c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and...
-
Upload
isabel-arnold -
Category
Documents
-
view
216 -
download
0
Transcript of c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and...

558 Research Paper
c-Myc activation in transgenic mouse epidermis results inmobilization of stem cells and differentiation of their progenyIsabel Arnold and Fiona M. Watt
Background: The epidermis is maintained throughout adult life by Address: Imperial Cancer Research Fund, 44Lincoln’s Inn Fields, London WC2A 3PX, Unitedpluripotential stem cells that give rise, via daughter cells of restricted self-Kingdom.renewal capacity and high differentiation probability (transit-amplifying cells),
to interfollicular epidermis, hair follicles, and sebaceous glands. In vivo,Correspondence: Fiona M. Watt
transit-amplifying cells are actively cycling, whereas stem cells divide E-mail: [email protected]. Experiments with cultured human keratinocytes suggest thatc-Myc promotes epidermal–stem cell differentiation. However, Myc is a Received: 17 January 2001
Revised: 26 February 2001potent oncogene that suppresses differentiation and causes reversibleAccepted: 1 March 2001neoplasia when expressed in the differentiating epidermal layers of
transgenic mice. To investigate the effects of c-Myc on the stem cellPublished: 17 Aprilcompartment in vivo, we targetted c-MycERe to the basal layer of transgenic
mouse epidermis.Current Biology 2001, 11:558–568
Results: The activation of c-Myc by the application of 4-hydroxy-tamoxifen 0960-9822/01/$ – see front mattercaused progressive and irreversible changes in adult epidermis. 2001 Elsevier Science Ltd. All rights reserved.Proliferation was stimulated, but interfollicular keratinocytes still underwentnormal terminal differentation. Hair follicles were abnormal, and sebaceousdifferentiation was stimulated at the expense of hair differentiation. Theactivation of c-Myc by a single application of 4-hydroxy-tamoxifen was aseffective as continuous treatment in stimulating proliferation and sebocytedifferentiation, and the c-Myc–induced phenotype continued to develop evenafter the grafting of treated skin to an untreated recipient.
Conclusions: We propose that transient activation of c-Myc driveskeratinocytes from the stem to the transit-amplifying compartment andthereby stimulates proliferation and differentiation along the epidermal andsebaceous lineages. The ability, demonstrated here for the first time, tomanipulate exit from the stem cell compartment in vivo will facilitate furtherinvestigations of the relationship between stem cells and cancer.
Background ture has defined a number of molecules that regulate exitfrom the stem cell compartment [1, 2, 5]. One is theThe epidermis is maintained throughout adult life by
pluripotential stem cells whose progeny differentiate to protooncogene c-Myc [5, 6]. The activation of c-Myccauses a progressive reduction in the growth rate of cul-form interfollicular epidermis, hair follicles, and sebaceous
glands [1–4]. Stem cell daughters that are destined to tured human keratinocytes without inducing apoptosis:this is due to a marked stimulation of terminal differentia-undergo terminal differentiation divide a small number
of times in the basal layer before withdrawing from the tion. c-Myc acts selectively on stem cells in culture anddrives them into the transit-amplifying compartment.cell cycle; these cells are known as transit-amplifying cells.
One role of transit-amplifying cells is, as their name im- Then, after a few rounds of division, they undergo termi-nal differentiation.plies, to increase the number of differentiated cells that
are generated by each stem cell division; in normal, un-damaged epidermis, stem cells divide infrequently,
In clonal cultures of human keratinocytes, only differenti-whereas transit-amplifying cells are actively cycling [1, 3].ation into interfollicular epidermis can be examined [1,A second feature of transit-amplifying cells, by analogy6], and so it is not possible to evaluate the effects of c-Mycwith the haemopoietic system [2], may be that their differ-on lineage commitment in vitro. In addition, stem cellsentiation potential is restricted to a particular lineage;are actively cycling in culture, and thus the ability of Mychowever, at present little is known about lineage commit-to recruit quiescent stem cells into the cell cycle cannotment in the epidermis.not be evaluated [6]. Furthermore, Myc is a potent onco-gene [7, 8] that has been reported to cause epidermalneoplasia [9]. Constitutive expression of c-Myc via theClonal analysis of human-epidermal keratinocytes in cul-

Research Paper c-Myc activation in epidermal stem cells Arnold and Watt 559
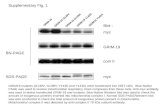
Figure 1
Transgene construction and expression.(a) Diagram showing the transgene cassette.(b) Dorsal skin of transgenic animal (founderline 2184C.1; treated daily with OHT for 4weeks; 3 months old when OHT treatmentwas started; hair cycle not synchronized)stained with an antibody to the murineoestrogen receptor (HL7). As a control,positive staining was specifically blocked bythe preincubation of the HL7 antibody with itsimmunogenic peptide (HL7 1 peptide). “HF”indicates a hair follicle; “BL” indicates thebasal layer of interfollicular epidermis; “SL”indicates the suprabasal layers. The scale barrepresents 50 mm. (c) Western blot analysiswas performed on keratinocytes cultured fromwild-type (WT) or transgenic mice by the useof a human c-Myc specific antibody (9E10)or, as a loading control, anti-actin.
loricrin promoter, which targets both the basal and su- the basal layer of interfollicular epidermis and along theentire length of the outer-root sheath of the hair follicleprabasal (differentiating) epidermal layers, results in su-
prabasal proliferation and reduced expression of terminal [11]. It thus includes both stem and transit-amplifyingcells [1, 4]. Immunostaining transgenic mouse skin con-differentiation markers in transgenic mice [10]. When an
inducible Myc transgene is selectively expressed in the firmed that the transgene was correctly expressed in thebasal layer of the epidermis and outer-root sheath of hairdifferentiating layers of mouse epidermis via the involu-
crin promoter, activation of c-Myc suppresses differentia- follicles (Figure 1b and unpublished data). Human c-Mycwas also detected by the Western blotting of extracts oftion and causes neoplastic changes; because cells express-
ing activated Myc are not permanent tissue residents, the keratinocytes cultured from transgenic but not from wild-type mice (Figure 1c).phenotype is, however, fully reversible [9].
While the effects of c-Myc on human keratinocytes in Although c-MycER protein was constitutively expressedin basal keratinocytes, as expected none of the founderculture are not consistent with the phenotype of trans-
genic mice expressing Myc in the epidermis, neither of lines showed any phenotype unless 4-hydroxy-tamoxifen(OHT) was applied to activate Myc function [6, 9, 12].the transgene promoters evaluated so far [9, 10] has selec-
tively targeted the basal layer, where the stem cells reside. In addition, we saw no systemic effects of topical OHTapplication to the dorsal skin of K14MycER mice, evenTherefore, to investigate the effects of c-Myc on the stem
cell compartment in vivo, we have used the keratin 14 though the K14 promoter is active in some internal epithe-lia [13]. OHT had no effects on the skin, including thepromoter [11] to express an inducible form of Myc,
c-MycERTM, in the basal layer of transgenic mouse epider- hair cycle, of wild-type mice. As a further control, twofounder lines expressing a mutant c-Myc with a deletionmis. The phenotype we observe is consistent with a role
for Myc in promoting exit from the stem cell com- in the transactivation domain (D106–143MycER) [6] weregenerated and found to exhibit no phenotypic responsepartment.to OHT (data not shown).
ResultsGeneration of transgenic mice c-Myc stimulates epidermal proliferation
The dorsal skin of K14MycER and wild-type animals wasFour founder lines of transgenic mice were generatedthat expressed the human c-Myc-2 cDNA fused to the treated daily with OHT for 4 weeks. The treated skin of
transgenic animals became greasy and stiff with some hairhormone binding domain (ERTM) of a mutant murine es-trogen receptor (MycER) [12] in a keratin 14 (K14) expres- loss, whereas wild-type mouse skin was unaffected by
OHT application (data not shown). Wild-type skin wassion cassette [11] (Figure 1a). Founder line 2184C.1 had70 copies of the transgene; 2184A had 25 copies; 2184C.2 histologically normal (Figure 2a–c), but transgenic skin
had thickened, hyperproliferative epidermis and abnor-had 2 copies; and 2184C.3 had 1 copy. ERTM lacks intrinsictransactivation activity; it responds to the synthetic steroid mal hair follicles; normal follicle architecture was no
longer visible, and the hair fibers were often missing (Fig-4-hydroxy-tamoxifen (OHT), but not to estrogen, and soin the absence of OHT MycER is inactive [6, 9, 12]. The ure 2d–f). Staining with the proliferation marker Ki 67
(which labels cells in late G1, S, G2, and M phases) re-K14 promoter directs transgene expression in all cells of

560 Current Biology Vol 11 No 8
Figure 2
Dorsal skin after 4 weeks of daily OHTtreatment. (a,b,c) Wild-type control mouse.(d,e,f) Transgenic mouse (2184 C.1). Micewere 3 months old when OHT treatment began;the hair cycle was not synchronized. (a,d)Hematoxylin and eosin staining. (b,e)Immunostaining with anti–Ki 67 as a markerfor proliferating cells. (c,f) Immunostainingwith an antibody to keratin 6. Stratum corneumstaining in (c) is nonspecific. “IE” indicatesthe interfollicular epidermis; “HF” indicates ahair follicle. The scale bars represent 50 mm.
vealed an increase in the number of actively cycling cells by impaired terminal differentiation [17]. To distinguishbetween these potential effects of c-Myc, we examinedin transgenic skin, the proliferative cells in interfollicular
epidermis being predominantly in the basal layer (Figure differentiation of three lineages founded by epidermalstem cells: interfollicular epidermis, sebaceous glands,2b,e). The interfollicular epidermis was also highly posi-
tive for keratin 6, a marker for epidermal hyperprolifera- and hair follicles [1, 3, 4].tion, which in wild-type animals is expressed exclusively
In normal interfollicular epidermis, keratin 1 is expressedin the hair follicles [14] (Figure 2c,f). Staining for markersin all of the suprabasal layers (Figure 3c), whereas inof T lymphocytes, neutrophils, and macrophages revealedneoplastic lesions keratin 1 expression is reduced or ab-only a small increase in immune infiltrate in OHT-treatedsent [9, 10, 17, 18]. Even though OHT-treated transgenictransgenic skin (data not shown). These results make itepidermis was much thicker than control epidermis (Fig-unlikely that inflammation was responsible for the epider-ure 3a,e), keratin 1 was expressed in all the suprabasalmal hyperproliferation. The phenotype was observed inlayers (Figure 3g). In addition, we noted an increasedall founder lines, although it was milder in the low thannumber of keratin 1–positive cells in the basal layer ofthe high copy number lines (data not shown). Transgenictransgenic epidermis (Figure 3c,g), which might be indica-mice aged between 7 weeks and 4 months were treatedtive of the premature initiation of terminal differentiation.with OHT; they developed the same phenotype regard-The expression of involucrin, filaggrin, and loricrin, mark-less of age or stage of the hair growth cycle.ers of later stages in the differentiation pathway [17, 18],was normal (Figure 3b,f and unpublished data), and theInterfollicular epidermal differentiation is not perturbed
by c-Myc activation outermost, cornified cell layers were anucleate in bothc-Myc is known to be a potent stimulus for cells to enter transgenic and wild-type skin (Figure 2a,d and 3a,e). Su-the cell cycle [15]. In cultures of human keratinocytes, prabasal expression of the a6b4 integrin is associated withboth stem and transit-amplifying cells are actively cycling, malignant progression in mouse skin carcinogenesis [19];and the activation of MycER with OHT does not, there- however, a6b4 remained confined to the basal layer offore, recruit stem cells into the cell cycle; in addition, it OHT-treated transgenic epidermis, as in normal epider-does not increase the number of rounds of cell division mis (Figure 3d,h). Thus, although OHT-treated trans-that transit-amplifying cells undergo prior to terminal dif- genic interfollicular epidermis was hyperproliferative, theferentiation, nor does it affect the length of the cell cycle keratinocyte terminal differentiation program was essen-[6]. In contrast to the situation in culture, in normal epider- tially normal, and the thickened epidermis comprised in-mis stem cells are rarely in cycle, whereas transit-ampli- creased layers of differentiating cells rather than an in-fying cells are actively cycling [16]. If the increase in creased number of basal layers.Ki 67–positive cells in OHT-treated transgenic mouseepidermis reflected the recruitment of quiescent stem Activation of c-Myc stimulates sebocyte differentiation
To examine differentiation of sebaceous gland cells (sebo-cells into the cycle and entry into the transit-amplifyingcompartment, the consequence would be excessive pro- cytes), we stained heavily affected transgenic and wild-
type skin with Oil Red O, a histochemical dye specificduction of terminally differentiated cells. Alternatively,if it reflected the neoplastic conversion of basal cells, the for lipid-containing cells, including sebocytes (Figure
4a,b). In wild-type skin, sebaceous glands were associatedincrease in Ki 67–positive cells might be accompanied

Research Paper c-Myc activation in epidermal stem cells Arnold and Watt 561
Figure 3 with the upper part of each hair follicle (Figure 4a, arrow).In transgenic mice, the number of sebocytes was greatlyincreased and filled the center of the abnormal hair folli-cle–derived structures (Figure 4b, arrows). There was noincrease in apoptotic cells in interfollicular epidermis (Fig-ure 4c,d), and this result is consistent with other studiesshowing that keratinocytes are resistant to c-Myc–inducedapoptosis [6, 9, 10]; although the lesions showing sebocytedifferentiation were strongly labeled with TUNEL, thestaining was diffuse throughout the cells rather than con-centrated in the nucleus and may therefore be nonspecific(Figure 4c,d). Further evidence that c-Myc promoted seb-ocyte differentiation came from examining the specializedsebaceous glands of the eyelid (exposed to OHT duringgrooming), the meibomian glands. Similar to the seba-ceous lesions of dorsal skin, the meibomian glands ofaffected mice were enlarged (arrows in Figure 4e,f). Somedifferentiated sebocytes continued to proliferate, and inOHT-treated skin some sebocytes migrated from the hairfollicles into the overlying epidermis (data not shown);this premalignant change is a feature of human sebaceouscarcinomas [20].
c-Myc stimulates sebocyte differentiation at the expenseof hair differentiationThe third type of differentiation we examined was theformation of hair follicles. Hair follicles continually un-dergo a defined cycle of growth (anagen), followed byinvolution (catagen) and a resting phase (telogen) [21].Although there was hair loss in some OHT-treated trans-genic mice, telogen follicles were capable of initiatinganagen and the number of hair follicles in the skin wasunaltered (unpublished data). However, treated anagenfollicles had abnormal morphology, and increased num-bers of sebocytes were observed (Figure 4h,j). Sebaceousdifferentiation was also stimulated by OHT in telogenfollicles (Figure 4g,i). Severely affected transgenic skintreated with OHT for 4 weeks and containing aberrantfollicles was stained with AE13 [22] and anti-Ha5 [23],which recognize hair-specific keratins in the cells of thehair matrix and cortex of normal anagen follicles. No posi-tive staining was observed (unpublished data). It thusappears that c-Myc stimulates sebaceous differentiationat the expense of hair differentiation.
Transient activation of c-Myc is as effective as sustainedExpression of differentiation markers in dorsal interfollicular epidermis activation in inducing epidermal hyperproliferationafter 4 weeks of daily OHT treatment. Mice were 3 months old when and sebocyte differentiationOHT treatment began, and the hair cycle was not synchronized. (a–d)
The use of the MycER transgene allowed us to examinewild-type control mouse. (e–h) Transgenic mouse (2184 C.1). (a,e)Hematoxylin and eosin staining. (b,f) Anti-involucrin (inv; BL, basal the effects of transient c-Myc activation. The initial half-layer; SL, suprabasal layers), (c,g) Anti–keratin 1 (K1; arrowheads life of OHT is less than 3 days [24], and when the appliedindicate examples of K1-positive cells in the basal layer); (d,h) Antibody dose of OHT was halved, some of the K14MycER micerecognizing the a6b4 integrin. The scale bar represents 50 mm.
failed to develop a skin phenotype (data not shown).Thus, within 3 days after the last dose of OHT, thetransgene should no longer be activated. Transgenic andwild-type animals were treated with OHT for up to oneweek and then monitored for another 6 days without

562 Current Biology Vol 11 No 8
Figure 4
(a–f) Sebaceous differentiation ofhyperproliferative lesions and (g–j) effectsof activated c-MycER on hair follicles.(a–d,g–j) dorsal skin; (e,f) eyelid (longitudinalsections). (a,c) Wild-type control mouse (4weeks of daily OHT treatment), (b,d)transgenic mouse (2184 C.1; 4 weeks of dailyOHT treatment). (a,b) Oil Red O staining withhematoxylin and eosin counterstain; note thatsubcutaneous fat stained positive with OilRed O in (a). (c,d) TUNEL staining (green)with propidium iodide counterstain (red).(a–d) Mice were 3 months old when OHTtreatment began; and the hair cycle was notsynchronized. (e–j) Hematoxylin and eosinstaining. (e) Wild-type control mouse (7 daysof OHT treatment). (f) Transgenic mouse(2184 C.1; 7 days of OHT treatment).Meibomian glands (MG) are outlined witharrows. (g,h) Wild-type control animals. (i,j)Transgenic animals (2184C.1), 7 days of OHTtreatment. Hair follicles in (g,i) telogen or (h,j)anagen are shown. Mice in (e–g,i,j) were 2months old at the onset of anagen, whenOHT treatment began. (h) shows anagenfollicles from a 3-month-old mouse. The scalebars represent 100 mm.
further application of OHT (Figure 5a). The transgenic during grooming. This transfer affected the oral epi-thelium.phenotype was not reversed but became much more se-
vere after termination of the OHT treatment (Figure 5a).Even a single treatment with OHT caused progressive To analyze later time points and to more stringently test
whether the epidermal phenotype progressed in the ab-changes in the epidermis (Figure 5b), and in some animals(whether receiving one or multiple doses of OHT) sponta- sence of OHT treatment, we gave wild-type and trans-
genic mice a single dose of OHT, and at intervals thereaf-neous wounds developed (see, for example, Figure 5b,day 15). The mice could not be monitored at later time ter we removed the treated areas and grafted them onto
immunocompromised recipient mice. The grafts were re-points; they were sacrificed because they exhibited exces-sive weight loss as a result of OHT transfer to the mouth covered 4 weeks from the date of OHT treatment. As

Research Paper c-Myc activation in epidermal stem cells Arnold and Watt 563
Figure 5
Effects of withdrawing OHT. (a) 7-week-old(hair follicles in telogen) transgenic(2184C.1) and wild-type (WT) control micewere treated daily for up to 7 days with OHT.OHT treatment was then stopped, and animalswere analyzed after a further 6 days. (b)transgenic (2184C.1) mice received a singledose of OHT and were analyzed 5, 9, 14,and 15 days later. (c) 4-month-old transgenic(2184C.1) and wild-type (WT) control micewere treated with a single dose of OHT. One(transgenic) or two (WT) weeks aftertreatment the animals were sacrificed, andpieces of skin containing telogen follicleswere either processed for histologyimmediately (upper panels) or were graftedsubcutaneously into nude mice and harvested4 weeks after the inital OHT treatment(bottom panels). “S” indicates areas ofextensive sebaceous differentiation. Dorsalskin sections stained with hematoxylin andeosin are shown. The scale bar represents100 mm.
illustrated in Figure 5c, the phenotype of the transgenic one dose of OHT and sacrificed 2 weeks later, ODCmRNA was absent, except in clusters of cells at the junc-skin was more severe than at the time of grafting and had
thickened, hyperproliferative epidermis and increased se- tion between the hair follicles and the epidermis (Figure6e,f). These cells have previously been shown to selec-baceous differentiation, whereas grafts of OHT-treated
wild-type skin had normal histology. tively express ODC in response to phorbol ester treatment[26]. Given that the abnormal follicle phenotype was morehighly developed in the one-dose animals examined 2Ornithine decarboxylase (ODC) is a well-documentedweeks later (Figure 6e) than in the animals treated contin-c-Myc target gene [25]. We therefore examined ODCuously with OHT for 7 days (Figure 6c) yet that ODCexpression in transgenics treated with a single dose ofmRNA was no longer detected in those follicles, we con-OHT as a marker of whether Myc activity could persistclude that continuous c-Myc activity was not required forin the absence of OHT (Figure 6). In wild-type epidermisthe progression of the phenotype.treated with OHT for 4 weeks, ODC mRNA was unde-
tectable in the interfollicular epidermis and upper portionof the hair follicles, as reported previously [9], although As the histological appearance of transgenic skin treated
continuously with OHT or with a single dose was similara signal was observed at the base of the follicles (Figure6a,b). In transgenic mice treated continuously for 7 days (Figure 5), it seemed likely that one dose of OHT was
sufficient to trigger prolonged epidermal hyperprolifera-(Figure 6c,d) or 4 weeks (unpublished data), ODC mRNAwas detected in the interfollicular epidermis and along tion. We examined this by staining for Ki 67 and by
labeling S phase cells with a 1 hr pulse of BrdU (Figurethe length of the hair follicles. In mice that were given

564 Current Biology Vol 11 No 8
Figure 6
ODC expression. In situ hybridization wasperformed on the dorsal skin of (a,b) a wild-type control mouse (the animal was 4 monthsold with an asynchronous hair cycle whenOHT treatment began), (c,d) a 4-month-oldtransgenic (2184A) mouse, (e,f) a 3.5-month-old transgenic (2184A) mouse.(b,d,f) are darkfield views of (a,c,e), respectively. Arrowheads indicate ODC mRNA in (a,b) the baseof hair follicles, (c,d) interfollicular epidermisand abnormal follicles, and (e,f) the boundarybetween hair follicle and interfollicularepidermis. Asterisks in (e,f) indicate abnormalfollicles that do not show elevated ODCmRNA (compare [c,d]). The scale barrepresents 100mm.
7). Five days after a single dose of OHT, Ki 67 and BrdU crease in the number of differentiated cell layers. Wepropose that transient c-Myc activation recruits quiescentlabeling were stimulated; the increase in labeling was still
evident 14 days after OHT treatment (Figure 7a–i and stem cells into cycle and thereby promotes entry into thetransit-amplifying compartment, which in turn leads tounpublished data). When epidermis that had a single
OHT dose was compared with epidermis treated continu- terminal differentiation along the sebaceous and interfol-licular epidermal lineages (Figure 8). Why sebocyte differ-ously for an equivalent period of time, the degree of
labeling was similar (see, for example, Figure 7b,g,e,j). entiation is stimulated at the expense of hair type differen-tiation is unclear at present, but it may reflect the needThus, a single dose of OHT resulted in sustained prolifer-
ation, accounting for the progressive thickening of the for additional inducing factors, in particular Wnts, for hair-type differentiation [27].interfollicular epidermis with time after treatment.
Discussion One characteristic of transit-amplifying cells is that theyundergo only a small number of rounds of division (vari-The aim of our experiments was to test whether activation
of c-Myc in basal keratinocytes in vivo stimulated exit ously estimated as 3–5 [1]) prior to terminal differentia-tion. If exit from the stem cell compartment is stimulatedfrom the stem cell compartment, as observed in vitro
[6], or whether it suppressed terminal differentiation, as at the expense of self-renewal, divisions in the transit-amplifying compartment should eventually be exhaustedobserved when Myc is selectively activated in the differ-
entiating epidermal layers [9]. A single application of and the epidermis should become thinner, with loss ofproliferation in the basal layer. However, even 30 daysOHT was as effective as repeated treatments in stimulat-
ing proliferation. However, the terminal differentiation after a single dose of OHT, the epidermis of K14MycERmice was hyperproliferative (Figures 5 and 7). There arepathway in interfollicular epidermis was executed nor-
mally, the thickening of the epidermis reflecting an in- two possible explanations for this. One is that, as the

Research Paper c-Myc activation in epidermal stem cells Arnold and Watt 565
Figure 7
Ki 67 and BrdU labeling of dorsal skin. (a–e)Ki 67 staining. (f–j) BrdU labeling (green)with a propidium iodide counterstain (red).Green fluorescence of hair shafts isnonspecific. (a,f) Untreated 7-week-oldtransgenic mouse (hair follicles in telogen);(b–d,g–i) 7-week-old transgenic mice intelogen were treated with one dose of OHTand sacrificed (b,g) 5 days, (c,h) 9 days, or(d,i) 14 days later. (e,j) 8.5-week-oldtransgenic mouse treated continuously withOHT for 6 days; the area shown is in telogen.The scale bar represents 50 mm.
keratin 14 promoter is expressed in both stem and transit- and transit-amplifying cells that are equivalent to thosecurrently available for human epidermis [1, 5, 6], it shouldamplifying cells [11], Myc activation increases the number
of rounds of division that transit cells undergo prior be possible to use the K14MycER mice to obtain anestimate of the size of the transit-amplifying compartmentto terminal differentiation. In culture, the activation of
MycER with OHT does not increase the number of transit and to determine whether the number of rounds of divi-sion that transit-amplifying cells undergo prior to terminalamplifying–cell divisions, nor does it affect the length of
the cell cycle [6]; however, in culture the stem cells are differentiation is fixed or responsive to environmentalmodulation. A further issue is whether Myc is acting solelyactively cycling, whereas in vivo they cycle infrequently.
The second possibility is that although the production of on the keratinocyte proliferation machinery or has addi-tional indirect effects, such as altered production oftransit-amplifying cells is stimulated, the stem compart-
ment continues to be replenished. growth factors [9], that could affect the interaction be-tween the epidermis and the underlying mesenchymalcells [28].By the discovery of markers of mouse epidermal stem

566 Current Biology Vol 11 No 8
Figure 8 be a consequence of c-Myc misexpression. The activationof Myc in basal keratinocytes could also contribute toneoplasia because any basal keratinocyte that has accumu-lated genetic lesions resulting in resistance to cell lossthrough Myc-induced terminal differentiation or apo-ptosis would clearly have a selective growth advantageand the potential to form a tumor.
ConclusionsThe phenotype of the K14MycER mice is consistent witha role for c-Myc in stimulating exit from the epidermalstem cell compartment. The mice allow us to manipulatethe adult stem cell compartment in vivo and are a valuabletool for exploring the factors that regulate lineage deci-sions by epidermal stem cells, as well as the relationshipbetween stem cells and cancer.
Model of the role of c-Myc in the epidermis. c-Myc is represented aspromoting differentiation of stem cells into transit-amplifying cellsspecific for the sebaceous and interfollicular epidermal lineages (red Materials and methodsarrows). The dotted red line and question mark indicate the possibility
Transgene constructionthat stem cell renewal is not inhibited.Human c-Myc-2 cDNA, fused at its carboxy terminus to the hormonebinding domain of a mutant murine estrogen receptor (c-MycER), wasexcised from pBluescript [12] as an EcoRI fragment, blunt-ended withKlenow DNA polymerase and cloned into the blunt-ended BamHI site
The observation that the effects of MycER on epidermal of a K14 expression cassette [11] (generous gift of E. Fuchs, HowardHughes Medical Institute, Chicago). The K14 expression cassette (Figureproliferation and differentiation progressed even after the1a) contains a 2100 bp AvaI fragment of the keratin 14 promoter/withdrawal of OHT is in contrast to the effects of MycERenhancer, a rabbit b-globin 59 untranslated region (UTR) together within suprabasal keratinocytes [9] or lineage-restricted hae- an intronic sequence upstream of a BamHI site, and the K14 39 UTR
mopoietic cells [29]. When MycER is expressed in su- followed by a polyadenylation site 39 downstream of the BamHI site.The transgene was excised from the parent plasmid as an EcoRI/HindIIIprabasal keratinocytes [9], phenotype reversal is evidentfragment, gel purified (Geneclean, Stratech Scientific), further purifiedwithin 7 days (S. Pelengaris, personal communication),with an elutip column according to the manufacturer’s instructionswhich corresponds to the estimated turnover time of(Schleicher and Schuell), and resuspended at a concentration of 5 mg/
mouse epidermis [30]. Whether the Myc-induced pheno- ml in sterile injection buffer (10 mM Tris-HCl, 0.1 mM EDTA [pH 7.4])type is reversible is likely to depend on whether the for pronuclear injection.targeted cell population consists of stem cells or differenti-ating cells. In the latter case, upon withdrawal of OHT, Generation of transgenic mice and determination of transgenecells with activated Myc will be lost from the tissue copy number
The transgene was injected into the male pronucleus of day-1-fertilizedthrough terminal differentiation, and the differentiation(CBA 3 C57BL/6) F1 embryos. Founders were backcrossed to establishcompartment will be replenished by a pool of stem cellslines of animals. Animals were screened for the presence of the transgenethat are not expressing MycER. In contrast, we are tar-by PCR of ear DNA with primers specific for the transgene (one primer
geting the stem cells, which are permanent tissue resi- was specific for the b-globin 59 UTR, 59-TACTCTGAGTCCAAACdents. In this context it is striking that the increased CGGGC-39; the other specific for c-myc-2, 59-AGCCTGGTAGGA
GGCCAGCTTCTCTGA-39). To determine transgene copy number, weproliferation in the basal layer is not dependent on contin-performed Southern blotting on genomic DNA isolated from tail snipsuous activation of the transgene. One potential mecha-and digested overnight with HindII and SacI. Blots were probed with a
nism by which transient activation of Myc could result in radiolabeled 1.2 kb human c-myc-2 cDNA probe and with a probe tosustained effects on the behavior of keratinocytes would the single copy gene interleukin 2 receptor a (as described previously)
[18].be Myc-induced histone acetylation and chromatin re-modeling [31], but this remains to be investigated.
Experimental treatments of miceThe c-MycER transgene was activated in transgenic mice by topicalWhile our data suggest that c-Myc promotes epidermalapplication of 4-hydroxy-tamoxifen (OHT; Sigma) to a shaved area ofdifferentiation, they do not exclude a proneoplastic role dorsal skin (1 mg dissolved in 0.2 ml ethanol; dose, 1 mg per mouse
for Myc in the epidermis. In normal epidermis c-Myc per day). Wild-type littermates were used as controls. No sex-specificeffects of OHT were observed. In some experiments, mice received anexpression is confined to the basal layer, whereas su-intraperitoneal injection of BrdU (0.1 mg/g body weight) 1 hr prior toprabasal, differentiating keratinocytes express memberssacrifice. In some experiments, pieces of dorsal skin (approximately 1of the Mad family [32–35], which are c-Myc antagonists. cm2) from OHT-treated wild-type and transgenic mice were grafted under
The neoplastic phenotype induced by the expression of the back skin of Balb/c nude mice, essentially as described previouslyfor grafts of reconstituted human skin [36].c-MycER in suprabasal keratinocytes [9] may therefore

Research Paper c-Myc activation in epidermal stem cells Arnold and Watt 567
6. Gandarillas A, Watt FM: c-Myc promotes differentiation ofHistology, immunostaining, in situ hybridizationhuman epidermal stem cells. Genes Dev. 1997, 11:2869-and TUNEL staining 2882.
Tissue samples were either fixed overnight in neutral buffered formalin 7. Grandori C, Cowley SM, James LP, Eisenman RN: The Myc/Max/and embedded in paraffin or frozen, unfixed, in OCT compound (Miles) on Mad network and the transcriptional control of cella frozen isopentane surface (cooled with liquid nitrogen). Five micrometer behavior. Ann Rev Cell Dev Biol 2000, 16:653-699.sections were prepared and stained with hematoxylin and eosin. 8. Pelengaris S, Rudolph B, Littlewood T: Action of c-Myc in vivo:
proliferation and apoptosis. Curr Opin Gen Dev 2000, 10:100-105.Immunostaining with antibodies to keratins, integrins, involucrin, and Ki
9. Pelengaris S, Littlewood T, Khan M, Elia G, Evan G: Reversible67 was performed essentially as described previously [18]. In addition,activation of c-Myc in skin: induction of a complexfrozen sections were stained with HL7, a rabbit antiserum to the murineneoplastic phenotype by a single oncogenic lesion. Mol Cellestrogen receptor (generous gift of H. Land, Imperial Cancer Research1999, 3:565-577.Fund), and with AE13 (generous gift of T.-T. Sun, New York University 10. Waikel RL, Wang X-J, Roop DR: Targeted expression of c-Myc
Medical School) and anti-Ha5 (kind gift of L. Langbein, DKFZ, Heidel- in the epidermis alters normal proliferation, differentiationberg), antibodies to hair keratins. For immunostaining with HL7, 0.5% and UV-B induced apoptosis. Oncogene 1999, 18:4870-4878.Triton X-100 was added to all the incubation buffers. For the confirmation 11. Vasioukhin V, Degenstein L, Wise B, Fuchs E: The magical touch:of the specificity of staining, HL7 antibodies were preincubated with genome targeting in epidermal stem cells induced bytheir immunogenic peptide (2 mg/ml) for 30 min at room temperature. tamoxifen application to mouse skin. Proc Natl Acad Sci USA
1999, 96:8551-8556.BrdU-labeled cells were detected in paraffin-embedded sections that12. Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI: Ahad been treated sequentially with HCl and trypsin [37] with a mouse
modified oestrogen receptor ligand binding domain as anmonoclonal antibody to BrdU (Becton Dickinson) and Alexa 488–improved switch for the regulation of heterologous proteins.conjugated goat anti mouse IgG.Nucl Acids Res 1995, 23:1686-1690.
13. Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E: Tissue-specificIn situ hybridization was performed as described by Pelengaris et al. [9] and differentiation-specific expression of a human K14with 35S-labeled riboprobes. Hybridization with a b-actin antisense probe keratin gene in transgenic mice. Proc Natl Acad Sci USA 1989,served as a positive control. 86:1563-1567.
14. Heyden A, Lutzow-Holm C, Clausen OPF, Brandtzaeg P, HuitfeldtApoptosis was detected in paraffin-embedded sections by the TUNEL HS: Expression of keratins K6 and K16 in regeneratingmethod with the Apoptosis Detection System and Fluorescein (Promega) mouse epidermis is less restricted by cell replication than
expression of K1 and K10. Epith Cell Biol 1994, 3:96-101.per the manufacturer’s instructions. TUNEL-labeled sections were coun-15. Elend M, Eilers M: Cell growth: downstream of Myc - to growterstained with propidium iodide. Oil Red O staining was performed on
or to cycle? Curr Biol 1999, 9:R36-R38.frozen sections, essentially as described by Catalano and Lillie [38].16. Potten CS, Morris RJ: Epithelial stem cells in vivo. J Cell Sci
Suppl 1988, 10:45-62.Western blotting 17. Hennings H, Glick AB, Greenhalgh DA, Morgan DL, Strickland JE,Keratinocytes were isolated from transgenic and wild-type newborn- Tennenbaum T, Yuspa SH: Critical aspects of initiation,mouse skin and cultured in low calcium FAD 1 FCS 1 HICE, essentially promotion, and progression in multistage epidermalas described previously [18] except that the skin trypsinization procedure carcinogenesis. Proc Soc Exp Biol Med 1993, 202:1-18.
18. Carroll JM, Romero MR, Watt FM: Suprabasal integrin expressionwas carried out at 40C and disaggregation of keratinocytes was per-in the epidermis of transgenic mice results informed without stirring. Cells were cultured to confluence, then lysed indevelopmental defects and a phenotype resemblingSDS-PAGE sample buffer. Protein lysates (20 mg) were separated onpsoriasis. Cell 1995, 83:957-968.a 10% SDS-PAGE gel, transferred to a Hybond C super membrane
19. Tennenbaum T, Weiner AK, Belanger AJ, Glick AB, Hennings H,(Amersham) by semi-dry electroblotting, blocked in 4% nonfat dried milkYuspa SH: The suprabasal expression of a6b4 integrin is
(Marvel, Cadbury) in PBS for 30 min, incubated for 2 hr in 2% Marvel/ associated with a high risk for malignant progression inPBS/0.1% Tween 20 containing the primary antibody (9E10 anti-Myc mouse skin carcinogenesis. Cancer Res. 1993, 53:4803-4810.antibody, Santa Cruz; AC-40 anti-actin antibody, Sigma), washed three 20. Lever WF, Schaumburg-Lever G: Histopathology of the skin, 6th
times in PBS/0.1% Tween 20, incubated for 1 hr in 2% Marvel/PBS/ edn. Philadelphia: J. B. Lippincott Company; 1983.0.1% Tween 20 containing the peroxidase-conjugated goat anti-mouse 21. Paus R, Cotsarelis G: The biology of hair follicles. N Engl J Med
1999, 341:491-497.secondary antibody (Pierce), and washed three times in PBS/0.1%22. Lynch MH, O’Guin WM, Hardy C, Mak L, Sun T-T: Acidic and basicTween 20 and once in PBS. Immunocomplexes were detected with the
hair/nail (“hard”) keratins: their colocalization in upperECL system (Amersham).cortical and cuticle cells of the human hair follicle and theirrelationship to “soft” keratins. J Cell Biol 1986, 103:2593-
Acknowledgements 2606.We are most grateful to Alberto Gandarillas, Lutz Langbein, Stella Pelengaris, 23. Rogers MA, Winter H, Langbein L, Krieg T, Schweizer J: GenomicRichard Pouslom, and Nick Wright for their help and to the staff of the ICRF characterization of the human type I cuticular hair keratinHistopathology and Transgenic Units for expert technical assistance. I. A. is hHa2 and identification of an adjacent novel type I hair keratinthe recipient of a European Union Marie Curie Fellowship and a European gene hHa5. J Invest Dermatol 1996, 107:633-638.Molecular Biology Organization (EMBO) Long Term Fellowship. 24. Furr BJA, Jordan VC: The pharmacology and clinical uses of
tamoxifen. Pharmac Ther 1984, 25:127-205.25. Bello-Fernandez C, Packham G, Cleveland JL: The ornithineReferences
decarboxylase gene is a transcriptional target of c-Myc.1. Watt FM: Epidermal stem cells: markers, patterning and theProc Natl Acad Sci USA 1993, 90:7804-7808.control of stem cell fate. Philos Trans R. Soc Lond B Biol Sci
26. Gilmour SK, Aglow E, O’Brien TG: Heterogeneity of ornithine1998, 353:831-837.decarboxylase expression in 12-O-tetradecanoylphorbol-2. Watt FM, Hogan BLM: Out of Eden: stem cells and their niches.13-acetate-treated mouse skin and in epidermal tumors.Science 2000, 287:1427-1430.Carcinogenesis 1986, 7:943-947.3. Taylor G, Lehrer MS, Jensen PJ, Sun T-T, Lavker RM: Involvement
27. Gat U, DasGupta R, Degenstein L, Fuchs E: De novo hair follicleof follicular stem cells in forming not only the follicle butmorphogenesis and hair tumors in mice expressing aalso the epidermis. Cell 2000, 102:451-461.truncated b-catenin in skin. Cell 1998, 95:605-614.4. Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y:
28. Szabowski A, Maas-Szabowski N, Andrecht S, Kolbus A, Schorpp-Morphogenesis and renewal of hair follicles from adultKistner M, Fusenig NE, et al.: c-Jun and JunB antagonisticallymultipotent stem cells. Cell 2001, 104:233-245.control cytokine-regulated mesenchymal-epidermal5. Watt FM: Epidermal stem cells as targets for gene transfer.
Hum Gen Ther 2000, 11:2261-2266. interaction in skin. Cell 2000, 103:745-755.

568 Current Biology Vol 11 No 8
29. Felsher DW, Bishop JM: Reversible tumorigenesis by MYC inhematopoietic lineages. Mol Cell 1999, 4:199-207.
30. Potten CS: Cell replacement in epidermis (keratopoiesis) viadiscrete units of proliferation. Int Rev Cytol 1981, 69:271-318.
31. Cole MD, McMahon SB: The Myc oncoprotein: a criticalevaluation of transactivation and target gene regulation.Oncogene 1999, 18:2916-2924.
32. Hurlin PJ, Foley KP, Ayer DE, Eisenman RN, Hanahan D, Arbeit JM:Regulation of Myc and Mad during epidermaldifferentiation and HPV-associated tumorigenesis. Oncogene1995, 11:2487-2501.
33. Hurlin PJ, Queva C, Koskinen PJ, Steingrimsson E, Ayer DE, CopelandNG, Jenkins NA, Eisenman RN: Mad3 and Mad4: novel Max-interacting transcriptional repressors that suppress c-mycdependent transformation and are expressed during neuraland epidermal differentiation. EMBO J. 1995, 14:5646-5659.
34. Vastrik I, Kaipainen A, Penttila TL, Lymboussakis A, Alitalo R, ParvinenM, Alitalo K: Expression of the mad gene during celldifferentiation in vivo and its inhibition of cell growth in vitro.J Cell Biol 1995, 128:1197-1208.
35. Gandarillas A, Watt FM: Changes in expression of members ofthe fos and jun families and myc network during terminaldifferentiation of human keratinocytes. Oncogene 1995,11:1403-1407.
36. Levy L, Broad S, Zhu AJ, Carroll JM, Khazaal I, Peault B, et al.:Optimised retroviral infection of human epidermalkeratinocytes: long-term expression of transduced integringene following grafting on to SCID mice. Gene Therapy 1998,5:913-922.
37. Kim TW, Porter KL, Foley JF, Maronpot RR, Smart RC: Evidencethat mirex promotes a unique population of epidermal cellsthat cannot by distinguished by their mutant Ha-ras genotype.Mol Carcinog 1997, 20:115-124.
38. Catalano RA, Lillie RD: Elimination of precipitates in Oil Red Ofat stain by adding dextrin. Stain Technol 1975, 50:297-299.