Chlormethine Gel for the Treatment of Skin Lesions in All Stages of Mycosis … · 2021. 5. 21. ·...
Transcript of Chlormethine Gel for the Treatment of Skin Lesions in All Stages of Mycosis … · 2021. 5. 21. ·...

REVIEW
Chlormethine Gel for the Treatment of Skin Lesionsin All Stages of Mycosis Fungoides Cutaneous T-CellLymphoma: A Narrative Review and InternationalExperience
Larisa J. Geskin . Martine Bagot . Emmilia Hodak . Ellen J. Kim
Received: February 26, 2021 / Published online: May 21, 2021� The Author(s) 2021
ABSTRACT
Mycosis fungoides (MF), the most commonform of primary cutaneous T-cell lymphoma, isa disease typically with an indolent course thatis initially characterized by localized patchesand plaques. In the early stages of the disease,treatment involves skin-directed therapies(SDTs) such as topical corticosteroids and reti-noids. Chlormethine gel (also known as
mechlorethamine) was the first SDT purposelydeveloped to treat MF and is currently endorsedby international guidelines for the treatment ofadult patients with MF as a first-line therapy.While chlormethine is an efficacious therapy,its usage may be complicated by the develop-ment of cutaneous reactions at the sites ofapplication. Herein, we discuss the supportiveguidelines for MF and the suitability ofchlormethine as a therapeutic option inpatients with MF. In addition, we present real-world experience on the use of chlormethinegel from clinics in the USA, Israel, and Francewith the aim of demonstrating the efficacy ofchlormethine gel in routine clinical practiceand outlining strategies that are being used tomanage emergent cutaneous reactions.
Keywords: Chlormethine gel; Cutaneous T-celllymphoma; Mycosis fungoides; Patientmanagement; Real-world
L. J. Geskin (&)Department of Dermatology, Columbia University,161 Fort Washington Ave, 12th Floor, New York, NY10032, USAe-mail: [email protected]
M. BagotDepartment of Dermatology, AP-HP, Universite deParis, Hopital Saint-Louis, Paris, Francee-mail: [email protected]
E. HodakDivision of Dermatology, Rabin Medical Center,Beilinson Hospital, Sackler Faculty of Medicine, TelAviv University, Tel Aviv, Israele-mail: [email protected]
E. J. KimDepartment of Dermatology, Perelman School ofMedicine at the University of Pennsylvania,Philadelphia, PA, USAe-mail: [email protected]
Dermatol Ther (Heidelb) (2021) 11:1085–1106
https://doi.org/10.1007/s13555-021-00539-3

Key Summary Points
Mycosis fungoides (MF) is a cutaneousT-cell lymphoma typically with anindolent course that is initiallycharacterized by localized patches andplaques
Chlormethine gel is a therapeutic optionrecommended by international guidelinesfor patients with MF skin lesions; a rangeof retrospective, prospective, andobservational clinical data supports its usein all disease stages
While chlormethine is an efficacioustherapy, its usage may be complicated bythe development of cutaneous reactionsat the sites of application
Real-world experience from clinicalpractice in the US, Israel, and France hasshown that chlormethine gel is used as askin-directed therapy in the first- andsecond-line setting in patients with early-stage MF and as an adjunctive therapy inpatients with advanced-stage disease
The emergent cutaneous adverse reactionscan generally be managed throughchlormethine gel dose adjustments or theuse of topical steroids
DIGITAL FEATURES
This article is published with digital features,including a summary slide, to facilitate under-standing of the article. To view digital featuresfor this article go to https://doi.org/10.6084/m9.figshare.14447193.
INTRODUCTION
Cutaneous T-cell lymphomas (CTCLs) are aheterogeneous family of T-cell lymphoprolifer-ative disorders, of which mycosis fungoides
(MF) is the most common. Early-stage MF fol-lows a slow, indolent course [1], with symptomspresent for extended periods of time. Due to theclinical similarity between benign skin diseases(such as eczema and psoriasis) and early-stageMF, as well as the lack of a singular diagnostictest or specific tumor markers, median timebetween MF symptom onset and biopsy-con-firmed diagnosis is 4–6 years [2]. Most patientswith early-stage MF have an average life expec-tancy following treatment but reduced qualityof life [3]. Median survival for those with stageIII or IV disease is low (\ 5 years), and C 50%die of their disease [4–7]. MF treatment goals aresymptom control and quality of life improve-ment [8], as there are no curative therapeuticoptions aside from allogeneic stem cell trans-plantation [9].
In this review, we will briefly present thetreatment guidelines for the management ofpatients with MF, discuss the role of topicalchlormethine gel as part of the treatmentparadigm, provide an overview of the clinicaldata demonstrating the effectiveness of the gel,and present real-world experience of chlorme-thine gel usage from four different dermatologypractices.
This review is based on previously conductedstudies and does not contain any new studieswith human participants or animals performedby any of the authors. Informed consent wasprovided by the patient whose case wasincluded.
CLINICAL MANAGEMENTGUIDELINES FOR MF
MF treatment guidelines (European Society forMedical Oncology [10], European Organisationfor Research and Treatment of Cancer [11],National Comprehensive Cancer Network [12],and British Association of Dermatologists/UKCutaneous Lymphoma Group [13]) base theirrecommendations on disease stage [10–13].
For asymptomatic patients with early-stageMF (stage IA), ‘‘watch and wait’’ is considered anappropriate option. For symptomatic patients,those with adverse prognostic factors (such asplaque stage disease or large cell
1086 Dermatol Ther (Heidelb) (2021) 11:1085–1106

transformation), or those with extensive diseaseinvolvement (stage IB), skin-directed therapies(SDTs) should be initiated. The most commonlyused SDTs for treating early-stage MF are topicalcorticosteroids [8, 14], topical chlormethine orretinoids [15, 16], and phototherapy; superficialradiotherapy may also be employed. Patientswith advanced-stage disease generally receivesystemic single-agent or combination therapywith SDTs. Addition of an effective SDT canalleviate symptoms and shorten time toresponse compared with systemic therapy alone[11].
CHLORMETHINE AS TOPICALCHEMOTHERAPY FOR MANAGINGMF
All major guidelines recommend the use ofchlormethine for first-line treatment in adultpatients with MF [10–13]. Chlormethine is abifunctional alkylating agent that inhibitsrapidly proliferating cells by binding andcrosslinking DNA strands. The original aqueousand ointment formulations were not approvedas a therapy for MF, and it was subsequentlydeveloped as a topical gel [15, 17]. This formu-lation was approved by the US Food and DrugAdministration (FDA) in 2013 for treating stageIA and IB MF in patients who received priorSDT, has been registered in Israel since 2016 (forthe same indication as in the USA), wasapproved by the European Medicines Agency in2017 for treatment of adult patients with MF[18], and is now available commercially in anumber of European countries. Chlormethinegel has been available in France since 2014under a ‘‘temporary authorization for use’’ pro-gram that ended in July 2019, with 876 patientshaving participated [19].
The chlormethine gel (chlormethine 0.016%w/w, equivalent to 0.02% chlormethinehydrochloride) formulation was designed tomaximize efficacy and tolerability. Its non-aqueous nature imparts high stability, and theactive solvent, diethylene glycol monoethylether (Transcutol�), promotes delivery of thedrug to the epidermis [20–22], although there isno evidence of systemic absorption of
chlormethine following application [15]. Effi-cacy is enhanced by inclusion of the excipient,KlucelTM hydroxypropylcellulose (Ashland)[23], which results in a fast-drying, nongreasyformulation with a viscosity that is more likelyto remain at the administration site, whichmakes it easier to apply at home. The intrinsicantimicrobial nature of the active ingredients[24] removes the need for antimicrobial preser-vatives (which frequently cause skin reactions),thus potentially reducing the risk of allergy,although further investigations are required toconfirm this.
Chlormethine may be used as monotherapyin early-stage MF, in combination with systemictherapy in advanced-stage disease [25–27], andas maintenance treatment [11, 28]. Thechlormethine gel label indicates daily applica-tion; however, the frequency of applicationmay be reduced according to the patient’sneeds. For severe skin reactions, treatmentshould be suspended (in some cases perma-nently) and upon improvement can be restartedgradually up to daily frequency, if tolerated.
CHLORMETHINE IN MFMANAGEMENT: A REVIEWOF THE LITERATURE
There is a substantial body of prospective andretrospective evidence underlying the recom-mendation of chlormethine as a treatmentoption for patients with MF in all stages of thedisease (summarized in Table 1 [15, 17, 26,27, 29–44]). These studies have demonstratedthat chlormethine is efficacious, with a durableresponse that may be sustained for up to 8 yearsin some cases.
The pivotal registration 201 study evaluatedchlormethine gel versus chlormethine oint-ment for the treatment of patients with persis-tent or recurrent stage I or II disease whoreceived no concomitant corticosteroids.Response rates for chlormethine gel were con-sistently higher than those for the ointment forthe primary endpoint of Composite Assessmentof Index Lesion Severity (CAILS), and once-daily(QD) treatment with chlormethine gel met allprespecified criteria for noninferiority. In the
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1087

Table
1Summaryof
efficacyandsafety
findingsof
studiesof
topicalchlorm
ethine
inthetreatm
entof
MF
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
Priceet
al.
(1977)
[29]
Retrospective
case
analysis
51Topicalchlorm
ethine
as
adjuvant
therapyafterTSE
B
therapyvs.T
SEBtherapy
alone
Disease-freeinterval:25months
forchlorm
ethine
plus
TSE
B
therapyvs.1
7monthsfor
TSE
Btherapyalone
Relapse-freesurvival:37%
vs.
29%
Patientsreceivingadjuvant
topicalchlorm
ethine
hada
lowincidenceof
contact
allergy
Vonderheid
etal.(1979)
[30]
Retrospective
case
analysis
243
Topicalchlorm
ethine
givenasa
dilute
aqueoussolution
with
orwithout
system
ic
chem
otherapy
Disease-freeinterval:[
3years
in32
(13%
)patients
Equallyeffectiveas
historical
treatm
entwithTSE
B
NA
Priceet
al.
(1983)
[31]
Retrospective
case
analysis
43:stageIA,1
7;stageIB,2
2;
stageII,2
;stageIII,2
Topicalchlorm
ethine
ointment
CRoccurred
in26
patientsover
a42-m
onth
evaluation
period
Contact
derm
atitisoccurred
in
1of
31(3%)chlorm
ethine-
naivepatientsand3of
12
(25%
)previouslytreated
patients
Ram
sayet
al.
(1984)
[32]
Retrospective
case
analysis
76Topicalchlorm
ethine
Of64
patientswho
continued
therapy,43
(67%
)achieved
CRand12
(19%
)achieved
PR
Mediantimesto
CRforstageI,
II,and
IIIdiseasewere6,
32,
and22
months,respectively
Allergiccontacthypersensitivity
reactionsoccurred
in51
(67%
)patients
Nodifference
inresponse
betweenpatientswithor
without
contactsensitivity
Zachariae
etal.
(1985)
[33]
Retrospective
case
analysis
33(withearlyMFin
plaque
stage)
Topicalchlorm
ethine
14patientsin
CRand7in
PR,
witharelapse-free
survivalof
50%
after6and12
years,
respectively
3deaths
dueto
disease
3patientsdiscontinu
eddueto
treatm
ent-relatedcontact
derm
atitis
NohematologicAEs
1088 Dermatol Ther (Heidelb) (2021) 11:1085–1106

Table
1continued
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
Hoppe
etal.
(1987)
[34]
Retrospective
case
analysis
123(m
edianage,59
years
[range
20–9
0];88%
White,
6%Black,5
%Hispanic,1%
Asian):stageIA,3
8;stage
IB,3
0;stageIIA,3
3;stage
IIB,1
3;stageIIIA,2
;stage
IIIB,7
Topicalchlorm
ethine
10–2
0mg/dl
giveneither
as
aqueoussolution
orointment
Efficacy
was
similarforaqueous
vs.o
intm
entform
ulations
ORRandCRrate:all,72%
and
32%;stageI,88%
and51%;
stageII,6
9%and26%
AmongthosewithCR,4
0%of
patientswithstageIdisease
and60%
withstageIIdisease
laterrelapsed
Subsequent
therapiesincluding
repeat
treatm
entswith
chlorm
ethine
weresuccessful
inachievinglaterskin
clearance
Long-term
remissionsnotedin
42%
ofpatientswithstageI
and31%
withstageIIdisease
NopatientswithstageIII
disease(n
=13)hadCR,and
allhadprogression
2of
9patientswithstageIV
diseasehadCR,but
both
later
relapsed
Whendeaths
occurred,they
weretypically
unrelatedto
diseasein
stageIpatients
Halfof
deaths
amongstageII
patientswereattributableto
disease
Among22
patientswithstage
IIIor
IVdisease,80%
were
attributableto
MF
Cutaneous
hypersensitivity
was
morecommon
withaqueous
than
withointment
form
ulation
14(11%
)patientsdeveloped
cutaneousmalignancies
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1089

Table
1continued
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
Ram
sayet
al.
(1988)
[35]
Retrospective
analysisof
medicalrecords
117(m
edianage,57
years;
56%
male):stageIA,2
8;
stageIB,3
5;stageIIA,1
2;
stageIIB,3
2;stageIIIA,1
;
stageIIIB,9
Chlormethine
10mgdissolved
in60
mlof
water
applied
QDun
til6
monthsafterCR,
tapering
over
thefollowing
18months;concom
itant
therapynotallowed
except
forlocalRT
forstageIII
disease
Mediantimeto
CR:stageI,
6.5months;stageII,
41months;stageIII,
39months
Clin
icallyapparent
remission
at
2years:stageI,76%;stageII,
45%;stageIII,49%
Delayed
hypersensitivity
reactions:58%
1patientdiscontinu
eddueto
hypersensitivity
Death
dueto
diseaseoccurred
in9cases,including3
unrelatedto
treatm
entand1
unknow
n
Vonderheid
etal.(1989)
[26]
Retrospective
analysisof
medicalrecords
331(m
eanage,
58±
0.7years;65%
male):
stageIA,8
9;stageIB,6
6;
stageIIA,4
6;stageIIB,3
9;
stageIII,37;stageIVA,3
8;
stageIVB,9
;lymphom
atoid
papulosis,7
Chlormethine
10–2
0mg
dissolvedin
40–6
0mlof
water
appliedQD
untilCR,
then
continuedQD
orEOD
depend
ingon
response;
adjunctive
therapyallowed
forslo
wlyresponsive,
extensivedisease,or
extracutaneous
involvem
ent
(e.g.,localRT,T
SEB
therapy,UVphototherapy,
methotrexate,or
other
alkylating
agents)
InitialCRdefin
edas
complete
disappearanceof
clinically
detectablediseaseC
2weeks
andconfi
rmed
byskin
biopsy:
stage1A
,80%
;stage
1B,68%
;
stageIIA,6
1%;stageIIB,
49%;stageIII,60%;stage
IVA,1
3%;stageIVB,1
1%
Sustainedremission
for4and
8years:65
and35
patients,
respectively
OfpatientswithCR[
8years,
12(35%
)experiencedACD
1090 Dermatol Ther (Heidelb) (2021) 11:1085–1106

Table
1continued
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
Licataet
al.
(1995)
[39]
Retrospective
case
analysis
164(who
hadreceived
TSE
B
therapybetween1974
and
1990;medianage,59
years
[range
20–8
8];62%
males;
88%
White,1
0%Black)
Evaluated
effectsof
therapy
beyond
TSE
B,including
topicalchlorm
ethine
NA
6patientsdevelopedmalignant
melanom
a12–9
5months
afterTSE
Btherapy,
including2treatedwith
chlorm
ethine
Duringmedianfollow-upof
6years,no
patientdied
of
second
arycutaneous
malignancy
Additionaluseof
chlorm
ethine
followingTSE
Btherapywas
associated
withnonm
elanom
a
skin
cancer
Esteveet
al.
(1999)
[40]
Multicenter,p
rospective
study
52(m
ixed
population
of
patientswithCTCL
including35
withMF;
age
18–8
7years;67%
males)
Topicalchlorm
ethine
0.02%
givenas
aqueoussolution
NA
Follow-updata
from
43
patients
Intoleranceto
chlorm
ethine
developedin
23patients(14
males,9
females)4days
to
9monthsafterinitiation,
including15
of35
(43%
)
patientswithMF
12patientsoverallwereableto
resumechlorm
ethine
after
resolution
ofsymptom
s
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1091

Table
1continued
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
Foulcet
al.
(2002)
[36]
Open-label,prospective
study
39withCTCL(including
34
withMF:meanage,63
years
[range
31–8
2];59%
males:
stageIA,8
;stageIB,1
4;
stageIIA,3
;stageIIB,8
;
stageIVA,1
)
Topicalchlorm
ethine
0.2%
dilutedin
10mlsolventand
50mlwater
andgiveneither
QD
orinterm
ittently
Responseratewas69%,w
ithno
difference
betweenQD
and
interm
ittent
application
CRin
54%;PR
in15%
CRin
59%
withstageIA
orIB
CRin
45%
withstageIIA
or
IIB
How
ever,responsedecreased
withshort-term
application
vs.com
parisonwithliterature
Cutaneous
intoleranceoccurred
in19
of39
(49%
)patients,
including6withACDaftera
meanof
9weeks;theother
13patientsdeveloped
contactderm
atitisaftera
longer
period
(3weeks
to
17months)
Kim
etal.
(2003)
[37]
Single-center,
retrospectivecohort
analysis
203(withstageI–IIIdisease;
medianage,56
years[range
12–8
7];61%
males;86%
White):stageIA,1
03;stage
IB,7
4;stageIIA,1
8;stage
IIB,4
;stageIII,4
Topicalchlorm
ethine
10–2
0mg/100mlaqueous
solution
orointment
ORRandCRrate:all,83%and
50%;stageI,93%
and65%;
stageII,7
2%and34%
Mediantimeto
CR:all,
12months;stageI,
10months;stageII,
19months
Mediantimeto
relapse:
12months
Whenadministeredas
salvage
therapy,response
rateswere
similarto
initialtherapy
Efficacy
similarforaqueousvs.
ointmentform
ulations
Contact
hypersensitivity
reactionsoccurred
in\
10%
whenused
asointment
8(4%)patientsdeveloped
second
arycutaneous
malignancies,withnone
attributed
tochlorm
ethine
Nosignificant
toxiceffectswere
observed
1092 Dermatol Ther (Heidelb) (2021) 11:1085–1106

Table
1continued
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
deQuatrebarbes
etal.(2005)
[38]
Multicenter,p
rospective,
nonrandomized
study
64(new
lydiagnosed,
early-
stagedisease;meanage,
63years[range
7–82];67%
males):stageIA,3
3;stage
IB,2
6;stageIIA,5
Topicalchlorm
ethine
0.02%
aqueousgiventwiceweekly
followed
bybetamethasone
cream
over
6months
CRin
58%
afterameanof
4months,including61%
withstageIA
disease,58%
withstageIB,40%
withstage
IIA
disease
Relapse
in17
(46%
)patients
afterameanof
7.7months
58%
ofpatientsdidnot
experience
anyadverse
cutaneousreactions
Severe
cutaneousreactions
requiringdiscontinu
ation
developedin
18(28%
)
patients,including
erythema,
severe
pruritus,o
rburning
sensationin
11cases,and
eczematousreaction
in7cases
Lindahl
etal.
(2013)
[27]
Retrospective
analysisof
medicalrecords(116)
116:
stageIA,11;
stageIB,68;
stageIIA,1
0;stageIIB,1
3;
stageIII,9;
stageIVA,4
;
stageIVB,1
Chlormethine
ointment
(concentration
not
mentioned)
ORR,9
2%;CRrate,5
3%
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1093

Table
1continued
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
Lessinet
al.
(2013)
[15]
PhaseIImulticenter,
rand
omized,o
bserver-
blinded,
noninferiority
trialin
patientswith
persistent/recurrent
stageI–II
disease
260(m
edianage,58
years
[range
11–8
8];59%
male;
74%
Caucasian):stageIA,
141;stageIB,115;stage
IIA,
4
Chlormethine
0.02%
gelor
ointmentappliedQD
for
12months;no
concom
itant
corticosteroidswere
perm
itted
CAILS(severityscoreforB
5
lesions;CR,1
00%
improvem
entfrom
BL;
PR,C
50to\
100%
improvem
entfrom
BL:gel,
14%
CRand45%
PR;
ointment,12%
CRand36%
PR
Chlormethine
gelwas
noninferiorto
ointmentby
prespecifiedcriteria
Nodrug-related
severe
AEs
werereported
Drug-relatedskin
AEsinthegel
andointmentarmswere
reported
by62%
and50%
of
patients,respectively
These
included:skin
irritation
(n=32
vs.1
8);pruritus
(n=25
vs.2
0);erythema
(n=22
vs.1
8);contact
derm
atitis(n
=19
vs.1
9);
skin
hyperpigmentation
(n=7vs.9
);folliculitis
(n=7vs.5
)
11patientsoverallincluding3
ingelarm
and8in
ointment
arm
werediagnosedwith20
second
arynonm
elanom
askin
cancers
Kim
etal.
(2014)
[17]
PhaseIIextensionstudy
withpatientswho
completed
theLessin
2013
[15]
study
(12monthsof
treatm
ent)butdidnot
achieveCR(N
=98)
98(m
eanage,53
±14
years;
55%
male;68%
Caucasian)
Chlormethine
0.04%
gel
appliedQD
for7months
(applicationfrequencycould
bereducedfortoxicity)
CAILS(severityscoreforB
5
lesions,confi
rmed
C4weeks
later):CRin
6patientsand
PRin
20patients
Mild-to-moderatedrug-related
skin
AEswerereported
by31
(32%
)patients
Mostcommon
drug-related
skin
AEswereskin
irritation
(11%
),erythema(10%
),and
pruritus
(6%)
1094 Dermatol Ther (Heidelb) (2021) 11:1085–1106

Table
1continued
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
Lindahl
etal.
(2014)
[41]
Population-based
cohort
study
303;
including110using
chlorm
ethine
(meanage,
69years[range
30–9
8];
69%
males:stageIA,1
4;
stageIB,3
9;stageIIA,8
;
stageIIB,25;
stageIIIA,15;
stageIV,9
)
Topicalchlorm
ethine
NA
Second
arycancerswerenot
significantlyincreased(H
R
0.8;
95%
CI0.5–
1.6)
in
patientsreceiving
chlorm
ethine
Subanalysesshow
edno
significantlyincreasedrisk
of
nonm
elanom
askin
cancers,
malignant
melanom
as,o
r
cancersof
therespiratory
organs
Mortalityandcause-specific
mortalitywerenotinfluenced
bytreatm
ent
Kim
etal.
(2020)
[42]
Prospective,
observational,
noninterventional
study
301(298
monitored)
stageIA–IIA:62%;stage
IIB–IV:8%
Topicalchlorm
ethine
gelin
combination
withother
therapies
ORRof
44%
inpatientswho
received
chlorm
ethine
gel
plus
corticosteroidsand/or
NBUVBphototherapy,o
ral
bexarotene,P
UVA,local
electron-beam
therapy,
topicalbexarotene,and
imiquimod
ORRof
45%
inpatientswho
received
chlorm
ethine
gelp
lus
anyothertreatm
ent
42%
experiencedC
1AE
Mostcommon
skin-related
AEs
were:derm
atitis(13%
);
pruritus
(10%
);skin
irritation
(7%);anderythema(5%)
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1095

Table
1continued
Firstauthor
(year)
Stud
ydescription
Patients,n
Treatment(s)
administered
Efficacy
findings
Safety
findings
Gilm
oreet
al.
(2020)
[43];
Alexand
er-
Savino
etal.
(2020)
[44]
PhaseII,nonrand
omized,
open-label,split-face,
two-arm
studyin
patientswithstageIA
andIB
disease
280.016%
w/w
topical
chlorm
ethine
gel(once
nightly)appliedover
a
minim
umof
8cm
2 ,over
a
4-month
period
0.016%
w/w
topical
chlorm
ethine
gel(once
nightly)plus
triamcinolone
0.1%
ointmentQD
applied
over
aminim
umof
8cm
2 ,
over
a4-month
period
CAILSscores
werecomparable
betweenthetwoarms
Additionof
triamcinolone
ointmentsignificantly
decreasedtheSC
ORAD
score
(p\
0.05)
32%
developedsevere
contact
derm
atitis
31%
developedACD
vs.6
6%
ofpatientsfrom
historical
data
usingaqueous
form
ulation
4%developedIC
D
The
trialswerecond
uctedun
dervaryingcond
itions,including
trialdesign,additionaltreatm
ents,and
responserates;AEscann
otbe
directlycomparedwithoneanotherandmay
not
reflect
theratesobserved
inclinicalpractice
ACD
allergic
contactderm
atitis;AEadverseevent;BLbaselin
e;CAILSCom
posite
Assessm
entof
IndexLesionSeverity;CIconfi
denceinterval;CRcompleteresponse;CTCL
cutaneousT-celllym
phom
a;EODeveryotherday;HRhazard
ratio;ICDirritant
contactderm
atitis;M
Fmycosisfungoides;NAdatanotavailable;NBUVBnarrow
band
ultravioletB;
ORRoverallresponserate;P
Rpartialresponse;PU
VApsoralen
andultravioletA;Q
Donce
daily;R
Tradiotherapy;SCORADSC
ORingAtopicDermatitis;T
SEBtotalskinelectron
beam
;UVultraviolet
1096 Dermatol Ther (Heidelb) (2021) 11:1085–1106

efficacy-evaluable population, overall responserates (ORRs) were 77% and 59% for the gel andointment, respectively [15]. Additionally, the95% confidence interval of the CAILS score inthe efficacy-evaluable population not onlyexceeded the noninferiority threshold (C 0.75),but also lies entirely above 1. On the basis of apost hoc approach of switching from noninfe-riority to superiority testing, these results areconsistent with superiority (p\ 0.05) findingsfor chlormethine gel.
The gel formulation had a faster time toresponse (50% response in 26 weeks) than theointment (42 weeks). Response rates at 52 weekswere 76% for the gel and 56% for the ointment.Maximum response to chlormethine gel treat-ment occurred between 8 and 10 months,emphasizing the importance of continuedtreatment and close follow-up of patients tomaximize the response potential [15]. A follow-up 7-month extension study evaluated a higherdose of chlormethine gel (0.04%) in patientswho did not have a complete response (CR)after previously receiving chlormethine gel orointment for 12 months. In total, 27% ofpatients had a confirmed response, which couldoccur as late as 16 months after initiation of thelower-dose chlormethine treatment [17],thereby reinforcing the value of continuedchlormethine treatment.
In the 201 study,[50% of patients in eachgroup experienced a skin-related adverse event(AE). Irritant contact dermatitis (ICD) was mostcommon, although this was managed withtreatment adjustments, such as suspension orreduction of chlormethine treatment and theuse of emollients/oral antihistamines. Notreatment-related serious AEs were reported,and there was no evidence of systemic absorp-tion of chlormethine [15, 17].
The prospective, observational, noninter-ventional US-based PROVe trial was designed toprovide information on the use of chlormethinegel in a real-world practice setting(NCT02296164). Patients who were diagnosedwith any stage of MF and were being treatedwith chlormethine gel in combination withother MF therapies were enrolled. At12 months, the proportion of stage IA and IBresponders (defined as C 50% reduction from
baseline in body surface area [BSA] involve-ment) was 44% in patients who receivedchlormethine plus topical corticosteroids plusother treatment and 45% in patients whoreceived chlormethine plus other treatment. Apeak response occurred at 18 months forpatients with stage IA and IB disease in thechlormethine plus other treatment group(67%). Overall, 28% of patients experienced atreatment-related AE; the most common skin-related AEs deemed to be therapy related weredermatitis (12%), pruritus (7%), skin irritation(7%), and erythema (4%) [45].
Other studies using compounded formula-tions of chlormethine (ointment or solution)have reported response rates of 58–69% inpatients with early-stage disease [26, 30, 36–38]and 13–53% in patients with advanced disease[26, 30, 37]. Moreover, one trial has reported a10-year overall survival rate of 71% in patientswith mainly T1 or T2 disease (96%). For thosepatients who attained a CR with topicalchlormethine, a 5-year relapse-free survival rateof 42% was observed [37]. Another study foundthat the probability of achieving clinicallyapparent remission rates at 2 years was 76% forstage I MF, 45% for stage II, and 49% for stage IIIdisease [35].
REAL-WORLD EXPERIENCEOF CHLORMETHINE GELIN THE MANAGEMENTOF PATIENTS WITH MF (TABLE 2)
Penn Dermatology Cutaneous T-CellLymphoma Clinic
At the Penn Dermatology Cutaneous T-CellLymphoma Clinic (USA), * 200 patients withnewly diagnosed MF are seen annually; of these,70% have early-stage disease. This center useschlormethine gel as a first-line SDT in patientswith early-stage disease for whom topical cor-ticosteroids have failed. It is applied as a local-ized spot treatment or, for patients with more-extensive BSA involvement, as full-body treat-ment (from the neck down). In advanced-stagedisease, chlormethine gel is used as an
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1097

Table 2 Summary of the real-world experience from four centers
Characteristic PennDermatologyCutaneousT-CellLymphomaClinic, USA
Cutaneous OncologyClinic, ColumbiaUniversity, USA
Rabin Medical Center,Israel
Hopital Saint-Louis,France
Number of
patients
with MF
seen/year
* 200 [ 350 300 * 320
Disease stage
of patients
with MF
Mostly early stage Ranging from stage IA or
IB to Sezary syndrome
Early stage Early and advanced stages
Chlormethine
gel usage
Early stage:
treatment after
corticosteroids
failure
Advanced stage:
adjunctive
treatment to
systemic
therapies or
other SDTs
For patients with early
stage, skin-limited
disease
Advanced disease: in
combination with
systemic therapies
Second-line treatment in
patients for whom at
least 1 previous SDT
failed (topical steroids
or phototherapy)
Early stage: second-line
treatment after failure of
high-potency
corticosteroids, mainly
when phototherapy is
not possible or
contraindicated
Late stage: in combination
with systemic treatments
when insufficient effect is
observed on
patch/plaques lesions
Chlormethine
gel initial
application
frequency
Alternative
evenings or 2
nights/week
1–2 times/week,
alternating with topical
steroids
Gradually, up to a
maximum of QD,
sometimes with 1–2
times/week topical
steroids
3 times/week alternating
with topical steroids. If
well tolerated and PR,
increase to QD
Response time 4–6 weeks;
4–24 months
to achieve CR
1–2 months; 80% of
patients respond
NA Beginning of response:
1–2 months. CR:
9–12 months; in some
patients, 12–15 months
required to achieve CR
Incidence of
dermatitis
ICD/ACD:
20–25% of
patients in first
6 months
ICD: * 30%;
10% develop severe ICD
ICD is the most
commonly diagnosed
AE, and is usually mild
Mostly ICD (25% of cases)
Real ACD rare
1098 Dermatol Ther (Heidelb) (2021) 11:1085–1106

adjunctive SDT to systemic therapy and otherSDTs. For at-home administration, patientsmust take appropriate precautions to avoidinadvertent mucosal exposure to chlormethinegel in other household members/pets.
Patients are instructed to apply chlorme-thine gel thinly, initially only every other nightor 2 nights/week, then slowly increase theapplication frequency as tolerance permits, tominimize irritant dermatitis. Patients may applytopical steroids every other day to the samearea, but this treatment is eventually tapered ifno AEs result from chlormethine gel treatment.Patients using full-body treatment are advisedthat they may notice new lesions during thefirst month; however, these are subclinical MFlesions unmasked by chlormethine gel, notnecessarily a sign of true disease progression.
In our experience, a response to chlorme-thine gel treatment can be expected within4–6 weeks. It takes 4–24 months to achieve aCR; the ORR is 70%, with 10% of these achiev-ing a CR and 90% achieving a partial response(PR). ICD or allergic contact dermatitis (ACD) isobserved in 20–25% of patients and occurs
mostly within the first 6 months of therapy.When dermatitis or other skin AEs occur, wetemporarily discontinue chlormethine geltreatment and apply potent topical steroids tothe affected area twice daily (BID) for 2–3 weeks.A ‘‘patch test,’’ where chlormethine gel isapplied daily to a small unaffected skin area, isthen performed. If dermatitis reappears, this issuggestive of ACD, and chlormethine gel isdiscontinued permanently. If no dermatitisappears, the prior reactions are most likely ICD,and chlormethine gel may be reintroducedslowly with applications every other night or 2nights/week. This practice of ‘‘starting low andgoing slow’’ with application frequency isanalogous to how we use other SDTs withknown irritant effects (e.g., topical retinoids).Patients who experience a moderate-to-severedermatitis reaction to chlormethine gel mayhave complete clearance of the original MFlesion once the dermatitis is cleared with potenttopical steroids, as has been observed in theliterature [46].
Table 2 continued
Characteristic PennDermatologyCutaneousT-CellLymphomaClinic, USA
Cutaneous OncologyClinic, ColumbiaUniversity, USA
Rabin Medical Center,Israel
Hopital Saint-Louis,France
Management
strategy for
dermatitis
Temporary
suspension of
treatment
Potent topical
steroids BID for
2–3 weeks
ICD: application of mid-
to-high-potency
ophthalmic steroid
(chlormethine gel
discontinuation if severe
ICD)
ACD: discontinue
chlormethine gel
Mild ICD: avoid
treatment
discontinuation if
possible; temporary
addition of topical
corticosteroids
Moderate-to-severe ICD:
topical steroid plus
temporary reduction or
discontinuation (only
for severe dermatitis)
Moderate-to-severe
dermatitis: chlormethine
gel discontinuation plus
topical steroids;
chlormethine gel
reintroduced after
reactions have
disappeared; and
frequency of application
has progressively increased
ACD allergic contact dermatitis; AE adverse event; BID twice daily; CR complete response; ICD irritant contact dermatitis;MF mycosis fungoides; NA data not available; PR partial response; QD once daily; SDT skin-directed therapy
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1099

The Cutaneous Oncology Clinicat Columbia University
At the Comprehensive Cutaneous OncologyClinic at Columbia University (USA), a range ofdisease stages are seen, from early-stage IA andIB MF-CTCL to stage IV disease, including Sez-ary syndrome. The patients are managedaccording to published algorithms in a stage-based approach. For patients with early-stageskin-limited disease, topical steroids, narrow-band ultraviolet B (NBUVB), and chlormethinegel are the first-line treatments. Based on theirschedule, personal preferences, or medical his-tory, and in consultation with their physician,patients choose a therapy that best fits theirlifestyle and disease state. Light therapy is aneffective way to treat cutaneous manifestationsof MF, but it may necessitate frequent visits tothe physician’s office and may not be conve-nient for people with busy work and familyschedules. Some of the benefits of chlormethinegel include the ability to apply the gel at home,reducing the need for office visits for lighttherapy in those patients unable to incorporatevisits into their daily schedule. Chlormethinegel treatment can continue away from home,provided refrigeration is available, so travelneed not interfere with the treatment schedule.Additionally, chlormethine gel is recommendedover NBUVB for patients with high risk of mel-anoma or nonmelanoma skin cancers, includ-ing patients with significant personal history ofthese diseases as well as light skin, numerousatypical nevi, or immunosuppression [47].Given that a link between chlormethine gel useand development of nonmelanoma skin cancershas been suggested, concurrent NBUVB andchlormethine gel treatment is not typicallyadvised in our patient population [48].
While chlormethine gel is FDA approved fortreating stage IA and IB MF in patients whoreceived one prior treatment, we also usechlormethine gel therapy in combination withsystemic therapies in more advanced disease,including Sezary syndrome.
At Columbia University, over 350 patientsper year are treated with chlormethine gel, allwith relatively low toxicity. The main AE isirritant dermatitis, seen in * 30% of treated
patients. ACD may be observed, and chlorme-thine gel should be discontinued in these cases.However, ICD is generally well controlled withmid- to high-potency topical steroid use, andchlormethine gel treatment can be continued inmost patients. Approximately 10% of patientsdevelop severe ICD, with lymphomatoid papu-losis observed in a few patients; this resolvesupon discontinuation of chlormethine gel [49].
Patients generally start chlormethine gelwith less frequent applications (one or twotimes/week, alternating with topical steroids). Ifthe patient can tolerate the gel without ICD orother concerns, the frequency is increased todaily use. In some patients, the gel may be usedBID depending on symptoms. In our experi-ence, response rates of up to 80% have beenseen in patients with early-stage disease. Initialresponse is typically observed 1–2 months afterstarting treatment, and therapy is continued for12 months in responders. Frequency can sub-sequently be reduced during the ‘‘maintenancephase,’’ which may last from several months toseveral years, or chlormethine gel can be dis-continued when cutaneous lesions disappearcompletely. A significant proportion of patientsuse skin-directed (mostly topical steroids) orsystemic agents in combination with chlorme-thine gel.
The Cutaneous Lymphoma Clinic at RabinMedical Center
In Israel, in daily practice the first-line treat-ment for early-stage MF (after topical steroids) isusually phototherapy, while chlormethine gel isused as a second-line treatment in patients forwhom phototherapy has failed or who havedeveloped intolerability. Chlormethine gel as afirst-line therapy (after topical steroids) isreserved for patients with early-stage MF whohave contraindications to phototherapy (e.g.,history of melanoma or multiple nonmelanomaskin cancers) or those who foresee adherenceproblems to phototherapy. Regional or whole-body application depends on the distribution oflesions and extent of cutaneous involvement.Since chlormethine gel has the potential tocause irritation, treatment initiation involves
1100 Dermatol Ther (Heidelb) (2021) 11:1085–1106

gradually increasing the application frequencyup to the maximal tolerable frequency, but nomore than QD, to minimize the occurrence anddegree of irritation. The process is similar to thatadopted with other topical treatments withirritant potential (e.g., retinoids) [50]. The skinfolds and face are generally more susceptible toirritant reactions; hence, the maximal recom-mended application frequency is usually alter-nate days.
ICD is the most common AE and is usuallymild. In the case of mild irritation, patients maybenefit from temporary addition of topicalsteroids without a change in chlormethine gelapplication frequency, although in somepatients, emollients are sufficient to alleviatesymptoms. If irritation is moderate to severe,topical steroid application is advocated along-side temporary reduction (moderate irritation)or temporary discontinuation (severe irritation)of chlormethine gel application. Once irritationis under control or resolved, the applicationfrequency is gradually increased or chlorme-thine gel is reintroduced at the highest tolerablefrequency. In many patients, topical steroidsmay be withdrawn or minimized to once-weekly application. In the case of severe irrita-tion, reintroduction of chlormethine gel may beattempted but is initially limited to a small areato assess tolerability.
The main differential diagnosis of ICD isACD. Patch tests are not done routinely, andthe diagnosis is based solely on clinical judg-ment. Key diagnostic features are: (1) timing ofappearance, with delayed-type hypersensitivityoccurring at least 2–4 weeks following treat-ment initiation versus primary irritation, whichmay develop as early as a few days after appli-cation; (2) distribution, where extension ofdermatitis beyond treated areas indicatesdelayed-type hypersensitivity versus primaryirritation, which is localized to treated areasonly. ACD is suspected in few patients. If theallergic reaction is mild to moderate, the pro-tocol is the same as for severe irritation. Forpatients with severe ACD, permanent discon-tinuation is required. It is important that anytype of dermatitis is distinguished clearly fromthe unmasking effect of chlormethine gel,where new lesions are observed in the treated
areas; this is seen in a small fraction of patientsand usually occurs during the first month oftreatment. Patients should be encouraged tocontinue with treatment, and whole-bodyapplication should be considered.
Hopital Saint-Louis
Hopital Saint-Louis (France) sees * 320patients per year with cutaneous lymphomas ofany MF stage; * 80% have early-stage and 20%have advanced-stage disease. In patients withearly-stage IA MF, chlormethine gel is pre-scribed after failure of high-potency corticos-teroids, whereas for patients with stage IB,chlormethine gel may be prescribed as a first-line therapy, particularly in patients for whomphototherapy is not possible or contraindicated.In patients with late-stage disease, chlorme-thine gel is used in combination with systemictreatments when insufficient effect is observedon patch or plaque lesions. Response tochlormethine gel may occur 9 months aftertreatment initiation, but in some patients aperiod of 12 or 15 months may be required toachieve remission. In our experience, 19% ofpatients achieve a CR, and 66% have a PR.
The most common AE is skin reactions,mostly contact irritation versus real allergicdermatitis. When severe skin reactions areobserved (e.g., moderately severe to severe der-matitis), chlormethine gel is discontinued andtopical steroids are prescribed. Once the reac-tions have disappeared, chlormethine gel maybe applied to a limited zone with persistinglesions on the trunk or the limb, with a reducedschedule (one or two times/week). If the patientpresents with real sensitization, contact allergywill develop on the limited area, thereby indi-cating the patient has a true allergy, andchlormethine gel is contraindicated. In mostpatients, however, this limited application iswell tolerated, and it is possible to applychlormethine gel to the whole skin, up to threetimes/week, and often every day. Real patchtesting to determine whether the response isICD or ACD may be very informative in suchsituations.
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1101

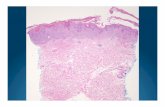
A 58-year-old male with a history of mela-noma on his back presented with stage IB MF.The patient had disseminated pruriginous ery-thematosquamous patches and plaques,although there were no adenopathies or bloodinvolvement. A cutaneous biopsy demonstrateda band-like infiltrate with epidermotropism andatypical lymphocytes, and the patient wasdiagnosed with stage IB MF. Treatment withtopical clobetasol yielded no response, whiletreatment with phototherapy was not possibledue to the history of melanoma. Consequently,this patient was treated with chlormethine gelQD. Due to the dissemination of lesions,chlormethine gel was applied to the whole
body, except for the face and scalp, where nolesions were present. After 9 months ofchlormethine gel treatment, the patient was infull remission and treatment was stopped(Fig. 1).
CONCLUSIONS
Chlormethine gel is a therapeutic option forpatients with MF skin lesions, and a range ofretrospective, prospective, and observationalclinical data supports its use in all disease stages.Its validity as a treatment is supported byinternational guidelines, which all recommend
Fig. 1 Patient case images. a Epidermotropism and atypical lymphocytes, diagnostic of mycosis fungoides. b Skin lesions onthe patient’s legs before and after 3 and 6 months of once-daily chlormethine gel application
1102 Dermatol Ther (Heidelb) (2021) 11:1085–1106

chlormethine gel as a first-line treatment forpatients with early-stage MF. In later stages ofMF, systemic treatments are usually indicatedand prescribed, although patch and plaquelesions in these patients may be only partiallyresponsive to systemic treatments; the additionof topical treatments, such as chlormethine gel,may be very useful in such cases. Moreover,chlormethine gel may be important as anadjunctive therapy in patients with late-stagedisease (especially for persisting patches andplaques) to palliate symptoms and to improvethe overall response and as a maintenancetreatment since systemic therapies do not typi-cally result in durable CRs.
Indeed, experience from clinical practice inthe USA, Israel, and France has shown thatchlormethine gel is used both as an SDT (oftenin the first line) in early-stage MF and as anadjunctive therapy in advanced-stage disease.The strategies employed by the centersdemonstrate that emergent cutaneous reactionscan be managed if the appropriate protocols arefollowed. Time to response varies slightlybetween centers, perhaps reflecting the diversityof patients who were seen (Table 2). ICD is themost frequently observed form of dermatitis,and all centers use topical steroids to managethis AE, although discontinuing chlormethinetreatment may be required for severe reactions.
Efforts are ongoing to gain a more in-depthunderstanding of the utility of chlormethine gelin patients with MF and the nature of theemergent skin reactions. The PROVe trial foundthat chlormethine gel is an effective treatmentacross all disease stages [45]. No chlormethinegel-related serious AEs occurred in the study,and the reported emergent skin-related AEswere manageable and less prevalent than in thepivotal clinical trial [15], possibly because offrequent dose adjustments and the co-adminis-tration of corticosteroids, which reflect the real-world experience reported herein.
The Mechlorethamine Induced ContactDermatitis Avoidance Study (MIDAS;NCT03380026), which evaluated the incidenceand severity of contact dermatitis followingtreatment with chlormethine gel alone or incombination with triamcinolone ointment inpatients with MF, aimed to gain a greater
understanding of chlormethine-related contactdermatitis. The study found that contact der-matitis with and without hypersensitivityresponses was seen, and histopathologyrevealed a superficial and deep lymphocyticinfiltrate with spongiosis and eosinophils simi-lar to arthropod assault [43, 44]. Evaluation ofthe patient samples is ongoing to provide moreinformation about the nature of the skin reac-tions, which should further help to guidemanagement of patients who develop contactdermatitis. Additional information that may beused to guide treatment and manage contactdermatitis in patients who receive chlormethinegel may come from the REACH trial (Study toDetermine the Aetiology of Chlormethine GelInduced-skin Drug Reaction in Early-stageMycosis Fungoides Cutaneous T Cell Lym-phoma; NCT04218825), which is currentlyrecruiting.
In conclusion, chlormethine gel is an effec-tive treatment for patients with all stages of MF.While contact dermatitis is an emergent skin-related AE, it can be managed effectively inmost cases if the appropriate strategies are inplace.
ACKNOWLEDGMENTS
Funding. Funding for this review the jour-nal’s and Rapid Service Fee were funded byHelsinn Healthcare SA, Lugano, Switzerland.
Medical Writing and/or Editorial Assis-tance. Editorial and medical writing assistancewas provided by Joanne Franklin, PhD, CMPP,from Aptitude Health, The Hague, The Nether-lands, funded by Helsinn Healthcare SA,Lugano, Switzerland.
Authorship. All named authors meet theInternational Committee of Medical JournalEditors (ICMJE) criteria for authorship for thisarticle, take responsibility for the integrity ofthe work as a whole, and have given theirapproval for this version to be published.
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1103

Authorship Contributions. All authorsequally contributed to the concept, design, anddrafting of the manuscript.
Disclosures. Larisa J. Geskin has receivedresearch support from and was Principal Inves-tigator for BMS, Galderma, Helsinn, InnatePharma, Johnson & Johnson, Kyowa Kirin,Mallinckrodt, Merck, miRagen, Soligenix, andStratpharma. She was also a speakers’ bureaumember for Helsinn, a scientific advisory boardmember for Helsinn, Kyowa Kirin, Mallinck-rodt, Recordati, Regeneron Pharmaceuticals,and Takeda, and has consulted for RegeneronPharmaceuticals and Sanofi. Martine Bagot is ascientific advisory board member for Helsinn/Recordati, Innate Pharma, Kyowa Kirin, andTakeda. Emmilia Hodak was a scientific advisoryboard member for Actelion, Helsinn, andTakeda and a speakers’ bureau member forHelsinn, Rafa, and Takeda. Ellen J. Kim hasreceived clinical trial grants from Galderma,Innate, Kyowa Kirin, and Soligenix. She has alsoconsulted for Galderma and Helsinn.
Compliance with Ethics Guidelines. Thisarticle is based on previously conducted studiesand does not contain any new studies withhuman participants or animals performed byany of the authors. Informed consent was pro-vided by the patient whose case was included.
Open Access. This article is licensed under aCreative Commons Attribution-NonCommer-cial 4.0 International License, which permitsany non-commercial use, sharing, adaptation,distribution and reproduction in any mediumor format, as long as you give appropriate creditto the original author(s) and the source, providea link to the Creative Commons licence, andindicate if changes were made. The images orother third party material in this article areincluded in the article’s Creative Commonslicence, unless indicated otherwise in a creditline to the material. If material is not includedin the article’s Creative Commons licence andyour intended use is not permitted by statutoryregulation or exceeds the permitted use, youwill need to obtain permission directly from thecopyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by-nc/4.0/.
REFERENCES
1. Diamandidou E, Cohen PR, Kurzrock R. Mycosisfungoides and Sezary syndrome. Blood. 1996;88:2385–409.
2. van Doorn R, Van Haselen CW, van Voorst VaderPC, et al. Mycosis fungoides: disease evolution andprognosis of 309 Dutch patients. Arch Dermatol.2000;136:504–10.
3. Lovgren ML, Scarisbrick JJ. Update on skin directedtherapies in mycosis fungoides. Chin Clin Oncol.2019;8:7.
4. Zackheim HS, Amin S, Kashani-Sabet M, McMillanA. Prognosis in cutaneous T-cell lymphoma by skinstage: long-term survival in 489 patients. J Am AcadDermatol. 1999;40:418–25.
5. de Coninck EC, Kim YH, Varghese A, Hoppe RT.Clinical characteristics and outcome of patientswith extracutaneous mycosis fungoides. J ClinOncol. 2001;19:779–84.
6. Kim YH, Liu HL, Mraz-Gernhard S, Varghese A,Hoppe RT. Long-term outcome of 525 patients withmycosis fungoides and Sezary syndrome: clinicalprognostic factors and risk for disease progression.Arch Dermatol. 2003;139:857–66.
7. Scarisbrick JJ, Prince HM, Vermeer MH, et al.Cutaneous Lymphoma International Consortiumstudy of outcome in advanced stages of mycosisfungoides and Sezary syndrome: effect of specificprognostic markers on survival and development ofa prognostic model. J Clin Oncol. 2015;33:3766–73.
8. Al Hothali GI. Review of the treatment of mycosisfungoides and Sezary syndrome: a stage-basedapproach. Int J Health Sci (Qassim). 2013;7:220–39.
9. DeSimone JA, Sodha P, Ignatova D, Dummer R,Cozzio A, Guenova E. Recent advances in primarycutaneous T-cell lymphoma. Curr Opin Oncol.2015;27:128–33.
10. Willemze R, Hodak E, Zinzani PL, Specht L, LadettoM, ESMO Guidelines Committee. Primary cuta-neous lymphomas: ESMO Clinical Practice Guide-lines for diagnosis, treatment and follow-up. AnnOncol. 2018;29:iv30–40.
11. Trautinger F, Eder J, Assaf C, et al. EuropeanOrganisation for Research and Treatment of Cancer
1104 Dermatol Ther (Heidelb) (2021) 11:1085–1106

consensus recommendations for the treatment ofmycosis fungoides/Sezary syndrome—update 2017.Eur J Cancer. 2017;77:57–74.
12. National Comprehensive Cancer Network. NCCNClinical Practice Guidelines (NCCN Guidelines�).Primary cutaneous lymphomas. Version 2.2020.https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf. Accessed 1 Feb 2021.
13. Gilson D, Whittaker SJ, Child FJ, et al. BritishAssociation of Dermatologists and U.K. CutaneousLymphoma Group guidelines for the managementof primary cutaneous lymphomas 2018. Br J Der-matol. 2019;180:496–526.
14. Zackheim HS, Kashani-Sabet M, Amin S. Topicalcorticosteroids for mycosis fungoides. Experience in79 patients. Arch Dermatol. 1998;134:949–54.
15. Lessin SR, Duvic M, Guitart J, et al. Topicalchemotherapy in cutaneous T-cell lymphoma:positive results of a randomized, controlled, multi-center trial testing the efficacy and safety of a novelmechlorethamine, 0.02%, gel in mycosis fungoides.JAMA Dermatol. 2013;149:25–32.
16. Breneman D, Duvic M, Kuzel T, Yocum R, Truglia J,Stevens VJ. Phase 1 and 2 trial of bexarotene gel forskin-directed treatment of patients with cutaneousT-cell lymphoma. Arch Dermatol. 2002;138:325–32.
17. Kim YH, Duvic M, Guitart J, Lessin SR. Tolerabilityand efficacy of mechlorethamine 0.04% gel inCTCL (mycosis fungoides) after initial treatmentwith topical mechlorethamine 0.02% gel. Presentedat: 6th Annual T-cell Lymphoma Forum; January23–25, 2014; San Francisco, CA, USA.
18. Ledaga [summary of product characteristics]. Lon-don, UK: Actelion Registration Ltd; 2017.
19. ATU Rapport de Synthese pour Valchlor/Ledaga�.Rapport nr. 9, version 1.0. Helsinn Birex Pharma-ceuticals Ltd. Dublin, Ireland. 2019.
20. Osborne DW, Musakhanian J. Skin penetration andpermeation properties of Transcutol�-neat or dilu-ted mixtures. AAPS PharmSciTech. 2018;19:3512–33.
21. Ritschel WA, Ye W, Buhse L, Reepmeyer JC. Stabil-ity of the nitrogen mustard mechlorethamine innovel formulations for dermatological use. Int JPharm. 2008;362:67–73.
22. Sullivan DW Jr, Gad SC, Julien M. A review of thenonclinical safety of Transcutol�, a highly purifiedform of diethylene glycol monoethyl ether (DEGEE)used as a pharmaceutical excipient. Food ChemToxicol. 2014;72:40–50.
23. Ashland Inc. KlucelTM hydroxypropylcellulose.Physical and chemical properties. 2017. https://www.ashland.com/file_source/Ashland/Product/Documents/Pharmaceutical/PC_11229_Klucel_HPC.pdf. Accessed 1 Feb 2021.
24. Soo VWC, Kwan BW, Quezada H, et al. Repurposingof anticancer drugs for the treatment of bacterialinfections. Curr Top Med Chem. 2017;17:1157–76.
25. Molin L, Thomsen K, Volden G, et al. Aspects of thetreatment of mycosis fungoides. A report from theScandinavian Mycosis Fungoides Study Group.Cutis. 1980;25(155–7):160–1.
26. Vonderheid EC, Tan ET, Kantor AF, Shrager L,Micaily B, Van Scott EJ. Long-term efficacy, curativepotential, and carcinogenicity of topical mechlor-ethamine chemotherapy in cutaneous T cell lym-phoma. J Am Acad Dermatol. 1989;20:416–28.
27. Lindahl LM, Fenger-Gron M, Iversen L. Topicalnitrogen mustard therapy in patients with mycosisfungoides or parapsoriasis. J Eur Acad DermatolVenereol. 2013;27:163–8.
28. Jennings T, Duffy R, Gochoco A, et al. Valchlormaintenance therapy for patients with mycosisfungoides who received low dose total skin electronbeam treatment. Chin Clin Oncol. 2019;8:13.
29. Price NM, Hoppe RT, Constantine VS, Fuks ZY,Farber EM. The treatment of mycosis fungoides:adjuvant topical mechlorethamine after electronbeam therapy. Cancer. 1977;40:2851–3.
30. Vonderheid EC, Van Scott EJ, Wallner PE, JohnsonWC. A 10-year experience with topical mechlor-ethamine for mycosis fungoides: comparison withpatients treated by total-skin electron-beam radia-tion therapy. Cancer Treat Rep. 1979;63:681–9.
31. Price NM, Hoppe RT, Deneau DG. Ointment-basedmechlorethamine treatment for mycosis fungoides.Cancer. 1983;52:2214–9.
32. Ramsay DL, Parnes RE, Dubin N. Response ofmycosis fungoides to topical chemotherapy withmechlorethamine. Arch Dermatol. 1984;120:1585–90.
33. Zachariae H, Thestrup-Pedersen K, Søgaard H.Topical nitrogen mustard in early mycosis fun-goides. A 12-year experience. Acta Derm Venereol.1985;65:53–8.
34. Hoppe RT, Abel EA, Deneau DG, Price NM. Mycosisfungoides: management with topical nitrogenmustard. J Clin Oncol. 1987;5:1796–803.
35. Ramsay DL, Halperin PS, Zeleniuch-Jacquotte A.Topical mechlorethamine therapy for early stage
Dermatol Ther (Heidelb) (2021) 11:1085–1106 1105

mycosis fungoides. J Am Acad Dermatol. 1988;19:684–91.
36. Foulc P, Evrard V, Dalac S, et al. Evaluation of a 1-hexposure time to mechlorethamine in patientsundergoing topical treatment. Br J Dermatol.2002;147:926–30.
37. Kim YH, Martinez G, Varghese A, Hoppe RT. Topi-cal nitrogen mustard in the management ofmycosis fungoides: update of the Stanford experi-ence. Arch Dermatol. 2003;139:165–73.
38. de Quatrebarbes J, Esteve E, Bagot M, et al. Treat-ment of early-stage mycosis fungoides with twice-weekly applications of mechlorethamine and topi-cal corticosteroids: a prospective study. Arch Der-matol. 2005;141:1117–20.
39. Licata AG, Wilson LD, Braverman IM, Feldman AM,Kacinski BM. Malignant melanoma and other sec-ond cutaneous malignancies in cutaneous T-celllymphoma. The influence of additional therapyafter total skin electron beam radiation. Arch Der-matol. 1995;131:432–5.
40. Esteve E, Bagot M, Joly P, et al. A prospective studyof cutaneous intolerance to topical mechlor-ethamine therapy in patients with cutaneous T-celllymphomas. French Study Group of CutaneousLymphomas. Arch Dermatol. 1999;135:1349–53.
41. Lindahl LM, Fenger-Grøn M, Iversen L. Secondarycancers, comorbidities and mortality associatedwith nitrogen mustard therapy in patients withmycosis fungoides: a 30-year population-basedcohort study. Br J Dermatol. 2014;170:699–704.
42. Kim EJ, Geskin L, Guitart J, et al. Real-world expe-rience with mechlorethamine gel in patients withmycosis fungoides-cutaneous lymphoma: prelimi-nary findings from a prospective observationalstudy. J Am Acad Dermatol. 2020;83:928–30.
43. Gilmore ES, Alexander-Savino CV, Chung CG,Poligone B. Incidence and types of contact der-matitis after chlormethine gel treatment in patientswith mycosis fungoides-type cutaneous T-cell lym-phoma: The MIDAS study. Presented at the 4th
World Congress of Cutaneous Lymphomas. 12–14February 2020; Barcelona, Spain. Abstract Y-04.Available at http://wcclbarcelona2020.com/images/site/PROTEGIDO/Abstracts_WCCl_2020_DIGITAL.pdf. Accessed 1 Feb 2021.
44. Alexander-Savino CV, Chung CG, Gilmore ES,Poligone B. Molecular signature patterns related tothe development of contact dermatitis in patientswith mycosis fungoides-type cutaneous T-cell lym-phoma treated with chlormethine gel. Presented atthe 4th World Congress of Cutaneous Lymphomas.12–14 February 2020; Barcelona, Spain. Abstract026. Available at http://wcclbarcelona2020.com/images/site/PROTEGIDO/Abstracts_WCCl_2020_DIGITAL.pdf. Accessed 1 Feb 2021.
45. Kim EJ, Guitart J, Querfeld C, et al. The PROVestudy: US real-world experience with chlormethine/mechlorethamine gel in combination with othertherapies for patients with mycosis fungoidescutaneous T-cell lymphoma. Am J Clin Dermatol.2021. https://doi.org/10.1007/s40257-021-00591-x.
46. Vonderheid EC, Ekbote SK, Kerrigan K, et al. Theprognostic significance of delayed hypersensitivityto dinitrochlorobenzene and mechlorethaminehydrochloride in cutaneous T cell lymphoma. J In-vest Dermatol. 1998;110:946–50.
47. Johnson MM, Leachman SA, Aspinwall LG, et al.Skin cancer screening: recommendations for data-driven screening guidelines and a review of the USPreventive Services Task Force controversy. Mela-noma Manag. 2017;4:13–37.
48. Du Vivier A, Vonderheid EC, Van Scott EJ, UrbachF. Mycosis fungoides, nitrogen mustard and skincancer. Br J Dermatol. 1978;99:61–3.
49. Trager MH, Chen C, Husain S, Geskin LJ. Nitrogenmustard gel-induced inflammation triggers lym-phomatoid papulosis in patients with mycosisfungoides. J Dermatol. 2020;47:546–50.
50. Latkowski JA, Heald P. Strategies for treating cuta-neous T-cell lymphoma: part 1: remission. J ClinAesthet Dermatol. 2009;2:22–7.
1106 Dermatol Ther (Heidelb) (2021) 11:1085–1106