Acoustic neuroma
-
Upload
entdost -
Category
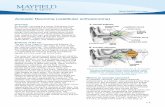
Health & Medicine
-
view
3.123 -
download
6
Transcript of Acoustic neuroma
- 1. By DR Mohammed Riyas
2. INTRODUCTION An acoustic neuroma (also called vestibular schwannoma) is a benign tumor arising from abnormally proliferative shwann cells , which envelope the lateral portion of the vestibular nerve in 3. Little things about CP angle 4. CP angle tumors Represents 10 % of all intracranial tumors. Fatal without treatment. VS account for 78 % of CPA tumors - mostly from vestibular branch of VIIIth Nerve. Variety of other tumors arise from this area like meningioma , CN swannomas , dermoid tumors , arachnoid cysts ,lipomas , 5. Anatomy of CP angle CPA Irregularly shaped potential space in the posterior fossa of the brain . Anteriorly posterior surface of temporal bone . Posteriorly anterior surface of the cerebellum. Medially cisterns of the pons & medulla and olive. Superiorly inferior border of pons 6. Anatomy of CP angle CN s V ( superiorly ) , IX,X,XI (inferiorly ) transverse the cephalic and caudal extent of the CPA. The central structures crossing the CPA to & from the IAC are CN VII & VIII s carrying with them a fine sheet of arachnoid tissue upto IAC. Schwann cells sorround these nerves beginning in the IAC , near the porus at the Obersteiner- Redlich zone. 7. Anatomy of CP angle CN s V ( superiorly ) , IX,X,XI (inferiorly ) transverse the cephalic and caudal extent of the CPA. The central structures crossing the CPA to & from the IAC are CN VII & VIII s carrying with them a fine sheet of arachnoid tissue upto IAC. Schwann cells sorround these nerves beginning in the IAC , near the porus at the Obersteiner- Redlich zone. 8. Anatomy of CP angle AICA is the main artery in the CPA and is the source of the labrynthine artery . The labrynthine artery courses via the IAC & is an end artery for the hearing and balance organs. 9. Vestibular schwannoma Nerve sheath tumors of the superior and inferior vestibular nerves. They arise in the medial part of the IAC or the lateral part of the CPA and cause clinical symptoms by displacing , distorting or compressing 10. Vestibular schwannoma Mean incidence range 9.1 tmr/yr to 13 tmr/yr( as per SB) 0.7 to 1.2 VS per lakh population/yr ( ballenger ) Types - Sporadic ( 95%) and non sporadic ( 5%) Age of presentation 40 to 60 yrs. Age of presentation is less in non sporadic ( 20-30 yrs ) 11. Tumor biology Equal frequency in sup and inf vestibular ( but recent japanese studies suggested 85 % from inf vestibular ) Arise from schwann cells within the IAC lateral to O-R zone in the area of scarpa ganglion. Schwannomas rarely arise from the cochlear nerve & are rarely 12. Tumor pathogenesis Owing to mutations in the gene for the tumor suppresor protein MERLIN located on chr 22q12. Formation of VS requires mutation of both copies of the merlin gene. Somatic mutations in both copies of merlin gene results in sporadic VSs . Familial VS occuring in NF 2 requires only one somatic mutation event .( inherit one ) 13. Tumor pathogenesis NF2 is autosomal recessive at gene level but inheritance is autosomal dominant ( pseudodominant ) A mutation in the normal allele leads to bilateral VS by the age of 20. Genetic screens for the NF2 mutation have been developed and are the basis for genetic counselling for family members of NF2 patients 14. Tumor pathogenesis Biochemical factors- VS express neuregulin ,which controls survival and proliferation of schwann cells and its receptors erbB2 & erbB3. FGF ,TGF B1 , PDGF & VEGF all these contribute to VS proliferation. VS may accelerate during pregnancy. 15. pathology GROSS : VS have a smooth surface with a yellow to gray color. Tumor is usually solid ,with occasional cystic components and therefore has a firm to soft texture depending on solid to cystic components. MICROSCOPIC : Capsule 3 to 5 micrometer in thickness. Two morphological tissue types Antony A & Antony B areas 16. T UMOR DEVELOPMENT Develops in nerve sheath Compresses rather than invading the nerve Gradually fills all the IAC Protrudes out of the porus 17. T UMOR DEVELOPMENT- extrameatally Extrameatal expansion into the large & empty pontine cistern Displacement and stretching of the VII & VIII th CN on the anterior Compress cerebellum and trigeminal N aspect of the tumor & of the AICA on the inferior aspect (During this time IAM continues to become more & more widened ) which leads gradually to hydrocephalus 18. T UMOR DEVELOPMENT- extrameatally Tumor may extent to the tentorium & can obstruct the cochlear aqueduct The AICA & lower cranial nerves are also displaced & become closely Overtime , the trigeminal & abducens Adherent to the inf surface of tumor. over the surface of the tumor and get thinned. 19. 1. 2. 3. 4. INTRACANALICULAR CISTERNAL COMPRESSIVE HYDROCEPHALIC 20. Tumor development.. Periods of growth are intermixed b/w slow growth & peroid of quescence. Occasionally tumor may undergo rapid expansion owing to cystic degeneration or hemmorhage into the tumor. The initial intracanalicular growth effects the vestibulocochlear nerve 21. Stage 1 Stage 2 Stage 3 22. Cerebellopontine Angle: 23. Large Acoustic Neuroma: Tumors over 2.5 centimeters (this one is 2.6 cm) become impacted into the brainstem and cerebellum. Complications associated with surgery and radiation are higher. It is difficult to deliver an adequate dose of radiation to control tumor growth without excessive dosing to the brainstem in tumors larger than this. 24. Symptoms & signs Intracanalicular: Hearing loss (UL progressive ), tinnitus, vertigo Loss of speech discrimination out of propotion to HL Cisternal: Worsened hearing and dysequilibrium Compressive: Occasional occipital headache CN V: Midface, corneal hypesthesia CN VII : Hitzelbergers sign, loss of taste and reduced lacrimation on Schirmers test ,facial weakness ( late) CN II , IV , VI : visual acquity and diplopia 25. Symptoms & signs Hydrocephalic: Fourth ventricle compressed and obstructed Headache, visual changes, altered mental status Nausea and vomiting On examination : ICP and pappiledema. Compression of CN IX & X Dysphagia , aspiration and hoarseness Poor gag reflex and VC paralysis. Cerebellar involvement( late ) Incoordination , widely based gate , tendency to fall owards affected side 26. Symptoms & signs Brainstem involvement: There is ataxia, weakness and numbness of arms and legs with exaggerated tendon reflexes. 27. Jackler Staging System STAGE TUMOUR SIZE Intra canalicular Tumour confined to IAC I (Small) 40mm 28. Tumor Growth Rate 29. Duration of Symptoms Prior to Diagnosis SYMPTOMS YEARS Hearing loss 3.9 Vertigo 3.6 Tinnitus 3.4 Headache 2.2 Dysequilibrium 1.7 Trigeminal 0.9 Facial 0.6 30. Diagnostic evaluation Average patient will require 4 years from the onset of symptoms to diagnosis. Majority will present with complaints of UHL, UT, Vertigo , dysequilibrium, facial numbness , weakness or spasm. Initial step in evaluation includes an audiologic assessment .if it suggests a retrocochlear lesion , then imaging of the CPA is performed . Vestibular testing lacks specificity in diagnosis of VS 31. AUDIOLOGICAL EVALUATION Includes PTA , Speech discrimination score (SDS) , Acoustic reflex threshold & acoustic reflex decay PTA of patients with VS shows assymetric , down sloping , high frequency SNHL in almost 70% of patients 32. AUDIOLOGICAL EVALUATION Retrocochlear HL causes SDS to be lower than predicted by the pure tone thresholds. This out of propotion is furthur accenuated when retested at a higher speech intensity ( roll over phenomenon ) Loss of acoustic reflex or acoustic reflex decay is noted in most patients with VS 33. audiological tests Cochlear Retrocochlear a) Pure tone audiometry Sensorineural hearing loss Sensorineural hearing loss b) Speech discrimination score 90% & specificity of > 90 % in detecting VS. 36. AUDITORY BRAINSTEM RESPONSE In 20-30 % there are no identifiable waveforms even with insignificant HL in higher frequencies. In 10-20 % only wave I is present. 37. BERA patterns in AN 38. Imaging studies VS is definitely identified MC via an imaging study. Earlier plain film radiograghs and polytomographs Introduction of CT in 1970 allowed axial imaging with improved bone & soft tissue evaluation. With the addition of iv iodinated contrast agent ,90 % of VS are enhanced furthur improving diagnostic accuracy. 39. Imaging studies Intracanalicular tumors & tumors extending less than 5 mm into the CPA frequently are missed with contrast enhanced CT. Accuracy improved by air-contrast cisternography. MRI was introduced in 1980 & has become the GOLD standard for VS 40. Imaging studies MRI : VII & VIII nerves as well as cerebellum ,brainstem , vasculature & other structures are well visualized on MRI The addition of gadolinium furthur enhanced the diagnostic accuracy Typically a series of T1 weighed images in which CSF is dark and fat is bright, T1 with gadolinium contrast , T2 In which CSF is bright is used. A hypointense globular mass centered over the IAC on T1 With enhancement on gadolinium. VS are iso-to hypointense on T2. T2 fast spin echo MRI without contrast as screening. 41. MRI Brain Isointense to brain, hyperintense to CSF Hyperintense to brain, hypointense to CSF 42. management options The primary management of VSs is surgical removal. Roles of observation and radiotherapy are currently for the pts,who cannot tolerate a surgical procedure or have a life span of < 5 yrs. Surgical approaches to the CPA include: Translabrynthine Retrosigmoid Middle fossa craniotomies. 43. management options The appropriate approach for a particular pt. is based on the hearing status , size of the tumor , extent of IAC involvement and experience of the surgeon The approaches are either hearing preservative or ablating. The retrosigmoid & middle fossa approaches are hearing preserving, while translabrynthine approach is otherwise. 44. management options The middle fossa approach is well suited for the pts with good hearing and tumor