate the signal. In other words, the coherence grating in OCT allows single back-scattered light as the signal while multiply scattered light is still contained as noise, which composes most of the backscattered light in turbid tissues [4–6].
To address this issue, adaptive optics has been applied in OCT [7–10]. However, they only adjust aberration, not multiple scattering, because complex wavefront correction is required to correct multiple light scattering. Spatial and frequency compounding methods, utilizing multiple uncorrelated OCT measurements, suppress the background speckle noise,
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7515

but do not increase the depth of penetration in OCT signals [11, 12]. Recently, deep tissue focusing was demonstrated using complex wavefront shaping in low coherence backscattered light [13]. More recently, our group has demonstrated that coherence-gated signals in spectral domain optical coherence tomography (SD-OCT) can be utilized to optimize an incident wavefront to selectively target enhanced light delivery to a target depth and position [14]. This was realized by utilizing a single mode fiber in combination with the SD-OCT signal which allows measurements of light scattered from a single scatter at a selected depth and position. By utilizing this signal as the feedback in a positive feedback loop, an optimized wavefront could be found to enhance delivery of light to the target scatter, and hence enhance the OCT signal. Another recent study showed that focusing at specific target depths is also accessible via the measurement of time-resolved reflection matrix of turbid media [15].
Here, we describe the extension of the principle to realistic applications where we have obtained a full cross-sectional tomography (B-scan) images rather than a single A-scan. To this end, synchronization between the wavefront optimizing protocol and SD-OCT image acquisition was essential, which is described in detail in the experimental setup section. We also demonstrate signal enhancement for different samples with varying transport mean free paths and also observed an increased penetration depth up to 92%. The present scheme utilizes a SD-OCT setup with the addition of just a single deformable mirror device (DMD) and a chirp compensating grating, which allows easy add-on to conventional systems.
2. Experiments and results
2.1 Experimental setup
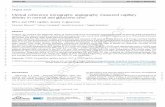
The experimental setup is based on a spectral-domain OCT system, in which a DMD is added for complex wavefront shaping [Fig. 1(a)]. Illumination is provided by a broadband super-luminescent diode (SLD-521, Superlum Diodes, Ltd., Ireland) with a center wavelength of 1025 nm, a full width at half maximum (FWHM) spectral bandwidth ∆λ of 110 nm, and an output power of 9.7 mW.
A collimated beam impinges onto a DMD (0.7 XGA, 1024 × 768, Texas Instruments, United States). The incident beam is modulated by Hadamard patterns at a frame rate of 17 kHz. The utilized modulated beam is the 4th order diffraction beam to obtain maximum diffraction efficiency. The effective grating period of the DMD is 400 lines/mm. The optical dispersion due to the DMD is compensated by the addition of the diffraction grating G1 (300 lines/mm, GR25-0310, Thorlabs, United States) after a 4-f telescopic lens system with × 4/3 magnification. The incident beam is then divided into a reference beam and a sample beam at a beam splitter BS. The beam for the sample arm is first steered by a 2-D galvano mirror GM (model #6210H, Cambridge Technology, United States) and then impinges onto a sample through an objective lens (LSM02-BB, Throlabs, United States). Through the 4-f telescopic lens systems, the sample plane is matched to the Fourier plane of the DMD. The reference beam is reflected by a mirror M3 and passes through a dispersion compensator. (LSM02DC, Thorlabs, United States)
The interference signal from the reference and sample arms is collected by a single-mode fiber and then measured by a spectrometer of SD-OCT. The basic principles of SD-OCT are discussed in the literature [16]. The spectrometer consists of a collimator lens C (0.24 NA, F810APC-1310, Thorlabs, United States), transmission grating G2 (1200 lines/mm, Wasatch Photonics, United States), achromatic doublet lens (L4) (100 mm, Thorlabs, United States), and a line CCD (SU1024-LDH2, 1024 lines InGaAs, 92 kHz frame rate, Sensors Unlimited Inc., United States). The imaging range of our system is measured as 1.5 mm in air. The theoretical axial resolution of the OCT system in air is 4.2 μm.
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7516

(b)
BS
G1
DMDM3
GM
sample
L1
L2
dispersioncompensator
line CCD
G2
M1
M2
L4
C
C
C
SLD
obj. NA=0.05
L4
L3
funct ion generator
(a)
69 μs
DMD
H1 H2
. . .
. . .
H7499 H7500
. . .O1 O2 O200
. . .
GM
H1 H2
. . .
. . .
. . .
An
CCD
O1, O2, , O200
. . .
An+1
Parallel Optimization
[Characterization]
[Opt imizing pat terns]
[Optimization]
15.4 s
DAQ board
Fig. 1. (a) Optical setup for SD-OCT with complex wavefront control capability. A DMD is added to a SD-OCT system for wavefront control. The diffraction grating (G1) is inserted for dispersion compensation and the objective back focal plane is conjugated to the DMD plane via two 4-f systems (L1-L2, L3-L4). L1, L2, L3, L4 are 75 mm, 100 mm, 150 mm and 150 mm achromatic lenses, respectively. C: collimator lens, M: mirror, G: grating, BS: beam splitter, GM: galvanometer mirror (b) The synchronization among DMD, CCD and GM via trigger signals. Hadamard patterns (H1, H2, …, H7500) are loaded on the DMD and corresponding OCT signals are measured at the CCD. The optimizing patterns (O1, O2, …, O200) for each depth are calculated in parallel, and the corresponding optimized signals are measured after characterization process.
In our previous study [14], we were able to acquire only a single A-line image using complex wavefront shaping in OCT. To obtain optimized B-scan images, it is critical to synchronize both the modulation and measurement instruments. Figure 1(a) describes the overall work flow of the system. Every pattern change on the DMD produces a trigger-out signal. A function generator (AFG3022C, Tektronix, United States) receives the trigger and duplicate trigger signals of matching impedance are generated and transmitted to a NI-DAQ board and the CCD. The NI-DAQ board counts the number of trigger-in signals and sends a trigger-out signal to the GM to scan the A-scan position as needed. A single trigger-in signal to the CCD results in recording of an A-line image. Consequently, a single A-line signal is acquired for every single modulation of the DMD pattern.
2.2 Principle of the wavefront shaping in OCT
Figure 1(b) shows the detailed measurement and optimization process. In the characterization process, we obtain OCT signals for each Hadamard pattern modulation. In all following experiments, we utilized the 300 Hadamard basis patterns with 25 phase shift, resulting in a total of 7,500 pattern modulations. For each Hadamard basis pattern, 25 phase shifts represent 5 lateral translations along the x- and y-directions on the DMD. The 5 shifts in one direction is equal to the spatial period of Hadamard basis pattern. For each basis pattern, the optimized phase shift is found at which the maximum energy is delivered to the target point. Once the optimized phase shifts are found for each basis patterns, the final optimized pattern is calculated by the coherent summation of all the Hadamard bases patterns with optimized phase shifts. Then, the final pattern is applied to the DMD, and the corresponding OCT signal is obtained.
Since the final optimized pattern should be applied to amplitude modulation (AM) only spatial light modulator (SLM) such as DMD, the superimposed pattern was then binarized by thresholding with respect to half of the entire number of bases, similarly done in the previous
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7517

work by Conkey et al. [17]. The wavefront optimization process used in the present work is comparable to binary-mode wavefront optimization [18].
To acquire raw A-line signals, we computed A-line OCT images from raw signals using a 1-D discrete Fourier transform function (Integrated Performance Primitives, Intel®) and found the optimizing patterns for each depth in parallel. The region of interest was taken to be 200 different depth positions, thus a total of 200 optimization patterns were produced and projected on the DMD. Finally the corresponding optimized signals for each depth are measured. Though the measurement time of a single raw A-line signal takes only 69 µs between successive pattern changes, the total optimization time for a single A-scan position was 15.4 s. This is due to the heavy calculation of 1-D discrete Fourier transform of A-line imaging and the optimization pattern calculation. Currently, this is far slower than the real-time imaging speed needed in practical applications; however, we anticipate that further system optimization can be applied by introducing graphics processing unit (GPU) or Field-programmable gate array (FPGA) systems. The optimizing time can further be reduced considerably by reducing the number of phase shifts of the Hadamard patterns from 25 to 9. In this case, obtaining the optimal wavefronts for 200 different depths of a single A-scan will take less than 0.1 sec with the same system. After all the optimized signals are obtained, the trigger-out signal is sent from the NI-DAQ board to the GM to switch the A-scan position. Then the same characterization and optimization process are consecutively repeated as described above to acquire the optimized A-scan image in a new position.
The optimized OCT signal is the intensity at a certain depth, determined by coherence gating in OCT and spatial gating in the single-mode fiber in the detection arm. During the optimization process, it is not needed to identify the signal and background areas a priori. Theoretically, the present wavefront shaping system in OCT should work irrespective of the signal intensity, since the principle is based on wave interference (coherent summation) of multiple waves. However, as an OCT signal is based on the coherence-gated detection of backscattered light, there has to be a scatter located at the depth of interest for the signal to exist. There is a possibility that backscattered signals from other depths could coincidentally match propagation lengths that can escape the coherence gating. Our system currently employs a single mode fiber as a spatial filter to block these unwanted signals when collecting the backscattered light and only collect light from the scatter located at specific depths. Therefore, a scatter has to be physically existing in the area of interest for the optimization to take place. We experimentally observed that the input basis patterns that are impinged into void areas show only noise level signals no matter the phase shift which results in a null enhancement even through coherent summation. Therefore, the scattering signal from single scattering object at the specific depth will be enhanced coherently, while other multiply scattered light paths occurring in shallower layers will be averaged out [14].
2.3 Sample preparation
To demonstrate the feasibility of the wavefront shaping technique for in-vivo applications, we fabricated tissue phantoms with various turbidity levels. Prepared samples are based on a fibrin matrix to which an intralipid solution (20% w/v) is added [19]. The fibrin matrix is a fibrous protein supporting blood clots and formed from fibrinogen (F8630, Sigma Aldrich, United States) by proteolysis induced by thrombin (T6634, Sigma Aldrich, United States). Intralipid is a well-known scatting material having a tissue-like scattering property [20]. Following the procedures presented in [19], we fabricated 5 different samples by varying the weight/volume concentration of intralipid from 4% to 13%. We measured the reduced scattering coefficient using a integrating sphere setup [21]. The illumination source was a laser diode with a center wavelength of 980 nm (L980E2P5, Thorlabs, United States). The obtained reduced scattering coefficients μ’s range from 0.55 mm−1 to 2.59 mm−1. These values were made to be within the range of human organ tissues [22] so that we could effectively check the feasibility for practical applications.
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7518

2.4 2-D images obtained using complex wavefront shaping
For demonstration of 2-D imaging capabilities of the present setup, we acquired optimized B-scan images of turbid samples as shown in Fig. 2. We prepared a sample consisting of three layers made of the fibrin network, a slide glass, and a plastic weighing dish [Fig. 2(a)]. We chose the plastic weighing dish as a target imaging object since it has a moderate reflectance. In the region of interest, the thickness of the fibrin network slowly gets thicker towards the right. As the fibrin network gets thicker, it can be seen that the structure underneath the slide glass gradually disappears due to the limited penetration depth.
I fibrin
40
80
120
160
I
II
III
(b) (c) [dB]
45
50
55
60
65
70
Dep
th (
2.9
μm/p
ix)
40 80 40 80
II slide glass
III weighing dish
(a)
Fig. 2. 2-D OCT images for a structured sample with the scattering tissue phantom slanted at the top-most layer (a). (b) Optimized image for the dashed-rectangular area. (c) The spatial compounding result using Hadamard basis patterns for the same area. The input illumination power is 0.55 mW for both cases.
Figure 2(b) shows the image taken with the optimized wavefront for the incident power of 0.55 mW. Figure 2(c) is obtained by the spatial compounding of OCT signals; 15 OCT signals obtained with uncorrelated Hadamard basis patterns are averaged. The input power is the same with the optimized result. Compared to Fig. 2(c), the optimized image shows the enhanced signal over all sample areas. The blurred image of the plastic dish below the slide glass clearly reveals its structure. From this result, we can conclude that the proposed OCT system successfully produces enhanced 2-D images by controlling the wave-front.
2.5 Quantitative analysis of signal profile
To quantify the signal enhancement, we obtained 2-D images of fibrin phantom samples. The representative B-scan images are shown in Fig. 3. All images are taken from the sample of reduced scattering coefficient μ’s = 1.25 ± 0.04 mm−1. Figure 3(a) shows the optimized image for the controlled wavefront with 0.55 mW incident power, while Fig. 3(b) shows the single measurement image for the uncontrolled wavefront with 1.1 mW incident power. In the optimized image, both signal to noise ratio (SNR) and the penetration depth increases compared to the uncontrolled wavefront despite the smaller incident power by two-folds. The single measurement image for the uncontrolled wavefront with an incident power of 0.55 mW is represented in Fig. 3(c), and the spatial compounding result with the same input power is shown in Fig. 3(d). Figures 3(c)-3(d) give unclear images with low signals compared to the optimized case even though they are all acquired for the same input power. For each image, 15 A-line signals were averaged and plotted in Fig. 3(e). The differences in the signal strength observed in the 2-D images are clearly exhibited in each case. The same integration time was
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7519

used for acquiring the OCT signals using both the controlled wavefront [Fig. 3(a)] and uncontrolled Gaussian beam [Fig. 3(d)].
40
80
120
160
45
50
55
60
150
(a)
150
(b)
150
(c)
150
(d)D
epth
(2.9
μm
/pix
) controlled wavefront, 0.5 mWGaussian beam, 0.5 mWGaussian beam, 1.1 mWspatial compounding, 0.5 mW
Am
plitu
de (a
.u.)
Depth (2.9 μm/pix) 0 50 100 150 200
100
200
300
400
500
600
(e)[dB]
Fig. 3. B-scan images under various conditions. (a) Optimized image by wavefront control with input power of 0.55 mW. (b) Image acquired for uncontrolled input beam of 1.1 mW. (c) Image acquired for uncontrolled input beam of 0.55 mW. (d) Image obtained by spatial compounding method. (e) The averaged A-scan profiles along 15 different A-scans in each case are plotted.
In addition, in order to compare the SNR for controlled and uncontrolled signal considering the total time to grab the data, we imaged a highly turbid sample, a dried leaf of cherry blossom Prunus serrulata (Fig. 4). The B-scan images were obtained with three different methods: (a) the wavefront shaping, (b) the spatial compounding method with 200 measurements, and (c) the spatial compounding method with 7,500 measurements. For the wavefront shaping method, the optimized wavefront was calculated from 7,500 measurements, and thus the total time (including the characterization, the computation, and the optimized measurement) to grab the data for the result (a) is the same as the total time it took to grab the data for the result (c). As clearly seen, the SNR of the spatial compounding method with averaging 7,500 uncontrolled signals is still worse than that of the wavefront shaping. Indeed, the spatial compounding method averaging 7,500 uncontrolled signals does not show significant improvements in comparison with the one averaging 200 uncontrolled signals. This clearly shows the simple incoherent summation of uncorrelated OCT signals does not improve the penetration depth and SNR as much as the wavefront shaping method does.
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7520

0 40 80 120 160
1000
2000
3000
Controlled wavefrontSpat ial compounding
(7,500 pat terns)Spat ial compounding
(200 pat terns)
Am
plitu
de (a
.u.)
Depth (2.9 μm/pix) 10
20
60
100
140
0 10 0 10
45
55
65
75
[dB]
0
(a) (b) (c)
Dep
th (2
.9 μ
m/p
ix)
(d)
Fig. 4. B-scan images under various conditions. (a) Optimized image by wavefront control. (b) Image obtained by spatial compounding method (averaged 7500 uncontrolled curves). (c) Image obtained by spatial compounding method (averaged 200 uncontrolled curves). (d) The averaged A-scan profiles along 10 different A-scans in each case are plotted.
2.6 Increase of penetration depth
To further characterize the setup, we conducted experiments to study the dependence of the signal enhancement on the sample scattering property and pupil segmentation of the DMD. The incident beam impinging upon the DMD has a diameter of 1.4 mm, and the effective modulation area of the DMD was grouped into 13 × 13, 16 × 16, or 21 × 21 segments for higher modulation SNR. Although Fig. 3(e) illustrates the enhancement of SNR and the increase of penetration depth qualitatively, we established a comparison criterion by introducing fitting functions to the obtained data to quantitatively study the increase of penetration depth. Since the reflected amplitude signal in OCT systems exponentially decreases as a function of depth [16], the signal profile can be fitted to a first order exponential function.
(a) (b)
0.55 0.87 1.25 1.87 2.590
25
50
75
100
125
Dep
th
Enha
ncem
ent
( b/
a )
(%)
Reduced scattering parameter, μs’ (mm-1)
13×1316×1621×21
Segmentation # in DMD
Depth enhancement (b/a) = 77.41%
Controlled wavefront, 0.55 mW
Depth (2.9 μm/pix)
Ampl
itude
(dB
)
0 40 80 120 160
44
48
52
Gaussian beam, 1.1 mW
Noise Level (42.9 dB)
a b
Fig. 5. (a) First-order exponential fits to the OCT signals in Fig. 3(e). (b) The penetration depth enhancement for various reduced scattering coefficients and DMD macro-pixel segmentations.
The resulting fitted functions for the red (wavefront control, 0.5 mW) and black curves (Gaussian beam 1.1 mW) in Fig. 3(e) are shown in Fig. 5(a) as red and black lines,
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7521

respectively. Here we define the penetration depth as the depth at which the fitting function and the noise threshold line intersect. The penetration depth for the uncontrolled wavefront is given by a, and the penetration depth for the controlled wavefront is given by a + b. We thus define a depth enhancement factor as b/a. The selection of noise threshold can greatly affect the depth enhancement factor. Here we chose the noise level as the mean noise signal in the region of the glass slide in Fig. 2(b), corresponding to 42.92 dB in this case. Ideally no signal should be detected from the glass slide due to its transparency, so we can safely assume that any signal from the glass slide is noise. By choosing this standard, we applied the same threshold for all the following experiments.
Figure 5(b) shows the depth enhancement for different scattering samples and various DMD pupil segmentations. The first interesting observation is that the depth enhancement increases in overall tendency as μ’s decreases. This is a rather intuitive result. As scattering get weaker, the tight focus can be easily formed inside the sample. Thus the penetration depth enhancement is driven in low scattering phantoms. It is important to note here that the incident power for the uncontrolled beam is twice compared to that of the controlled case. The depth enhancements in Fig. 5(b) were obtained while using only half the power of a conventional Gaussian input wavefront.
The second characteristic is the existence of optimal segmentation. For high scattering phantoms, the optimal enhancement is observed for a larger number of macropixels used for the segmentation. This can be understood as a higher number of controlled modes is needed to effectively compensate multiple scattering. For lower scattering phantoms the optimal number of segmentations is reduced to 16 × 16. In this case, although 300 Hadamard patterns were chosen as the input basis, the macropixels only allowed 256 numbers of degrees of freedom which was still enough to compensate the lower degree of scattering. For phantoms with μ’s higher than 1.25, depth enhancement was only shown for the case with the segmentation number of 21 × 21.
3. Discussions and conclusions
We have demonstrated the first experimental results of complex wavefront shaping SD-OCT capable of 2-D imaging. The obtained signal is shown to increase among different A scans of the sample resulting in a higher SNR and deeper penetration depth of the system. Such a development enabled imaging of hidden structures that were unseen in a conventional system through wavefront controlled input of the incident beam. The sample dependent study also infers that these enhancements can be achieved for various human tissues such as breast, liver and aorta tissues.
Though we successfully obtained enhanced 2-D OCT images, the measurement time is still a great barrier for real applications. In the current system, the total measurement and optimization time is 12 minutes for 50 A-scans. Although the achieved acquisition time is too slow to be applicable in-vivo imaging, there is still room for enhancing the acquisition speed. The current main bottle-neck in the optimization process is the calculation of 1-D Fourier transform and the extraction of optimum enhancement pattern. Using well developed FPGA or GPU, it is expected that these calculation times can be faster than the pattern switching time on the DMD through parallel programing. We can also additionally directly reduce the time by reducing the number of phase shifts to find the optimum pattern from 25 to 9 by which we expect to achieve a single A-scan in under 0.1 s with currently available technology. There is still room to further improve the speed of wavefront shaping OCT using deformable mirror SLMs or utilizing a swept source OCT (SS-OCT) platform. In our experiments, the detector (line camera) and SLM (DMD) have the maximum frame rates of 92 kHz and 22 kHz, respectively. To overcome the speed limit of the present system, a deformable mirror SLM can be utilized which has a refresh rate of up to 400 kHz. Current state-of-the-art SS-OCT systems have even faster acquisition speeds with up to 20 million A-scans per second frame rate [23]. Considering these numbers, when the present complex
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7522

wavefront shaping technique is combined with SS-OCT, it can potentially achieve a frame rate of 2 kHz. Furthermore, by adapting a hybrid scheme where a conventional OCT is first taken to choose specific areas of interest to optimize based on the pre-acquisition image, the number of optimizations can be drastically reduced to accelerate an imaging speed of the present approach.
Due to its enhanced penetration depth and universal applicability to various turbid tissues, we hope that our proposed method will open a new stage for the wide application of OCT to in-vivo tissue imaging. Because of large degree of freedoms in multiple scattering in complex media [24–26], the present method can also be adapted to spectroscopic and polarization sensitive OCT. Furthermore, the spatial resolution of OCT can be further enhanced by exploiting multiple light scattering [27–29]. We expect that imaging of previously unseen structures inside tissues can greatly strengthen non-invasive diagnosis through OCT, which will be our next goal.
Acknowledgments
This work was supported by Samsung Advanced Institute of Technology, KAIST, the Korean Ministry of Education, Science and Technology (MEST), and the National Research Foundation (2012R1A1A1009082, 2013K1A3A1A09076135, 2013M3C1A3063046, 2009-0087691, 2012-M3C1A1-048860).
#207318 - $15.00 USD Received 27 Feb 2014; accepted 13 Mar 2014; published 24 Mar 2014(C) 2014 OSA 7 April 2014 | Vol. 22, No. 7 | DOI:10.1364/OE.22.007514 | OPTICS EXPRESS 7523