Aplastic Anemia Review Etiology
description
Transcript of Aplastic Anemia Review Etiology
-
plastic anemia is defined as the failure ofbone marrow to produce blood cell compo-nents. The hallmarks of the disease are pancy-topenia and a hypocellular bone marrow.
Aplastic anemia is a rare disease, and approximately2000 patients are diagnosed in the United States everyyear.1 The estimated incidence is approximately twocases per million in Europe, and the incidence in Asiais two to three times higher.2 This geographic variationis more likely caused by environmental rather thangenetic elements, although individual susceptibility isan important factor.
ETIOLOGY
The most common causes of aplastic anemia are list-ed in Table 1. Inherited forms of the disorder are rareand consist of Fanconis anemia, dyskeratosis congenita,and Schwachman syndrome. Among patients with theacquired disorder, idiopathic aplastic anemia, in whichno cause is apparent, accounts for approximately 65%of all cases of aplastic anemia.
Secondary Aplastic Anemia
Secondary aplastic anemia occurs after exposure toenvironmental factors and in certain disorders. The fol-lowing factors have been implicated as causes of sec-ondary aplastic anemia: chemicals, drugs, infectiousagents, radiation, rheumatic disease, and pregnancy.
Chemicals. A definitive linkage between benzeneand aplastic anemia has been established from clinicaland epidemiologic data, as well as from animal and invitro studies.3,4 Despite this association, benzene is stillwidely used as a solvent and in the manufacture ofother chemicals, drugs, dyes, explosives, leather goods,and rubber. Chemicals used in insecticides (chlorophe-nothane), glue (toluene), and Stoddard solvent(petroleum distillates) have also been associated withaplastic anemia.
Drugs. Chloramphenicol was, at one point, the mostcommon cause of drug-induced aplastic anemia in the
United States. Anticonvulsant medications, in particularcarbamazepine and hydantoins, are also associated withthe development of aplastic anemia. The toxic metabol-ic intermediate of carbamazepine has been implicatedin fatal cases of aplastic anemia.5 Treatment with anti-neoplastic cytotoxic agents carries a high risk of aplasticanemia, and drugs such as gold salts, D-penicillamine,phenylbutazone, quinacrine, and acetazolamide havealso been implicated. Commonly used drugs such aspenicillin, furosemide, allopurinol, and nonsteroidalanti-inflammatory drugs (NSAIDs) are linked to a lesserdegree with aplastic anemia.
Infectious agents. Some viral infections, notablyinfectious mononucleosis caused by Epstein-Barr virus,have been associated with aplastic anemia. Whetheranemia results from a direct effect by the virus on thebone marrow or from a host immunologic response isunclear. The association between hepatitis and aplasticanemia is also strong, but anemia does not appear to berelated to infection with hepatitis viruses A, B, or C, andmay be caused by an unknown virus.6 Human par-vovirus B19, the virus that causes fifth disease, has beenlinked with pure red cell aplasia but not with severeaplastic anemia. Although some cases of aplastic ane-mia have been reported with human immunodeficien-cy virus (HIV) infections, most patients with HIV infec-tion have a cellular bone marrow, despite varyingdegrees of peripheral cytopenia.
Radiation. Repeated exposure to low doses of radi-ation has been associated with aplastic anemia. Singleexposure to high doses of radiation (such as after a nu-clear explosion) is more likely to lead to leukemiarather than aplastic anemia.
A
Dr. AlKhouri is Attending Physician, Department of Medicine, DoctorsHospital, Wentzville, MO. Dr. Ericson is Assistant Professor, Depart-ments of Medicine and Microbiology/Immunology and Blood andMarrow Transplantation Program, Mary Babb Randolph CancerCenter, West Virginia University, Morgantown, WV.
46 Hospital Physician May 1999
P r a c t i c e S t r a t e g i e s
Aplastic Anemia:Review of Etiology and Treatment
Nabiel AlKhouri, MDSolveig G. Ericson, MD, PhD
-
Rheumatic diseases. Connective tissue disorders suchas rheumatoid arthritis and systemic lupus erythematosushave been associated with aplastic anemia. However, itis not certain whether the drugs used to treat these dis-orders (NSAIDs, gold salts, allopurinol, D-penicillamine)cause the anemia or whether the activated immune sys-tem, which is a feature of these diseases, is the responsi-ble factor.
Pregnancy. Many cases of aplastic anemia have alsobeen found in association with pregnancy. However,because these cases showed variable clinical courses,the relationship between pregnancy and aplastic ane-mia is yet to be defined.
PATHOPHYSIOLOGY
The pancytopenia in aplastic anemia reflects failure ofthe hematopoietic process manifested as a severe de-crease in the numbers of all hematopoietic progenitorcells. Two mechanisms have been suggested for bonemarrow failure. The first mechanism is direct hematopoi-etic injury by chemicals (eg, benzene), drugs, or radia-tion to both proliferating and quiescent hematopoieticcells. The second mechanism, supported by clinicalobservations and laboratory studies, is immune-mediatedsuppression of marrow cells;7 examples of this mecha-nism are bone marrow failure after graft-versus-host-disease (GVHD), eosinophilic fasciitis, and hepatitis. Themechanism for idiopathic, pregnancy-associated, andsome cases of drug-associated aplastic anemia is not clearbut may involve immunologic processes as well. CytotoxicT cells are thought to mediate the suppressive effect on hematopoietic cells through the production ofhematopoiesis-inhibiting cytokines such as interferon-and tumor necrosis factor-.8 An immune-mediated sup-pressive effect on hematopoiesis may explain why mostpatients with acquired aplastic anemia respond to treat-ment with immunosuppressive therapy.
At presentation, patients with aplastic anemia usuallydo not have more than 10% of the normal number ofstem cells. However, laboratory studies show that stro-mal cells from patients with aplastic anemia can supportgrowth and development of normal hematopoieticstem cells and can also produce normal or increasedquantities of hematopoietic growth factors. Althoughthe success of bone marrow transplantation dependson adequately functioning stromal elements, clinicaland in vitro data do not support the use of hematopoi-etic growth factors alone in the treatment of aplasticanemia.
The pathophysiology of aplastic anemia, therefore,suggests two major approaches for treatment: replace-ment of deficient stem cells by bone marrow transplan-
tation and suppression of a destructive immunologicprocess.
CLINICAL PRESENTATION
The signs and symptoms of patients presenting withaplastic anemia are typically related to the decrease orabsence of peripheral blood cellular components.9
The clinical presentation ranges from insidious to dra-matic. Because platelets are depleted early in theprocess of the disease, dependent petechiae, bruising,gum bleeding, buccal hemorrhage, epistaxis, or retinalhemorrhage may be among the first presentations.Because of anemia, patients may complain of shortness
A l K h o u r i & E r i c s o n : A p l a s t i c A n e m i a : p p . 4 6 5 2
Hospital Physician May 1999 47
Table 1. Etiologic Classification of Aplastic Anemia
Acquired aplastic anemia
Idiopathic
Secondary
Chemicals
Benzene
Insecticides
Glue
Solvents
Drugs
Cytotoxic agents
Antibiotics
Nonsteroidal anti-inflammatory drugs
Anticonvulsive agents
Gold salts
Radiation
Viruses
Epstein-Barr virus
Non-A, non-B, non-C hepatitis viral agent (?)
Human immunodeficiency virus
Immune and rheumatologic diseases
Graft-versus-host disease
Rheumatoid arthritis
Systemic lupus erythematosus
Paroxysmal nocturnal hemoglobinuria
Pregnancy
Inherited aplastic anemia
Fanconis anemia
Dyskeratosis congenita
Schwachman syndrome
-
of breath, fatigue, or chest pain. Neutropenia or leuko-penia may result in fever, chills, or infections. Hepa-tosplenomegaly, lymphadenopathy, or bone pain areless common in patients with aplastic anemia, butthese findings should alert the physician to other diag-noses, such as infection, leukemia, or lymphoma.
DIAGNOSISDifferential Diagnosis
Pancytopenia is a common feature of many illness-es. Although the medical history, physical examination,and basic laboratory studies can often exclude aplasticanemia, the distinction is more difficult in certainhematologic diseases, and further testing is required.
Causes of pancytopenia that need to be consideredin the differential diagnosis include Fanconis anemia,paroxysmal nocturnal hemoglobinuria (PNH), myelo-dysplastic syndrome (MDS), myelofibrosis, aleukemicleukemia, agranulocytosis, and pure red cell aplasia.Each of these conditions is briefly reviewed in the fol-lowing discussion.
Fanconis anemia. This congenital form of aplasticanemia is an autosomal recessive inherited condition inwhich 10% of patients present beyond childhood.10,11
Typical physical stigmata include short stature, skinhyperpigmentation, microcephaly, thumb or radiushypoplasia, urogenital abnormalities, and mental retar-dation. Fanconis anemia is confirmed by cytogeneticanalysis of peripheral blood lymphocytes, which showchromosome breaks after culture with substances thatpromote chromosome stress (eg, diepoxybutane or mi-tomycin C).
Paroxysmal nocturnal hemoglobinuria. PNH is an
acquired disorder that is characterized by anemiacaused by intravascular hemolysis and manifested bytransient episodes of hemoglobinuria and life-threaten-ing venous thromboses.12 A deficiency of CD59, an ery-throcyte surface antigen that inhibits reactive lysis, islargely responsible for the hemolysis.13 Approximately10% to 30% of patients with aplastic anemia developPNH later in the clinical course.14 It is possible thatthe majority of patients with PNH have an underlyingaplastic process.15 The diagnosis of PNH is currentlymade by demonstrating decreased expression of thecell surface antigen CD59 by flow cytometry, replacingpreviously used screening tests such as the sucrosehemolysis test and examination of the urine for hemo-siderin.16
Myelodysplastic syndromes. The MDSs are a groupof clonal hematopoietic stem cell disorders that arecharacterized by abnormal bone marrow differentia-tion and maturation, which leads to bone marrow fail-ure with peripheral cytopenias, dysfunctional bloodelements, and probability of leukemic conversion. Thebone marrow in MDS is typically hypercellular or nor-mocellular, although hypocellularity may also be de-tected. It is important to distinguish hypocellular MDSfrom aplastic anemia because the diagnosis dictatesclinical management and prognosis. A critical featurethat identifies hypocellular MDS is an associated clonalcytogenetic abnormality (such as deletions in chromo-some arms 5q and 7q).17
Idiopathic myelofibrosis. The two major features ofidiopathic myelofibrosis are extramedullary hema-topoiesis (in spleen, liver, and other organs) and bonemarrow fibrosis. The extramedullary hematopoiesiscauses hepatosplenomegaly in the majority of patients.Bone marrow biopsy specimens show varying degreesof reticulin or collagen fibrosis, with prominent mega-karyocytes.
Aleukemic leukemia. Aleukemic leukemia, a rarecondition characterized by the absence of blast cells inthe peripheral blood of patients with leukemia, occursin fewer than 10% of all leukemic patients and is gen-erally seen in very young children or in elderly pa-tients. Bone marrow aspirate and biopsy demonstratethe blast cells.
Pure red cell aplasia. This rare disorder that in-volves only erythrocyte production is characterized bysevere anemia, a reticulocyte count of less than 1%,and a normocellular bone marrow containing less than0.5 % mature erythroblasts.
Agranulocytosis. Agranulocytosis is an immune dis-order that affects the production of blood granulocytesbut not that of platelets or erythrocytes.
48 Hospital Physician May 1999
A l K h o u r i & E r i c s o n : A p l a s t i c A n e m i a : p p . 4 6 5 2
Table 2. Diagnostic Criteria for Severe Aplastic Anemia*
At least two of the following:
Absolute neutrophil count
-
Diagnostic Evaluation
The hallmark of aplastic anemia is pancytopenia anda hypocellular bone marrow.2 A complete blood count isthe initial diagnostic study, and this study reveals varyingdegrees of anemia, thrombocytopenia, and leukopenia.Because of the hypoproliferative marrow, the reticulo-cyte response is low or absent despite the anemia.
Aplastic anemia is classified as mild, moderate, orsevere on the basis of the severity of the pancytopenia.Criteria for classifying aplastic anemia as severe are listedin Table 2. The most important prognostic factor is theabsolute neutrophil count (ANC). Patients with an ANCless than 0.5 x 109/L have a high risk of developinginfection and patients with an ANC less than 0.2 x 109/Lhave a poor prognosis.
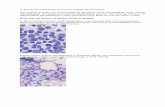
Bone marrow aspiration and biopsy must be per-formed to rule out other possible causes for pancytope-nia, such as MDS or leukemia. In normal bone mar-row, 40% to 60% of the marrow space is typicallyoccupied with hematopoietic cells (depending on theage of the person) (Figure 1A); by contrast, the bonemarrow in patients with aplastic anemia typically con-tains very few hematopoietic cells and consists primari-ly of fatty space and stromal cells (Figure 1B).
Human leukocyte antigen (HLA) typing should beperformed on all patients as soon as the diagnosis ofaplastic anemia is entertained. Tests for exposure toviruses, especially cytomegalovirus, should also be per-formed early because these tests assist in the choice ofblood products for transfusion.
Table 3 lists the relevant diagnostic studies that mustbe undertaken in patients with aplastic anemia.
TREATMENT Treatment Considerations and Supportive Care
The severity of aplastic anemia and the age of the pa-
tient are, in general, the two major considerations thatguide treatment.18 As an initial treatment strategy, all un-necessary medications that could be suppressing bonemarrow function should be discontinued. Occasionally,patients with mild to moderate aplastic anemia respondto treatment with androgens, which act by stimulatingerythropoiesis.19
Some patients with aplastic anemia may have de-creased production of cytokines that are required in he-matopoiesis. Administration of hematopoietic growthfactors (such as granulocyte colony-stimulating factorand granulocyte-macrophage colony-stimulating factor)as the sole treatment for aplastic anemia, however, hasnot been very successful.18 Growth factor administrationas an adjunct to immunosuppressive treatment or afterbone marrow transplantation has been useful in de-creasing periods of absolute neutropenia.
While diagnostic testing for aplastic anemia is beingundertaken and treatment options are being contem-plated, patients may require transfusions of bloodproducts. It is important that patients, particularly po-tential candidates for bone marrow transplantation,receive as few blood products as possible in order todecrease the risk of sensitization. Also, blood donationsfrom a family member should be avoided during thistime period. Younger patients may tolerate hemoglo-bin levels of 7 to 8 g/dL; older patients or patients witha history of coronary artery disease may require hemo-globin levels to be maintained at more than 8 g/dL.Platelet transfusions should be given when the patienthas an active bleeding episode or when the plateletcount is severely depressed (less than 10 x 109/L).Blood transfusion guidelines for patients with aplasticanemia are presented in Table 4.
Precautions for neutropenia should be followed inpatients with aplastic anemia. These patients should
A l K h o u r i & E r i c s o n : A p l a s t i c A n e m i a : p p . 4 6 5 2
Hospital Physician May 1999 49
A B
Figure 1. Bone marrow biopsy specimens from A) a healthy patient and B) a patient with aplastic anemia.
-
avoid fresh fruits and vegetables, focus on carefulmouth and dental care, wash hands frequently andthoroughly, minimize invasive procedures, and use astool softener. In patients with febrile neutropenia, abroad-spectrum antibiotic should be administeredafter cultures have been obtained from blood, urine,and any anatomic location in which infection is sus-pected.
In patients with severe aplastic anemia, supportivecare alone carries a poor prognosis. Hematologic re-covery occurrs in only 20% of patients, and approxi-mately 50% of patients die within 6 months of diagno-sis from complications such as bleeding or infections.20
Bone Marrow Transplantation
Bone marrow transplantation (BMT) from an HLA-matched sibling is the treatment of choice for severeaplastic anemia in patients age 60 years or younger(Figure 2). HLA typing of the patient and any poten-tial sibling donor(s) should be performed as soon asBMT has been identified as a treatment option. Sur-vival rates of 70% to 90% after BMT have been report-ed in a number of studies, with higher survival rates in patients younger than 40 years who receive HLA-identical BMT.2 The benefit of concomitant immuno-suppressive treatment was reported for a series of 40 patients with severe aplastic anemia who received aBMT from their genetically identical twins between1964 and 1992.21 Patients who received initial treat-ment with immunosuppressive agents (such as cyclo-
phosphamide or total body irradiation) had improvedrates of hematologic recovery (12 of 17), comparedwith patients who received genetically identical mar-row without preceding immunosuppressive treatment(seven of 23). Of the latter group, 13 of 15 patientswho received two to five additional transplants after im-munosuppressive treatment subsequently recoveredtheir marrow function.21 Thus, clinical findings frompatients receiving genetically identical BMT show thataplasia can result not only from a stem cell defect butalso from immunologically mediated suppression ofmarrow function.
Despite the excellent survival after HLA-matchedallogeneic BMT, the procedure carries potential risks.To prevent GVHD, treatment regimens include high-dose cyclophosphamide with or without antithymo-cyte globulin (ATG), which leads to prolonged peri-ods of immunosuppression and places the patient athigh risk for opportunistic infections. Other potentialproblems are graft failure, in which the donor bonemarrow either fails to take (primary graft failure), orgraft loss, in which the graft is rejected weeks or evenmonths after the BMT (delayed graft failure).1 The riskof graft failure increases with the number of bloodtransfusions before BMT. Intensive immunosuppres-sive therapy must be administered to prevent primaryand delayed graft failure.
Increasingly, matched unrelated donor (MUD)transplants have been performed in patients youngerthan age 40 years who do not have a matching siblingdonor, and response rates of 25% to 35% have beenobtained. MUD transplants for patients with aplasticanemia carry a high risk of GVHD or graft rejection,which can result in early death of the patient. Inpatients who are younger than age 40 years and wholack an HLA-identical related donor, immunosuppres-sive treatment should be started without awaiting the
50 Hospital Physician May 1999
A l K h o u r i & E r i c s o n : A p l a s t i c A n e m i a : p p . 4 6 5 2
Table 4. Blood Transfusion Guidelines for Patients withAplastic Anemia
Transfuse irradiated, leukocyte-filtered blood products
Transfuse cytomegalovirus-negative blood products to all patients until cytomegalovirus antibody titers are available
For cytomegalovirus-negative patients, administer cytomegalovirus-negative units only
Transfuse packed erythrocytes for severe or symptomatic anemia
Transfuse single-donor platelets if the platelet count is < 10 x 109/L or for active bleeding
Table 3. Diagnostic Studies and Additional LaboratoryTests for Aplastic Anemia
Diagnostic studies
Complete blood count with differential
Platelet count
Reticulocyte count
Bone marrow aspirate and biopsy (cytogenetic analysis and flow cytometry studies)
Additional laboratory tests
Human leukocyte antigen typing
Viral titers
Cytomegalovirus
Hepatitis A, B, and C viruses
Herpes simplex virus 1 and 2
Human immunodeficiency virus
Human parvovirus B19 viral antibody titers
Flow cytometric evaluation of CD59
-
result from a search for an unrelated donor becausethis process typically takes approximately 6 months.
Immunosuppressive Therapy
The risk of morbidity and mortality after BMT in-creases with age. Hence, immunosuppressive treatmentis considered first-line treatment for older patients andfor younger patients for whom a matched sibling donoris not available.
Immunosuppressive therapy, which typically consistsof ATG, cyclosporine, and corticosteroids, is aimed atsuppressing T-cell subsets that might exert a suppres-sive effect on bone marrow function. ATG, which isprepared from horse serum, can lead to allergic reac-tions or serum sickness, with patients developing fever,arthralgia, and skin rash.22 Steroid treatment canreduce or eliminate many of these symptoms. Cyclo-sporine inhibits interleukin-2 (IL-2) production by T cells and also inhibits proliferation of T cells inresponse to IL-2.19 Patients treated with cyclosporinerequire close monitoring because the drug may causerenal dysfunction or hypertension and also leads tointeractions with other drugs.
Intense immunosuppression with simultaneous ad-
ministration of ATG and cyclosporine has resulted inhematologic recovery in 70% to 80% of patients.22,23 In aseries of 51 patients, 67% of patients showed a responseto treatment within 3 months, and this number in-creased to 78% by 1 year.22 A patient was considered aresponder if the patients peripheral blood count nolonger met the criteria for severe disease. In a series of227 patients with severe aplastic anemia who were treat-ed with immunosuppressive therapy at a single centerover a period of 23 years (1978 to 1991), 78 patients(34%) achieved a complete or partial response, 23 pa-tients (10%) had a minimal response, 122 patients(54%) did not respond, and four patients (2%) werenot evaluable. Of the 122 nonresponders, 29 died within3 months of the start of treatment.24
PROGNOSIS
Before the advent of BMT and intensive immuno-suppressive therapy, the prognosis for patients withsevere aplastic anemia was dismalmore than 25% ofpatients died within 4 months of diagnosis and 50%died within 1 year.19 In previously untransfused patients,BMT has a cure rate of 75% to 85%; in patients whoreceive multiple transfusions before BMT, the cure
A l K h o u r i & E r i c s o n : A p l a s t i c A n e m i a : p p . 4 6 5 2
Hospital Physician May 1999 51
Figure 2. Treatment guidelines for patients with severe aplastic anemia.
Severe aplastic anemia
Yes
Yes
Yes No
No
No
Human leukocyte antigen-matched sibling
Immunosuppressive treatment
Bone marrow transplantation
Immunosuppressive treatment
Age < 40 years
Age < 60 years
Determine whether a matched unrelateddonor is available
Consider bone marrow transplantation froma matched unrelated donor if immunosup-
pressive treatment is unsuccessful
Continue immunosuppres-sive treatment
-
rates are 55% to 65%.19 However, 20% to 30% of allpatients undergoing BMT may suffer from severeGVHD. Approximately 15% of patients suffer a relapseof aplastic anemia. Older immunosuppressive regimensled to a significant improvement in blood counts in50% of patients,19 but with the recent intensive regi-mens, response rates as high as 78% by 1 year havebeen reported.22 Although 36% of the patients res-ponding to intense immunosuppressive treatment hada risk of relapse at 2 years, most of these patients res-ponded to additional courses of immunosuppression.22
As many as 40% of patients who initially respond toimmunosuppressive agents progress over a 10-year peri-od to PNH, acute myeloid leukemia, or MDS becauseof the underlying stem cell disorder.19
SUMMARY
Severe aplastic anemia is a rare disease, but this dis-ease must be included in the differential diagnosis of pa-tients presenting with pancytopenia. With prompt diag-nostic evaluation and appropriate initial supportive care,the majority of patients respond to aggressive treatmentwith immunosuppressive agents or to BMT. HP
REFERENCES1. Gajewski JL, Champlin RE: How to manage severe aplas-
tic anemia. Contemp Oncol May 1994:6982.2. Young NS, Bessler M, Casper JT, Liu J: Biology and ther-
apy of aplastic anemia. In Education Program of the Amer-ican Society of Hematology. McArthur JR, Schechter GP, eds.Orlando, FL: American Society of Hematology, 1996:113.
3. Shahidi NT: Acquired aplastic anemia: classification andetiologic considerations. In Aplastic Anemia and OtherBone Marrow Failure Syndromes. Shahidi NT, ed. New York:Springer-Verlag, 1988:2537.
4. Snyder R, Kocsis JJ: Current concepts of chronic ben-zene toxicity. CRC Crit Rev Toxicol 1975;3:265288.
5. Gerson WT, Fine DG, Spielberg SP, Sensenbrenner LL:Anticonvulsant-induced aplastic anemia: increased sus-ceptibility to toxic drug metabolites in vitro. Blood 1983;61:889893.
6. Brown KE, Tisdale J, Barrett AJ, et al: Hepatitis-associatedaplastic anemia. N Engl J Med 1997;336:10591064.
7. Young NS, Maciejewski J: The pathophysiology ofacquired aplastic anemia. N Engl J Med 1997;336:13651372.
8. Young NS: Immune pathophysiology of acquired aplas-tic anemia. Eur J Haematol Suppl 1996;60:5559.
9. Kelly JP, Jurgelon JM, Issaragrisil S, et al: An epidemio-logical study of aplastic anemia: relationship of drug
exposures to clinical features and outcome. Eur JHaematol Suppl 1996;60:4752.
10. Liu JM, Buchwald M, Walsh CE, Young NS: Fanconi ane-mia and novel strategies for therapy. Blood 1994;84:39954007.
11. Auerbach AD, Rogatko A, Traute M, Schroeder-KurthTM: International Fanconi Anemia Registry: relation ofclinical symptoms to diepoxybutane sensitivity. Blood1989;73:391396.
12. Rotoli B, Luzatto L: Paroxysmal nocturnal hemoglobin-uria. Baillieres Clin Haematol 1989;2:113138.
13. Yamashina M, Ueda E, Kinoshita T, et al: Inherited com-plete deficiency of 20-kilodalton homologous restrictionfactor (CD59) as a cause of paroxysmal nocturnal hemo-globinuria. N Engl J Med 1990;323:11841189.
14. Tichelli A, Gratwohl A, Wursch A, et al: Late haemato-logical complications in severe aplastic anemia. Br JHaematol 1988;69:413418.
15. Hillmen P, Lewis SM, Bessler M, et al: Natural history ofparoxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:12531258.
16. Hartmann RC, Jenkins DEJ: The "sugar-water" test forparoxysmal nocturnal hemoglobinuria. N Engl J Med1965;275:155157.
17. Kampmeier P, Anastasi J, Vardiman JW: Issues in thepathology of the myelodysplastic syndromes. HematolOncol Clin North Am 1992;6:501522.
18. Young NS, Barrett AJ: The treatment of severe acquiredaplastic anemia. Blood 1995;85:33673377.
19. Shadduck RK: Aplastic anemia. In Williams Hematology.Beutler E, Lichtman MA, Coller BS, Kipps TJ, eds. NewYork: McGraw-Hill, 1995:238251.
20. Champlin R, Ho W, Gale RP: Antithymocyte globulintreatment in patients with aplastic anemia: a prospectiverandomized trial. N Engl J Med 1983;308:113118.
21. Hinterberger W, Rowlings PA, Hinterberger-Fischer M,et al: Results of transplanting bone marrow from geneti-cally identical twins into patients with aplastic anemia.Ann Intern Med 1997;126:116122.
22. Rosenfeld SJ, Kimball J, Vining D, Young NS: Intensiveimmunosuppression with antithymocyte globulin andcyclosporine as treatment for severe acquired aplasticanemia. Blood 1995;85:30583065.
23. Bacigalupo A, Broccia G, Corda G, et al: Antilymphocyteglobulin, cyclosporin, and granulocyte colony-stimulatingfactor in patients with acquired severe aplastic anemia(SAA): a pilot study of the EBMT SAA Working Party.Blood 1995;85:13481353.
24. Doney K, Leisenring W, Storb R, Appelbaum FR:Primary treatment of acquired aplastic anemia: out-comes with bone marrow transplantation and immuno-suppressive therapy. Seattle Bone Marrow TransplantTeam. Ann Intern Med 1997;126:107115.
52 Hospital Physician May 1999
A l K h o u r i & E r i c s o n : A p l a s t i c A n e m i a : p p . 4 6 5 2
Copyright 1999 by Turner White Communications Inc., Wayne, PA. All rights reserved.