Anatomy and Pathology of the Cerebellar Peduncle - asnr. · PDF fileof the upper brains tem...
Transcript of Anatomy and Pathology of the Cerebellar Peduncle - asnr. · PDF fileof the upper brains tem...

Anatomy and Pathology of the Anatomy and Pathology of the CerebellarCerebellar Peduncle PeduncleToshio Moritani MD, PhD, Akio Hiwatashi MD, Henry Z Wang MD, PhD, Yuji Toshio Moritani MD, PhD, Akio Hiwatashi MD, Henry Z Wang MD, PhD, Yuji NumaguchiNumaguchi, MD, PhD, Leena Ketonen MD, PhD, Sven E Ekholm , MD, PhD, Leena Ketonen MD, PhD, Sven E Ekholm MD,PhDMD,PhD, Per-Lennart A Westesson MD, PhD, DDS, Per-Lennart A Westesson MD, PhD, DDS
Division of Diagnostic and Interventional Neuroradiology, Department of Radiology, University of Rochester Medical Center, Rochester NY Division of Diagnostic and Interventional Neuroradiology, Department of Radiology, University of Rochester Medical Center, Rochester NY FF E-mail: [email protected] E-mail: [email protected]
Infarction Infarction (Figures 2-5)
The inferior cerebellar peduncle is mainly supplied by the posteriorinferior cerebellar artery (PICA). The middle cerebellar peduncle issupplied by the anterior inferior cerebellar artery (AICA) and partly by thesuperior cerebellar artery (SCA). The superior cerebellar peduncle ismainly supplied by the SCA. These arteries vary greatly in origin, size,course, and supply area, the aera of infarction are variable in extent andlocation, ranging from a small infarct local ized into the cerebellarpeduncle (Figure 2 and 3) to large involvement of the cerebellarhemisphere, and can be associated with the involvement of pons,midbrain, thalamus and occipital lobe (Figure 4) (4). Bilateral AICAterritory infarcts are very rare (Figure 5), and can occur due tohypoperfusion in the watershed area between the AICA and the SCA (6).
Wallerian degeneration of the pontocerebellar tractsWallerian degeneration of the pontocerebellar tractssecondary to pontine hemorrhage or infarction secondary to pontine hemorrhage or infarction (Figure 6).
Wallerian degeneration secondary to pontine hemorrhage or infarction isusually bilateral. Differentiating from infarction is important. This is becauseof damage to both ipsilateral pontine nuclei (which deliver axons to thecontralateral cerebellar hemisphere) and the ipsilateral axons (whichoriginate from the contralateral pontine nuclei and course into the ipsilateralcerebellar hemisphere) (Figure 6G) (3). The T2 prolongations were firstrecognized from 26 days to 4.5 months after insult Theoretically, thewallerian degeneration of the pontocerebellar tracts should extend out tothe mossy fibers in the cerebellar cortex, as seen on the pathologicalspecimen; however this is beyond the resolution of MRI(3). Hypertrophicolivary degeneration occasionally coexists with these lesions, because thelesion can also involve the area in the Guillain-Mollaret triangle (Figure 6H)(7).
Figure 3. An infarct in the inferior cerebel lar peduncle. 57-year-old man with ataxia and diplopia.
A,B. T2WI and DWI at 24hrs after onset clearlyreveal homogenousround hyperintensityareas representing acuteinfarcts in the bilateralmiddle cerebellarpeduncles and bothcerebellar hemispheres.
A, B, FLAIR image andDWI show ahyperintense lesion,representing a smallacute infarct in the leftinferior cerebellarpeduncle.
Figure 5. Bilateral infarcts in the middle cerebellar peduncle. 80-year-old man with ataxia and vertigo.
A B
A B
G. Cerebellar connections. The cortico-ponto-cerebellar pathway(corticopontine tract and pontocerebellar tract) is major afferent fibersthrough the middle cerebellar peduncle. Olivocerebellar fibers (afferentfibers) are through the inferior cerebellar peduncle. Efferent fibersmainly arise from the dentate nucleus to the red nucleus through thesuperior cerebellar peduncle (cerebello-rubro-thalamic tract).
Osmotic Osmotic myelinolysismyelinolysis (Figure 7).
Symmetrical lesions in bilateral middle cerebellar peduncles without centralpontine myelinolysis (CPM) was reported as cerebellar peduncle myelinolysis(8). However, in our case, bilateral cerebellar peduncle lesions accompaniedby CPM (Figure 7). These lesions are presumed to be due to myelinolysisitself or secondary degeneration related to CPM.
Figure 6. Wallerian degeneraion in the middle cerebellarpeduncle. 50-year-old man with quadriparesis and loss ofconsciousness after chiropract ic.
F. On the pathologicalspecimen of anotherpatient, walleriandegeneration of thepontocerebellar tractsis recognized assymmetric roundedlesions (arrows) inbilateral middlecerebellar pedunclesand extends out to themossy fibers in thecerebellar cortex(open arrows)
(Ref. 3. Int J Neuroradiol1998;4:171-177.Toshihiro O'uchi MDwith permission).
A, B. DWI and T2WI shows hyperintense lesions in the right cerebellarhemisphere and the left side of the pons at 10 days after onset, whichrepresents subacute hemorrhagic infarcts.C, D. On follow-up MRI at 8 months after onset, old infarcts show very highintensity on T2WI and low intensity on FLAIR image as CSF. Symmetricalround hyperintense lesions in bilateral middle cerebellar peduncles are seenon T2WI and FLAIR image (arrows). These lesions represent walleriandegeneration of the pontocerebellar tracts secondary to pontine infarction.E. T2WI at the level of the medulla demonstrates symmetric hyperintenselesions corresponding to the bilateral olivary nuclei, representing hypertrophicolivary degeneration (arrows).
A B
F
G. Wallerian degenerationin bilateral middlecerebellar peduncles
The lesion involving thepontine nucleus can alsoinvolve the ponto-cerebellar tract from thecontralateral pontinenucleus, which results inwallerian degeneration inbilateral middle cerebellarpeduncles.
C D E
A. B. T2WI shows a hyperintense lesion in the central pons representing CPM.T2WI also shows symmetrical round lesions in the bilateral middle cerebellarpeduncles (arrows). These lesions maybe due to myelinolysis itself orsecondary degeneration.
Figure 7. CPM50-year-old femalepresenting with loss ofconsciousness afterrapid correction ofhyponatremia.
A B
Neurofibromatosis Neurofibromatosis (Figure 19)(Figure 19)
Hamarto matous lesions are observed in 80% of al l patients withneurofibromatosis type 1. Multiple lesions in the basal ganglia, opticradiation, brainstem, and cerebellar peduncles are common (Figure 19).Pathologically, these lesions are foci of hyperplastic or dysplastic glialproliferation and considered malformations rather than neoplasms.
Diffuse axonal injury (DAI) Diffuse axonal injury (DAI) (Figure 20)(Figure 20)
The gray-white matter interface, the corpus callosum, and dorsal aspectof the upper brainstem including the superior cerebellar peduncle arethree specific areas for the occurrence of DAI. Other less frequentlocations include the caudate nuclei, thalamus and internal capsule.Cerebellar involvement including the middle cerebellar peduncle isuncommon (Figure 20).
TumorTumor (Figures 17 and 18).
Benign tumors such as astrocytoma or cavernous angioma can involvein the cerebellar peduncle (Figures 17). An extra-axial tumor such asbenign acoustic schwannoma occasionally displaces the middlecerebellar peduncle (Figure 18). Malignant tumors such as metastasisor glioblastoma multiforme also o ccur in the cerebellar peduncle.Tumors involving the ventricular portion or the cerebellar portion of themiddle cerebellar peduncle can be removed by surgery (3).
A. FLAIR image shows ahyperintense lesion in the leftmiddle cerebellar peduncle tocerebellar hemisphere due toDAI.
B. DWI shows hyperintenselesions in the corpus callosumand bilateral internalcapsules, which is typicalfindings of DAI.
Figure 20. DAI. 29-year-old woman with DAI, presenting with loss ofconsciousness after motor vehicle accident.
A B
A,B T2WI and FLAIRimage showmultiple asymmetriclesions in the pons,middle and inferiorcerebellarpeduncles, andcerebellum.
Figure 19. Neurofibromatosis type 1. 4-year-old girl presentingwith developmental delay.
A B
Re feren ces
1) Carpenter MB. Cerebellum. In, Core Textbook of Neuroanatomy. 5th edition. Baltimore, MD: Williams &Wilkins, 1996;583-623.
2) Tomita T. Surgical management of cerebellar peduncle lesions in children. Neurosugery 1986;18:568-575.
3) O'uchi T. Wallerian degeneration of pontocerebellar tracts after pontine hemorrhage. Int J Neuroradiol 1998;4:171-177.
4) Amenco P, Rosengart A, DeWitt D, Pessin MS, Caplan LR. Anterior inferior cerebellar artery teritory infarcts. Mechanisms and clinicalfeatures. Arch Neurol 1993;50:154-161.
5) Milandre L, Rumeau C, Sangla I, Peretti P, Khalil R. Infarction in the territory of the anterior inferior cerebellar artery: report of five cases.Neuroradiology 1992;34:500-503.
6) Akiyama K, Takizawa S, Tokuoka K, Ohnuki Y, Kobayashi N, Shinohara Y.Bilateral middle cerebellar peduncle infarction caused bytraumatic vertebral artery dissection. Neurology 2001;56:693-694.
7) Kitajima M, Korogi Y, Shimomura O, Sakamoto Y, Hirai T, Miyayama H, Takahashi M.Hypertrophic olivary degeneration: MR imaging andpathologic findings. Radiology 1994;192:539-543.
8) Mangat KS, Sherlala K. Cerebellar peduncle myelinolysis: case report.Neuroradiology 2002;44:768-769.
9) Aydin K, Sencer S, Demir T, Ogel K, Tunaci A, Minareci O.Cranial MR findings in chronic toluene abuse by inhalation. AJNR Am JNeuroradiol 2002;23:1173-1179.
10) Uchino A, Kato A, Yuzuriha T, Takashima Y, Heijima S, Murakami M, Endoh K, Yoshikai T, Kudo S. Comparison between patientcharacteristics and cranial MR findings in chronic thinner intoxication. Eur Radiol 2002;12:1338-1341.
11) Thajeb P, Shih BF, Wu MC. Crossed cerebellar diaschisis in herpes simplex encephalitis. Eur J of Radiol 2001;38:55-58.
12) Pantano P, Baron JC, Samson Y, et al. Crossed cerebellar diaschisis: further studies. Brain 1986;109:677-694.
13) Chung HD. Retrograde crossed cerebellar atrophy. Brain 1985;108;811-889.
14) Rollins NK, Wen TS, Dominguez R. Crossed cerebellar atrophy in children: a neurologic sequela of extreme prematurity. Pediatr Radiol1995;25:S20-25.
15) Savoia rdo M , Strada L, Gir otti F, Zimm erma n RA, G risoli M, T esta D, Petr i llo R .Olivo pontocerebella r atr ophy:MR dia gnosis an d relationshipto mu ltisystem a troph y. R adiology 1990;174:693-696.
16) Nakagawa N , Katayama T , Makita Y, et al. A case of spinocerebella r ataxia type 6 mimicking olivo pontocerebellar at rophy. N eur orad iology199 9;41:503 -505.
17) Lee J, Lacomis D , Co mu S, et al. Acquired hepatocer ebr al dege nera tion : MR and pathologic findings. AJNR AM J Neur orad iol199 8;19:485 -487.
18) Brun berg JA , Jacquemont S, Hag erma n RJ, et al. Fr agile X prem utation car riers: C haracter i stic MR im aging findings of ad ult male patientswith pr ogr essive cereb ellar and cognitive dysfun ction. AJNR Am J Neur or adiol 2 002;23:1757-1766
19) van der Knaap MS, Ba rth PG , G abreels F J, et a l. A ne w leukoencepha lo pathy with vanish ing white matte r. Ne urology 19 97;48:845-855.
20) Ormerod IE, Miller DH, McDonald WI, du Boulay EP, Rudge P, Kendall BE, Moseley IF, Johnson G, Tofts PS, Halliday AM. The role ofNMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quantitative study. Brain 1987;110:1579-1616.
21) Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositivepatients: neuroimaging with clinical and pathologic correlation. Radiology 1993;187:233-40.
ConclusionConclusionLesions in the middle cerebellar peduncle include various pathologicalconditions, ranging from infarction, tumor, infection, trauma anddemyelination to primary and secondary degeneration. Understandingthe anatomy, pathology, imaging characteristics is important for thedifferential diagnosis of lesions in the middle cerebellar peduncle.
A. Coronal FLAIR image. B,C,D, Sagittal T1WI. E, Axial T2WI.
F. FLAIR image occasionally shows slightly high signal intensity in thenormal superior (not shown), inferior and middle cerebellar peduncles.
middle cerebellar peduncle (m), superior cerebellar peduncle (s),inferior cerebellar peduncle (i).
brain stem portion (B), ventricular portion (V), and cerebellar portion (C)of the middle cerebellar peduncle.
A B
C D
E F
m m
s s
i i
m m
m
i
i i
s
BVC
IntroductionIntroductionLesions in the cerebellar peduncle include variou s pathologicalconditions: infarction, various primary or secondary degeneration,demyelinatiing disease, toxic metabolic diaease, trauma, and benignand malignant tumors. Their differential diagnoses are occasionallydifficult. We illustrate the anatomy, pathology and imaging findings of thecerebellar peduncle.
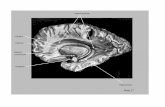
AnatomyAnatomyGross anatomy (Figure 1A-F).
The cerebellum is connected to the brainstem by three cerebellarpeduncles: 1) the inferior cerebellar peduncle (restiform body andjuxtrarestiform body) 2) the middle cerebellar peduncle (brachiumpontis), and 3) the superior peduncle (brachium conjunctivum) (1). Thewall and lateral roof of the 4th ventricle are formed by the inner surfacesof the cerebellar pedun cles; the rost ral port ion by the superiorpeduncles; and the caudal portions by the inferior peduncles (Figure1A-F). The middle cerebellar peduncle is lateral to inferior and superiorpeduncles and is not directly exposed to the cavity of the 4th ventricle.The middle cerebellar peduncle can be divided into three portions: brainstem portion, ventricular portion, and cerebellar portion (Figure 1E).This classification can be a useful application in surgery (2).
Cerebellar connections (Figure 1G).
The cerebellum is linked to other parts of the brain by numerous efferentand afferent fibers that are grouped together on each side of thecerebellum in three peduncles (1). Most of the afferent tracts enter thecerebellum via the inferior and middle cerebellar peduncles. A few entervia the superior cerebellar peduncle. Afferent fibers are far morenumerous that efferent fibers by a ratio of 40:1 (3). The cortico-ponto-cerebellar pathway, composed of the corticopontine tra ct and thepontocerebellar tract, i s major afferent f ibers through the middlecerebellar peduncle. It arises from the cerebral cortex and enter theipsilateral pontine nucleous and almost entirely crossed to thecontralateral cerebellum. Olivocerebellar fibers form the large stcomponent of the inferior cerebellar peduncle. Most of efferent tracts ofthe cerebellum are via the superior cerebellar peduncle. They mostlyarise from dentate nucleus and decussate at the levels through theinferior collicu lus. Most of the fibers enter the contralateral red nucleusand project the cerebral cortex via the thalamus.
Symptomatology
Lesions of the cerebellar peduncle result in variable clinical symptoms,ranging from vertigo or vomiting as the only clinical presentation to facialpalsy, ataxia, nystagmus, diplopia, dysphagia, dysarthria, deafness,contralateral motor weakness, trigeminal sensory loss, dysmetria of thelimb, loss of pain and temperature sense, Horner's syndrome, and"locked-in" syndrome (1,4,5).
Imaging of normal anatomy(Figure 1F).
The posterior fossa is difficult to evaluate on CT because of poorcontrast resolution and artifacts. MRI more clearly demonstrates theanatomy and pathology of the middle cerebellar peduncle in theposterior fossa than does CT. FLAIR images occasionally show a slightincrease in signal intensity in normal middle cerebellar peduncles(Figure 1F).
Figure 1. Normal anatomy.
Crossed cerebellar diaschisis and atrophy (Figures 10 and 11)
Crossed cerebellar diaschisis and atrophy presumed to be associated withtransneuronal metabolic depression in the cerebellum through cortico-ponto-cerebellar pathways (middle cerebellar peduncle) or other pathwayssu ch as cerebello-rubro-thalamic tract (superior cerebellar peduncle)(Figure 10) (12,13) Unilateral atrophy of the middle cerebellar peduncleand cerebellar hemisphere occurs as a sequela of ischemic or destructiveinjury of the contralateral cerebral hemisphere (14). These findings arefound in chi ldren with a history of extreme prematurity, perinatalintracranial hemorrhage, and recurrent seizures (Figure 11).
Solvent encephalopathySolvent encephalopathy (Figure 8)
There have been a few case reports of middle cerebellar peduncle lesionsin solvent encephalopathy (chronic toluene intoxication) in which thecerebral and cerebellar white matter, thalamus, basal ganglia, internalcapsule, and brain stem are also involved (9, 10) (Figure 8). Thesepatients' symptoms are usually composed of pyramidal tract and cerebellarsigns. The middle cerebellar peduncle lesions can be primary orsecondary degeneration.
A. T2WI shows right cerebral atrophy with ventricular dilatation representinga sequela of perinatal intracranial hemorrhage.
B. T2WI through the posterior fossa shows atrophy of the contralateralcerebellar middle cerebellar peduncle (arrow) and hemisphere.Wallerian degeneration of ipsilateral brain stem is also seen (arrow).
Figure 11. Crossedcerebellar atrophy
19-year-old female.She had a history ofrecurrent seizures andperinatal intracranialhemorrhage.
A B
Herpes encephalitis (Figure 9)
Bilateral middle cerebellar peduncle lesions were present in a patient withherpes encephalitis with bilateral temporal lobe involvement (Figure 9).The cause of these lesions is unknown. Secondary transneuronaldegeneration via bilateral cortico-ponto-cerebellar pathways may be one ofthe possible explanations for these lesions (F igure 6G) (11).
Figure 9.Herpesencephalitis
40-year-old malepresenting witha seizure.
A. T2WI shows diffuse hyperintense lesions in the cortex and white matterin bilateral temporal lobes, which represents herpes encephalitis.
B. Coronal FLAIR image shows hyperintense lesions in the bilateral middlecerebellar peduncles (arrows) and the temporal lobes.
A B
A,B A mass lesion is locatedin the ventricular portion tothe cerebellar portion ofthe left middle cerebellarpeduncle. It is high signalon T2WI and low signalon T1WI and with noenhancement (notshown). This lesion canbe removable by surgery.
A,B Axial T2WI and sagittal T1WI shows an extra-axial cerebellopontineangle mass lesion which deviates the left middle cerebellar peduncleposteriorly and superiorly (arrows).
Figure 18.Acousticschwannoma.
17-year-old femalepresenting withhearing loss andprogressive ataxia
Figure 17. Low grade astrocytoma. 4-year-old boy presenting withautism.
A B
A B
Figure 8. Solvent encephalopathy. 38-year-old man presenting with blurredvi sion, ataxi c speech and bilateralpyramidal signs.T2WI shows diffuse hyperintense lesions inthe white matter of both temporal lobes andmildly hyperintense lesions in the pons andbilateral middle cerebellar peduncles (arrows).
H. Guillain-Mollarettriangle and hypertrophicolivary degenerationThe lesion involving thepontine nuclei can extendinto the areas within theGuillain-Mollaret triangle,which causes hypertrophicolivary degeneration.
A, B, T2WI and DWI show ahyperintense lesion,representing an acuteinfarct in the right middlecerebellar peduncle.
A, B, FLAIR image and DWIshow hyperintensitylesionsin the leftcerebellar hemisphre,and the midbrainincluding the left superiorcerebellar peduncle.
A B
A B
Figure 2. An infarct in the inferior cerebellar peduncle. 72-year-oldman with vertigo.
Figure 4. infarcts involving the superior cerebellar peduncle. 58-year-old man with loss of consciousness.
A. FLAIR image shows hyperintensity lesions in the right cerebellarhemisphere (arrows) and contralateral diffuse cerebral hyperintensityassoci ated with status epilepticus.
B,C. FLAIR images at the level of the brain stem show a hyperintenselesion in the right superior cerebellar peduncle (arrows). Thesefindings suggest that crossed cerebellar diaschi sis of this case isrelated to retrograde transneuronal degeneration through thecerebello-rubro-thalamic tract.
Figure 10. Crossed cerebellar diaschisis
27-year-old male, presenting with status epilepticus. He has a historyof recurrent generalized seizures.
A B C
DemyelinatingDemyelinating disease: disease: multiple sclerosis (MS), acutemultiple sclerosis (MS), acutedisseminated encephalomyelitis (ADEM) and progressive disseminated encephalomyelitis (ADEM) and progressive multifocalmultifocalleukoencephalopathyleukoencephalopathy (PML) (PML) (Figures 14-16)(Figures 14-16)
Brain stem and cerebellar involvement including cerebellar peduncles iscommon in patients with MS and ADEM. Cerebellar symptoms and signsare commonly seen in 50-80% in MS patients. On MRI brainstem lesionsin 68% and cerebellar lesions in 49%-88% were detected (20). Theselesions in MS or ADEM are often bilateral but asymmetric (Figures 14 and15). In PML involvement of the posterior fossa including the cerebellarpeduncles is also common (32%). Isolated disease in the posterior fossa isin 10% of PML patients (21) (Figure 16).
A, B.T2WI shows multipleasymmetric hyper-intenselesions in the pons, middlecerebellar peduncles(arrows) cerebellarhemispheres, and in thedeep white matter, which ischaracteristicof MS.
Figure 14. MS. 40-year-old woman with multiple sclerosis, presentingwith speech disturbance and ataxia.
A B
LeukodystrophyLeukodystrophy and and leukoencephalopathyleukoencephalopathy (Figure 13).
Some kinds of leukodystrophy and leukoencephalopathy can also involve inthe cerebellar peduncles. This leukoencephalopathy with vanishing whitematter is an autosomal recessive disorder with chronic and progressiveepisodes of rapid deterioration, provoked by fever and minor head trauma.This is primarily an axonopathy, with myelin being secondarily affected (19)(Figure 13).
A. T2WI shows diffuse whitematter signal abnormalitiessimilar to CSF intensity.
B. T2WI also showshyperintense lesions in thecentral tegmental tracts(arrows), pyramidal tracts(arrows), and inferior andmiddle cerebellar peduncleswith atrophy (arrows).
Figure 13.Leukoencephalopathy with vanishing white matter.
An 11 year-old boy.
A B
Olivopontocerebellar atrophy (OPCA) and other primaryOlivopontocerebellar atrophy (OPCA) and other primarydegenerative diseases degenerative diseases (Figure 12).
OPCA is a degenerative disease characterized by atrophy of the pons,middle cerebellar peduncles, and cerebellar hemispheres. There arecharacteristic histologic changes, such as loss of specific fiber tracts andthe presence of gliosis in the pons, middle cerebellar peduncles andcerebellum (15). The fibers affected in the pons are the transverse pontinefibers, while the pyramidal tracts and tegmentum are spared (Figure 12).Increased hyperintensity in the middle cerebellar peduncles are alsoreported in other multiple system atrophy, autosomal do minantspinocerebellar atrophy (16), Wilson’s disease, non-Wilsonianhepatocerebral degeneration (17), and fragile X syndrome (18).
A, B. Axial and sagittal FLAIR images show hyperintensity in the middle(arrows) and inferior cerebral peduncles (arrows).
C. Axial FLAIR image also shows cruciform hyperintensity in the transversepontine fibers on the anterior and lateral aspect of the pons. Thetegmentum (arrows ) and the pyramidal tracts (arrows) are spared.
Figure 12. Ol ivopontocerebellar atrophy (sporadic type). 54-year-old woman presenting with dysarthric speech and dizziness.
A B C
A. T2WI shows an isolatedhyperintense lesion inthe right middlecerebellar peduncleextending into thecerebellar hemisphere.
B. Gd-enhanced T1WIshows this lesion ashypointensity with noenhancement.
Figure 16. PML. 25-year-old man presenting with right-sidedweakness and headache. He has had a history of HIV infection.
A B
Figure 15. MS. 57-year-old man presenting with speech disturbanceand ataxia.
A B
C D
A, B.T2WI and FLAIRimage showshyperintense lesions inthe pons, and inferiorcerebellar peduncles(arrows), and in thecallosomarginalinterface in the deepwhite matter, which ischaracteristic of MS.
C, D. Hyperintenselesions are also seenin the midbrain, andthe superior cerebellarpeduncle (arrows).