Chromatography. Match these key words with the correct definitions: Starter SOLVENT SOLUTE AQUEOUS...
-
Upload
miles-conley -
Category
Documents
-
view
245 -
download
5
Transcript of Chromatography. Match these key words with the correct definitions: Starter SOLVENT SOLUTE AQUEOUS...

Chromatography

Match these key words with the correct definitions:
Starter
SOLVENT
SOLUTEAQUEOUS SOLUTION
SOLUTION
NON-AQUEOUS SOLUTION

Match the key words with the correct definitions:
Starter
SOLVENT
SOLUTE
AQUEOUS SOLUTION
SOLUTION
NON-AQUEOUS SOLUTION
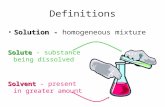
solution where a solute is dissolved in water
solution where the solute is dissolved in a solvent that isn’t water (e.g ethanol)
a liquid in which chemicals dissolve to make a solution (e.g. water, ethanol)
a substance that is dissolved in a solvent.
a solute dissolved in a solvent

Learning Objectives
• Understand when chromatography is used
• Describe the method of chromatography
• Know how to carry out paper
chromatography
• Analyse chromatograms
• Calculate Rf values

When is chromatography used?
Paper chromatography is a technique for separating components of a mixture.
Which kind of mixtures ?
Inks Colourings
agents Dyes/paints
Water soluble mixtures such as:

Simple Chromatography
• Chromatography is an analytical technique that separates components in a mixture between a mobile phase and a stationary phase
• The mobile phase moves, it can be a liquid or a gas.
• The stationary phase doesn’t move. It can be a solid or a suspended liquid.

How it works
• Some substances are more soluble than others.
• Substances that don’t dissolve, don’t move.
• Substances that dissolve the most move the farthest. origin
solvent front
Insoluble pigment
most soluble pigment
partly soluble pigment

How it works
• With a solid stationary phase, the pigments are adsorbed onto the surface of the solid particles or fibres.
• The relative forces of attraction between the stationary phase, the mobile phase and the pigment mean that separation happens Insoluble
pigment
pigment with weakest forces of adsorption
Pigment with strong forces of adsorption

How does it work?
• The water is drawn up by capillary action (sticky attractive force between water molecules and the paper).
• When the solvent reaches the ink spots they are dissolved and carried with the water as it moves up the paper.
• The most soluble ink travels the fastest, whilst the less water-soluble inks are left near the bottom of the paper.
chromatogramSolvent
front
Separated dyes
Base line (pencil line)

Start Finish
1) How many colours in the red dye?2) Which colours is the purple dye made
out of?3) Which dyes are made using yellow
colour?
Analysing chromatograms

1) How many dyes were used in Black?2) Are the yellows in brown and orange the same? How do you know?
Analysing chromatograms

Inks i, ii, iii and iv were made from colours A, B, C and DTry to work out which colours were mixed for each of the inks (i-iv)

Rf values
• The ratio between the distance travelled by the pigment and the distance travelled by the solvent front is a constant, Rf.
• (provided temperature, mobile phase and stationary phase are the same)
origin
solvent front
most soluble pigment B
partly soluble pigment A

Rf Values (retention factor)
• Distance travelled by component
• Distance travelled by mobile phase
• Specific to each plate• Comparable• Each component on
plate has unique Rf value

Calculating Rf values
• A chemical can also be identified by its Rf
(Retention Factor)• It is the ratio of the distance travelled by the
sample (spots) to the distance travelled by the solvent (water).
• The formula is:
The answer is never greater
than 1.

Calculate Rf for the three spots.
Substance
Distance moved by
sample (cm)
Distance moved by solvent
front (cm)
Rf
E120 7.2 10 0.72E133 4.1 10 0.41E124 9.5 10 0.95
Calculating Rf values

Past Paper Questions

Past paper question

Mark scheme

Challenge question - 1This question relates to the chromatogram shown in the earlier question. Refer back…

Mark scheme

Challenge question 2

Mark scheme – check your answer