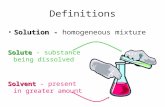
A. Definitions Solution - Solution - homogeneous mixture Solvent Solvent - present in greater...
-
Upload
alexina-norris -
Category
Documents
-
view
228 -
download
4
Transcript of A. Definitions Solution - Solution - homogeneous mixture Solvent Solvent - present in greater...

A. DefinitionsA. Definitions
Solution - Solution - homogeneous mixture
Solvent Solvent - present in greater amount
Solute Solute - substance being dissolved

A. DefinitionsA. Definitions
Solute Solute - KMnO4Solvent Solvent - H2O

B. SolvationB. Solvation
Solvation – Solvation – the process of dissolving
solute particles are separated and pulled into solution
Solid solute is surrounded by solvent particles

B. SolvationB. Solvation
StrongElectrolyte
Non-Electrolyte
solute exists asions only
- +
salt
- +
sugar
solute exists asmolecules
only
- +
acetic acid
WeakElectrolyte
solute exists asions and
molecules

B. SolvationB. Solvation
NONPOLAR
NONPOLAR
POLAR
POLAR
““Like Dissolves Like”Like Dissolves Like”““Like Dissolves Like”Like Dissolves Like”

C. SolubilityC. Solubility
SolubilitySolubility• maximum grams of solute that will
dissolve in 100 g of solvent at a given temperature
• g solute/100 g solvent• varies with temp• based on a saturated soln

C. SolubilityC. Solubility
Saturated Solution – contains the maximum amount of solute for a given amount of Solvent.

C. SolubilityC. Solubility
Unsaturated Solution – a solution that containsless solute than the maximum that can be
dissolved.

C. SolubilityC. Solubility
Supersaturated Solution – A solution consisting of more solute dissolved than the maximum solubility.

C. SolubilityC. Solubility
SATURATED SOLUTION
no more solute dissolves
UNSATURATED SOLUTIONmore solute dissolves
SUPERSATURATED SOLUTION
becomes unstable, crystals form
concentration