A Rendering Scanning Electron Microscopy … Methodfor Rendering Scanning Electron Microscopy ......
Transcript of A Rendering Scanning Electron Microscopy … Methodfor Rendering Scanning Electron Microscopy ......

Proc. Nat. Acad. Sci. USAVol. 70, No. 12, Part I, pp. 3599-3603, December 1973
A Wet Chemical Method for Rendering Scanning Electron MicroscopySamples Conductive and Observations on the Surface Morphologyof Human Erythrocytes and Ehrlich Ascites Cells*
(Centrifugal Cytology/artifacts)
MARK A. GOLDMANt AND ROBERT C. LEIFtTt Institute of Molecular Biophysics, Florida State University, Tallahassee, Fla. 32306; and I Papanicolaou CancerResearch Institute, Miami, Florida 33123
Communicated by Michael Kasha, July 20, 1973
ABSTRACT An alternate method to the common tech-nique of evaporating a metallic coating on cells to renderthem conductive for scanning electron microscopy is de-scribed. This wet chemical technique is less expensive andeasier to use, but is not as widely applicable as the evap-orative technique. It should prove invaluable in the identi-fication of artifacts by comparison of micrographs of ma-terial prepared in both manners. The first step in our ap-plication of this wet chemical method is to use the Cen-trifugal Cytology bucket to deposit the cells onto conduc-tive polyethylene. After centrifugation and fixation with4% glutaraldehyde, the sample is placed in a 50% glutar-aldehyde solution. After a rinse in distilled water it istreated with ammoniacal silver nitrate. The reduced silverrenders the sample conductive and, thus, for erythrocytes,eliminates artifacts due to charging and reduces theseartifacts to virtually acceptable levels for larger cells. Thesurfaces of erythrocytes appear smooth with this tech-nique while those coated by conventional vapor depositionof Au-Pd alloys often appear slightly wrinkled. Ehrlichascites cells apparently can be divided into two classes bysurface morphology. The surface structure of Ehrlich as-cites cells rendered conductive by the wet method appearsto be finer than conventionally prepared ones.
One of the most common problems encountered in scanningelectron microscopy of biological specimens is that of de-termining if a structure existed in a living state or whether itis an artifact generated during the preparation or visualiza-tion of the specimen (1). Biological materials, because theyare quite delicate, act as insulators, and often contain muchfine detail, may be altered or destroyed during the processingof these samples for observation. A conductive sample isnecessary in scanning electron microscopy to prevent charging,the buildup of a negative charge by the impinging of thescanning electron beam on the sample (1, 2). If this chargeis not grounded, the result is a very distorted image or noneat all.The standard method of rendering an insulating (e.g., bio-
logical) sample conductive is to place it in an evacuated belljar and to evaporate a thin coating of conductive material,often gold or gold alloy, onto the surface of the sample (1, 2).Possible sources of artifacts due to the deposition of the metal
* This is the second paper of a series "Centrifugal Cytology."The first paper is ref. 3.1 Reprint requests should be sent to Dr. Robert C. Leif at the:Papanicolaou Cancer Research Institute, 1155 Northwest 14thSt., P.O. Box 6188, Miami, Fla. 33123.
3599
coating include: etching of the cell surface by the collidingatoms of metal vapor; uneven distribution of the coatingwhich could result in both the filling in of fine surface detailsand the accentuating or thickening of coarse details by selec-tive buildup; and, finally, the formation of microcrystalswhich provide a grainy appearance to the cell surface. Thus,it would prove most advantageous to have an alternatemethod for rendering a sample conductive, preferably onequite different in methodology from the metal-depositionprocedures presently used. We describe such a method inthis communication. The advantage of a wet chemical methodover vacuum metal deposition is that the artifacts mentionedabove should be eliminated, because solution concentrationsare easy to control and reproduce and placing a sample in asolution exposes it evenly and isotropically. A further possibleadvantage of using wet chemistry is that by varying both themetal and the reducing agent additional information on thesample surface and the areas slightly underlying it may begleaned by the relative reactivities (or lack thereof) of thechemicals.
MATERIALS AND METHODS
The samples were obtained and first prepared by means ofCentrifugal Cytology, a new technique (3) which uses a specialswinging bucket rotor for preparing, from cellular suspen-sions, glutaraldehyde-fixed dispersions on conventional glassmicroscope slides or other substrates. The Centrifugal Cytologybuckets were designed and function so that all solutions in-serted into the chambers remained and the cells are not driedby air. For these studies, conductive polyethylene§ was sub-stituted for the nonconductive coverslip.The Centrifugal Cytology bucket and procedure have been
described in detail (3). Briefly, human blood was obtainedby finger puncture. The blood was collected in calcium- andmagnesium-free phosphate-buffered tissue-culture medium199 containing 1% by weight bovine-serum albumin to whichhad been added a small quantity of sodium Chelex, an ion-exchange resin that is selective for doubley and tripleycharged cations. Ehrlich ascites cells, originally obtainedfrom T. S. Hauschka, were propagated in the peritonealcavity of female HA/ICR Swiss mice. The cells were har-
§ Available from M. Goldman Consultants, P.O. Box 1472, Hilo,Hawaii 96720.

3600 Biophysics: Goldman and Leif
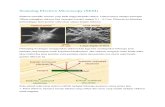
FIG. 1. Uncoated conductive polyethylene substrate. In-strument magnification set at 4,100.
FIG. 2. Human erythrocyte (a X11,100; b X44,400) pre-
pared by wet chemical procedure.
FIG. 3. Human erythrocyte (a X 11,100; b X39,590) coatedwith 60% Au-40% Pd.
vested from the mice between 7 and 10 days after the intra-peritoneal injection of 0.25 ml of a 1/100 dilution of the origi-nal crude cell suspension. The cells were collected directlyinto Ca- and Mg-free medium 199 containing 1% bovine-serum albumin. The cells were then pelleted, washed twicewith the culture medium which now contained medium 199with Ca and Mg and 1% bovine-serum albumin, and thencounted with a Coulter Counter. The erythrocytes and as-cites cells were, respectively, diluted to about 500,000 and100,000 cells per ml with the standard diluting solution forCentrifugal Cytology, which consists of (by volume): 75%medium 199 containing 0.2% bovine-serum albumin, 10% di-methylsulfoxide, and 15% H20. This medium slightly swellsand flattens the cells, which aids in their identification bylight microscopy. 0.25 ml of cell suspension was inserted intoeach of the chambers of the Centrifugal Cytology bucket, andthe cells were centrifuged for 15 min at room temperature atabout 2000 X g. After centrifugation, the Centrifugal Cytologybuckets were removed from the centrifuge, the cells wereoverlayed with fixing solution, which consists of 4% glutar-aldehyde and 12% dimethylsulfoxide and contains 680 mg ofKH2PO4, 870 mg of K2HPO4, and 1 g of bovine-serum al-bumin per liter. The samples in Centrifugal Cytology bucketswere centrifuged as before, but for 45 min.
Proc. Nat. Acad. Sci. USA 70 (1978)

Scanning Electron Microscopy 3601
FIG. 4. Ehrlich ascites cell (a X 5,476; b Xi18,130) with small button-like projections. This cell was made conductive by the chemicalmethod.
The following procedure may then be used immediatelyafter the glutaraldehyde fixation step or later after air dryingfrom xylene (we have used cells stored for over 6 weeks withno difficulty). The sample is placed for 3 hr in a standard50% glutaraldehyde solution (Sigma Chemicals no. G6254,grade VI) which has been decanted from the BaCO3. The sam-ple is then dipped for a few seconds in distilled water to re-move excess aldehyde, placed in a 0.12 M solution of am-moniacal silver nitrates for 10 min, and placed in (KodakKodafix) photographic fixer for 3 min. If the last step is notperformed, the sample is coated with a particulate precipi-tate. The cells were then dehydrated by immersion for 3 mineach in successive ethanol-water solutions of 25, 50, 70, 95,and 100 volume percent ethanol, and then for 3 min each in50% ethanol-50% o-xylene and in pure o-xylene. The in-dividual squares were cut apart, glued onto stubs with aconductive silver epoxide (Epoxy Products Co., E-Kote no.3030, New Haven, Conn.), and viewed with a CambridgeMark Ha scanning electron microscope at 20 kV beam volt-age and 150 ptA beam current. All magnifications given inthe figures are in terms of instrument settings. All of thepreparation steps were done at ambient temperature.
RESULTS AND DISCUSSIONThe use of conductive polyethylene is particularly advan-tageous for several reasons. Primarily this substrate pro-vides a path for the conductive cell to ground, and is easilycut with scissors to any desired shape.
If the conventional evaporative technique is used, thegranular surface of Fig. 1 can serve as a standard referencefor comparison with any sample on which an excess of metalmay have been deposited. Any large excess of metal is im-mediately apparent by the loss of fine detail. Two minor dis-advantages are that when drying from the final xylene solu-tion, the polyethylene tends to curl slightly and it has poorcompatability with the silver epoxide currently used in thesestudies.
Fig. 2 is of a human erythrocyte prepared by the presentwet chemical method. A similar cell, but subsequently coatedwith 60% Au-40% Pd, is shown in Fig. 3. The slight wrinklingof the erythrocyte membrane shown in Fig. 3 may be due tothe formation of microcrystals and uneven distribution ofthe alloy since the membrane appears to be quite smooth inthe wet chemical preparations (Fig. 2). It should be noted,however, that some of the conventionally coated erythro-cytes were also quite smooth (3). We believe that the smoothappearance of the chemically-treated erythrocytes is real andnot an artifact, because the most common artifact inducedby this treatment would be shrinking, not swelling (4).The Ehrlich ascites cell shown in Fig. 4 was prepared
chemically and shows signs of charging. Thus it providesnot quite as good a picture as the conventionally coated cellshown in Fig. 5. The two cells shown in Figs. 4 and 5 are ex-amples of one of the major types of Ehrlich ascites cellsfound in this study, the "lumpy" cell. However, in spite ofthe slight charging, one observes (Fig. 4) that the fine struc-ture of this cell consists of discrete small button-like projec-tions rather than the series of ridges shown in Fig. 5. Theseprojections are probably due to a buildup of metal from theevaporative process.
Figs. 6, 7, and 8 each show an example of the other majortype of Ehrlich ascites cell found in this study, the "hairy"cell. The chemically prepared cell in Fig. 6 also shows somesigns of charging, but the structure of the surface "hairs" isfiner than that shown in Figs. 7 and 8 on the cells preparedby the evaporative technique. Cells intermediate between thetwo types have also been observed. It is not known why theEhrlich ascites cells prepared by the wet method are not asgood conductors as erythrocytes prepared by this method.Possible reasons for this decrease in conductivity are thatthe glutaraldehyde-treated Ehrlich ascites cell possesses alower capacity to reduce silver ions or presents a longer re-sistance path for the electrons than the erythrocytes. Thetwo types of Ehrlich ascites cells observed also showed slightdifferences in their relative amounts of charging, with thelumpy cell showing the most charging.The silver deposited by this wet method diffuses into the
cell, giving a conductive coating not limited to the surface.
¶ This solution was prepared by first making a 0.12 M AgNO3solution in a 98%o water-2%0 ethanol solvent. NH40H was thenadded until the precipitate formed was redissolved.
Proc. Nat. Acad. Sci. USA 70 (1973)

3602 Biophysics: Goldman and Leif Proc. Nat. Acad. Sci. USA 70 (1973)
w_
FIG. 5. Similar but not identical Ehrlich ascites cell (a X5,328; b X 15,910) from the same sample as in Fig. 4 but coated with Au-Pd.
FIG. 6. Ehrlich ascites cell (a X4,588; b X28,120) with "hairy" surface. This cell was rendered conductive by the chemical method.
FIG. 7. Similar but not identical Ehrlich ascites cell (a X4,736; b X 29,600) from the same sample as in Fig. 6 but coated with Au-Pd.

Scanning Electron Microscopy 3603
FIG. 8. Similar Ehrlich ascites cell (a X5,180; b X37,000) from another sample with a heavy coating of Au-Pd. Notice the loss ofdetail on the cell surface and the filling in of the conductive polyethylene substrate in Fig. Sa compared with Fig. 1. Notice (Fig. 8b) thatthe heavy coating reduces the overall resolution so that the figure appears to be overmagnified.
Such a penetrating coating should leave fine detail in a morenatural state and, thus, less artifically thickened than in thecell coated by evaporation.
In summary, when the above results are compared, severalfeatures may be emphasized:
(1) The wet chemical method gives excellent results forerythrocytes, but its applicability to the study of other cellsremains to be investigated. Improvements of the above tech-nique, which result in increased amounts and/or concentra-tions of reduced silver may be necessary for studies of otherbiological materials. Other modifications we have tried areusing a formaldehyde-glutaraldehyde mixture (5) and re-placing the ethanol with dimethylsulfoxide in the silver solu-tion. Neither worked as well as the procedure described.
(2) Although the evaporative technique should be ap-plicable to all material, there are disadvantages due to theartifacts mentioned above and difficulty in reproducibility, aswell as the inherent expense of the deposition system itselfwhich includes vacuum pumps and bell jars and their main-tenance and operation by trained personnel. The chemicalmethod, by comparison, is easily reproducible and can beexecuted with a minimum of training.
(3) The results of these two dissimilar methods of ren-dering the cells conductive are sufficiently similar to indicatethat the common structural details, such as the "hairs" ob-served on the surface of one type of Ehrlich ascites cell,cannot be an artifact of preparation and that the diversityof structure observed on the surfaces of cells is a real phenome-non. The only cells so far that we have observed not to becovered by some sort of projection are human and chickenerythrocytes. Subsequent studies of other cells (Thornth-
waite, J., Zucker, R. M. & Leif, R. C., unpublished), wherethe critical-point evaporation technique was compared withair drying from xylene, indicated no readily observable dif-ference in specimens coated by evaporation.
This work was supported in part by a contract between theDivision of Biology and Medicine, U.S. Atomic Energy Com-mission, and the Florida State University. We wish to thank Mr.R. L. Warters and Mr. L. A. Dunlap for the cell samples and theirpreparation via Centrifugal Cytology, Mr. C. Railey for printingthe pictures, Dr. L. Beidler for use of the scanning electron micro-scope, and Dr. J. Hines and Mr. R. Parker for assistance in itsoperation. We also wish to thank Dr. M. Kasha for many helpfuldiscussions, and Dr. D. Smith and Dr. H. Lipner for their sug-gestions on this manuscript.
1. Pfefferkoen, G. (1969) "Preparation methods and artifactsin scanning electron microscopy," Proceedings Second An-nual Stereoscan® Colloquium, 1969, Sponsored by EngisEquipment Co., pp. 81-87.
2. Russ, J. C. & Kabaya, A. "Preparation of samples for scan-ning electron microscopy," Proceedings Electron MicroscopySociety of America, Twenty-Eighth Annual Meeting, ed.Arceneaux, C. J. (Claitors Publishing Division, Baton Rouge,Louisiana), pp. 380-381.
3. Leif, R. C., Easter, H. N., Warters, R. L., Thomas, R. A.,Dunlap, L. A. & Austin, M. F. (1971) "Centrifugal cytology1. A quantitative technique for the preparation of glutar-aldehyde-fixed cells for the light and scanning electron micro-scope," J. Histochem. Cytochem. 19, 203-215.
4. Morel, F. M. M., Baker, R. F. & Wayland, H. (1971) "Quan-titation of human red blood cell fixation by glutaraldehyde,"J. Cell Biol. 48, 99-100.
5. Vassallo, G., Capella, C. & Solica, E. (1971) "Grimelius silverstain for endocrine cell granules, as shown by electron micro-scope," Stain Technol. 46, 7-13.
Proc. Nat. Acad. Sci. USA 70 (1973)