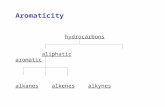
Naming Saturated Hydrocarbons Aliphatic Alkanes. Some Simple Alkanes (C n H 2n+2 ) 2-methylpropane.
3_part 1_3.doc · Web viewChapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons...
Transcript of 3_part 1_3.doc · Web viewChapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons...

Chapter 3 Alkanes and Cycloalkanes
Two types
◦ Saturated hydrocarbons
◦ Unsaturated hydrocarbons
3.1 Alkanes
Also referred as aliphatic hydrocarbons
General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
Chm 201 - Dang1

Alkanes (RH) Alkyl Substituent (R-) Other Substituent (R-)
(“parent” carbon chains)CH4 methane
CH3CH3 ethane CH3CH2CH3 propane
CH3(CH2)2CH3 butaneC5 pentane C8 octaneC6 hexane C9 nonaneC7 heptane C10 decane
(groups attached to parent) CH3 meth (Me) CH2CH3 ethyl (Et) CH2CH2CH3 propyl (Pr)
(groups attached to parent) F fluoro Cl chloro Br bromo I iodo
Structure Presentation
E.g. methane: CH4
All single carbon has four bonding position, completely saturated by the four hydrogen atoms. There is only one possible arrangement of the atoms.
Condensed formula 2D formula 3D formula
CH4
Chm 201 - Dang2

E.g. Ethane: C2H6
Only two carbon atoms are connected to each other and there are six possible bonding sites. These are filled by the six hydrogen atoms
Condensed formula 2D formula 3D formula
Because single bonds allow rotation, there are number of ways that alkanes can be drawn using slightly different representation.
Constitutional isomers: Compounds that have the same molecular formula but different structural formulas (a different connectivity of their atoms).
• For the molecular formulas CH4, C2H6, and C3H8, only one structural formula is possible. There are no constitutional isomers for these molecular formulas.
E.g. How many alkane structures can you draw from C4H10? (*Hint: always start with a straight chain carbon-carbon backbone)
E.g. How many isomers can you draw from C4H9Cl?
* Do cycloalkanes have isomers? YES
E.g. There are two possible ways to make ring using four carbon atoms of C4H8.
E.g How many cyclic constitutional isomers can you draw from C5H10?
Chm 201 - Dang3

3.2 – 3.4 IUPAC Rules for naming alkanes
1. Find the longest carbon chain (if there is a tie, choose chain with the most substituent). Name parent
2. Number the carbon chain, starting from the end closest to the first substituent
3. Name and number the subtituents (use di, tri, tetra etc.., prefixes for groups that appear more than once).
4. Alphabetize and list substituents before the parent name. Ignore all prefixes other than iso.
E.g.
If there are two substituents on the cycloalkane, number the ring by the beginning with the substituent of lower alphabetical order.
DON’T FORGET to add the pre-fix “cyclo” to the open-chain hydrocarbon (the parent)
If there are three or more substituents, number the ring so as to give the lowest set of numbers, and then list the substituents in alphabetical order.
Chm 201 - Dang4

3.5 - Classification of Alkyl Halides, Alcohols and Amine
Alkyl Halides Alcohol Amine
Chm 201 - Dang5
Type of carbons:
Primary (1o) attached to one carbon
Secondary (2o) attached to two carbons
Tertiary (3o) attached to three carbons

The functional group is a halogen (X = F, Cl, Br, I)
The functional group is hydroxyl (-OH)
R-OH
The functional group is a nitrogen atom.
RNH2, R2NH or R3N
Nomenclature
Chm 201 - Dang6

3.6 Rotation occurs about the carbon-carbon single bond
-rotation about any σ bond without changing the amount of orbital overlapping
- to get different conformational isomers
Chm 201 - Dang7

E.g Consider the rotation at C2-C3 of butane, CH3CH2CH2CH3
Energy diagram
Chm 201 - Dang8

3.9 Cycloalkanes: Ring Strain (kcal/mol)
sp3 bond angle can’t be 109.5o (angle strain)
Conformation: Any three-dimensional arrangement of atoms in a molecule that results by rotation about a single bond.
Cyclohexane – no ring strain with two conformations
Chm 201 - Dang9

In a chair conformation,
Chm 201 - Dang10

E.g Following is a chair conformation of cyclohexane with carbon atoms numbered 1 through 6.
◦ Draw methyl groups that are equatorial on carbon 1,2 and 4
◦ Draw methyl group that is axial on C2, C3 and equatorial on C6
Conformations and stabilities
If one substituent, then it will prefer conformer in equatorial position
If two substituents on same position, prefer equatorials
Chm 201 - Dang11

If two position on different position, largest and bulkiest substituent prefer equatorial
3.12-Conformers of disbsubsituted cycloalkanes- cis/trans geometric isomers
The cis isomer has its substituents on the same side of the ring
The trans isomer has its substituent on opposite side of the ring
What about the 1,2-dimethylcyclohexane geometric isomers?
Chm 201 - Dang12

E.g Which substitution patterns are trans?
1,2 a,a 1,3 a,e 1,4 aa
E.g Determine whether each of the following is a cis isomer or a trans isomers
E.g Draw a chair conformation of the compound below
Chm 201 - Dang13