Binary Ionic Polyatomic Ionic Acids Molecular...Binary Ionic Polyatomic Ionic Acids Molecular Iron...
Transcript of Binary Ionic Polyatomic Ionic Acids Molecular...Binary Ionic Polyatomic Ionic Acids Molecular Iron...
Chapter 4
Naming Binary and Complex Compounds
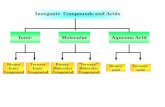
Binary Ionic Polyatomic Ionic Acids Molecular
Iron (III) Oxide
Sodium Carbonate
Nitrogen Trichloride
CuSO3
LiC2H3O2
Magnesium Sulfite
Ba3(PO4)2
Hydrochloric Acid
CaBr2
Aluminum Hydroxide
HNO3
CuI
Ammonium Phosphate
I2Cl2
Lead (II) Acetate
Compounds
Chapter 4
Naming Binary and Complex Compounds
Binary Ionic
Iron (III) Oxide
CaBr2
CuI
Compounds
Sodium Carbonate
CuSO3
LiC2H3O2
Magnesium Sulfite
Ba3(PO4)2 Aluminum Hydroxide
Ammonium Phosphate
Lead (II) Acetate
Polyatomic Ionic
Hydrochloric Acid HNO3
Acids
Nitrogen Trichloride
I2Cl2
Molecular
Chapter 4
Naming Binary and Complex Compounds
1. To learn to name binary compounds of a metal and nonmetal
2. To learn to name binary compounds containing only nonmetals
3. To summarize the naming of all types of binary compounds
Objectives
1. AxBy where A is a metal, B is a non-metal
2. CxDy where C and D are non-metals
Chapter 4
Naming Binary and Complex Compounds A. Naming Compounds That Contain a Metal and a
Nonmetal • Binary ionic compounds contain positive cations and
negative anions. – Type I compounds
• Metal present forms only one cation – Type II compounds
• Metal present can form 2 or more cations with different charges
Chapter 4
Naming Binary and Complex Compounds A. Naming Compounds That Contain a Metal and a
Nonmetal
Chapter 4
Naming Binary and Complex Compounds A. Naming Compounds That Contain a Metal and a
Nonmetal • For compounds containing both a metal and a nonmetal,
the metal is always named first. The nonmetal is named from the root element name.
Chapter 4
Naming Binary and Complex Compounds A. Naming Compounds That Contain a Metal and a
Nonmetal Type I Binary Ionic compounds
Use your Machine to identify and name the following: NaCl, MgO, LiBr, CaF2, Al2O3
Rules A
Chapter 4
Naming Binary and Complex Compounds A. Naming Compounds That Contain a Metal and a
Nonmetal Type II Binary Ionic compounds
• Since the metal ion can have more than one charge, a Roman numeral is used to specify the charge.
Rules B
Gold (I) Chloride Gold (III) Chloride
Chapter 4
Naming Binary and Complex Compounds A. Naming Compounds That Contain a Metal and a
Nonmetal
Type II Binary Ionic compounds
Chapter 4
Naming Binary and Complex Compounds
What are the Formulas that would be formed from the following ions and what are the Names of the compounds?
Cu+ and F-
Cu2+ and F-
Ni3+ and I-
Fe2+ and O2-
Pd4+ and Cl-
Ti4+ and P3-
Chapter 4
Naming Binary and Complex Compounds
Name the following compounds:
FeCl2
CoS
CoBr3
PbI2
CuO
SnO2
Fe2O3
Chapter 4
Naming Binary and Complex Compounds B. Naming Binary Compounds That Contain Only
Nonmetals (and Metalloids)
Type III Compounds – Non-metal/Non-metal
Rules C
Chapter 4
Naming Binary and Complex Compounds B. Naming Binary Compounds That Contain Only
Nonmetals (and Metalloids) Type III Compounds Name:
N2O CO2
PCl5
SiO2
NI3
SeO B2O3
XeF4
SO3
O2F2
Chapter 4
Naming Binary and Complex Compounds
Binary Compounds
What are the names of these compounds? (Use your machine to identify the type first)
CuO Ag2S
SrO ClF
B2O3 Na3P
TiCl4 MnF2
K2S NH3
OF2 CuCl2
Chapter 4
Naming Binary and Complex Compounds
Binary Compounds
Write the formula for the following compounds:
Scandium oxide
Xenon tetrafluoride
Nickel (III) sulfide
Dihydrogen monoxide
Sodium oxide
Copper (II) chloride
Chapter 4
Naming Binary and Complex Compounds
1. To learn the names of common polyatomic ions 2. To learn to name compounds containing polyatomic
ions 3. To learn how the anion composition determines an
acid’s name 4. To learn names for common acids 5. To learn to write the formula for a compound, given its
name
Objectives
Chapter 4
Naming Binary and Complex Compounds
A. Naming Compounds That Contain Polyatomic Ions
• Polyatomic ions are charged entities composed of several atoms bound together (“charged molecules”).
• Compounds should be electrically neutral • Use parentheses (when appropriate) in writing formulas
e.g Ni(CN)2
• They should be considered as other ions in determining names of compounds
• They have special names which should be memorized.
Chapter 4
Naming Binary and Complex Compounds
A. Naming Compounds That Contain Polyatomic Ions
• Naming ionic compounds containing polyatomic ions follows rules similar to those for binary compounds. – ammonium acetate
Rules D
Chapter 4
Naming Binary and Complex Compounds
Naming Compounds That Contain Polyatomic Ions
Name the following compounds (are they Type I or II metals?): - NaOH - Na2CO3
- CuOH - Cu(OH)2
- Cu2SO4
- Zn(NO3)2
- Fe3(PO4)2
- NaC2H3O2
- (NH4)2CO3
Chapter 4
Naming Binary and Complex Compounds
Give formulas for the following compounds
Sodium hydroxide
Barium phosphate
Magnesium acetate
Copper (I) nitrate
Iron (II) sulfate
Cobalt (III) carbonate
Chapter 4
Naming Binary and Complex Compounds
B. Naming Acids
• An acid is a molecule with one or more H+ ions attached to an anion. When dissolved in water the H+ ion and anion separate. Rules E
Chapter 4
Naming Binary and Complex Compounds
Name, and give the formula for, the acids made from the following anions: - Chloride
- Nitrate
- Bromide
- Cyanide
- Acetate
- Sulfite
- Sulfate
- Fluoride
- Phosphate
Chapter 4
Naming Binary and Complex Compounds
Acids You Should Know
• Hydrochloric Acid (HCl) – many industrial uses, stomach acid
• Sulfuric Acid (H2SO4) – major commercial chemical ~200 million Tons annually
• Nitric Acid (HNO3) – used to make fertilizers and explosives
• Acetic Acid (HC2H3O2) – vinegar • Carbonic Acid (H2CO3) – ocean acidification, sodas • Hydrofluoric Acid (HF) – etches glass, very toxic
Chapter 4
Naming Binary and Complex Compounds
Binary Ionic Polyatomic Ionic Acids Molecular
Iron (III) Oxide
Sodium Carbonate
Nitrogen Trichloride
CuSO3
LiC2H3O2
Magnesium Sulfite
Ba3(PO4)2
Hydrochloric Acid
CaBr2
Aluminum Hydroxide
HNO3
CuI
Ammonium Phosphate
I2Cl2
Lead (II) Acetate