3. Acidic and Basic Character of Organic Compounds
description
Transcript of 3. Acidic and Basic Character of Organic Compounds
Structural Effects on Acidity
Acidic And Basic Character of Organic CompoundsChemistry @ MBCCScience Year 21
3.1 Explain the difference in acidity of alcohols, phenols and carboxylic acids
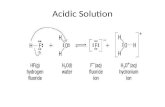
AcidityAcidity may be defined by:
the tendency of compound to yield hydrogen in H2O (Arrhenius acid)
The ability to donate H+ (Bronsted-Lowry acid)
the ability to accept an electron pair to form a covalent bond (Lewis acid)3Acid StrengthThe strengths of weak acids are measured on the pKa scale
The smaller the pKa, the stronger the acid is pKa = -log10Ka
The larger the Ka, the stronger the acid
In terms of acidity: Ethanoic acid > phenol > ethanol
4Acidity of AlcoholsAlcohols are extremely weak acids
5Acidity of AlcoholsSee page 316 of Maraj and Samai.Can you explain the trend in the table below?What is an alpha () carbon?
6Acidity of AlcoholsAs the number of alkyl groups attached to the -C increases:Electron density of the -C increases.Alkyl groups are donating electron density and pushes electron density onto the -C (positive inductive effect)
Oxygen in the alkoxide ion becomes more negatively charged and wants to hold on to the hydrogen more (polarity of O-H bond decreases)
The alkoxide anion is less stable and the alcohol is less ready to lose a proton
In terms of acidity of alcohols:10 alcohol > 20 alcohol > 30 alcohol
7Acidity of Phenols Acidity of phenols is dependent on the stability of the phenoxide ionIn the phenoxide ion, the lone pairs on oxygen becomes delocalised and overlaps with the pi ring system of the benzene ring The electron on the oxygen of the phenoxide ion is therefore less available to accept a proton and go back to the phenolThe pH of a typical dilute solution of phenol in water is likely to be around 5 - 6 (depending on its concentration)
8Acidity of Phenols Any substituents on the benzene ring which stabilizes the phenoxide ion makes the compound more acidic
Can you explain the trend in acidity as shown below:
Hint: Are the groups on the benzene ring Electron Donating Groups (EDGs) or Electron Withdrawing Groups (EWGs)?
9Acidity of Phenols Answer: EDGs such as alkyl groups and alkoxide groups makes the benzene ring more electron rich and reduces the extent of delocalization of the oxygen lone pair into the aromatic ring. The electron density on oxygen remains and is available to accept a proton less acidic
EWGs such as COOH, NO2 and Cl make the benzene ring more electron poor. The lone pair of the oxygen is therefore delocalized to a large extent into the pi ring system and is less available more acidic
10Acidity of Carboxylic AcidsCarboxylic acids partially ionise in solution and the strength depends on equilibrium constant
Acidity depends on the stability of the carboxylate ion. The more stable the ion, the less readily it will recombine with H+ ions to reform the carboxylic acid
11Acidity of Carboxylic AcidsThe carboxylate ion is resonance-stabilized unlike the alkoxide ion in phenolsThe negative charge is spread (delocalized) over both the oxygen atoms and will be unavailable to accept a protonCarboxylate ion is protonated less easily than phenoxide ion and alkoxide ion, therefore, carboxylic acid is more acidic than phenols and alcohols
12Phenols vs. Carboxylic AcidsIn carboxylate ion, the negative charge is equally distributed over two electronegative atoms (oxygen atoms) while in phenoxide ion, it is present only on one oxygen
Carboxylate ion is more stabilized than phenoxide ion. Hence, carboxylic acids ionize to the greater extent than phenols furnishing higher concentration of H+ions
Therefore carboxylic acids behave as stronger acids than phenols
Acidity of Carboxylic AcidsCarboxylic acidpKaCH3COOH4.76CH2ClCOOH2.86CHCl2COOH1.29CCl3COOH0.65Electronegative atoms for eg. Cl on the carbon chain pulls electron density to itself and away from the carboxylate group
This leads to a loss of electron density from the oxygen atom, which decreases the ability of the COO- ion to accept a proton
The greater the amount of electronegative atoms and the closer in proximity to the COO- group, the stronger the acid
The more electronegative the atom, the stronger the acidCarboxylic acidpKaCH2FCOOH2.66CH2ClCOOH2.86CH2BrCOOH2.90CH2ICOOH3.1714Acidity of Carboxylic AcidsCarboxylic acidpKaCH3CH2CH2COOH4.82CH3CH2CHClCOOH2.84CH3CHClCH2COOH4.06CH2ClCH2CH2COOH4.52The chlorine is effective at withdrawing charge when it is next-door to the -COO- group, and much less so as it gets even one carbon further awayThe greater the amount of electronegative atoms and the closer in proximity to the carboxylate group, the stronger the acid
15Acidity of Carboxylic AcidsCarboxylic AcidpKaHCOOH3.75CH3COOH4.76CH3CH2COOH4.87CH3CH2CH2COOH4.82The less the charge is delocalized, the less stable the ion, and the weaker the acidAlkyl groups have a tendency to "push" electrons away from themselves. That means that there will be a small amount of extra negative charge built up on the -COO- groupAny build-up of charge will make the ion less stable, and more attractive to hydrogen ionsThe more alkyl groups present, the weaker the acidCan you explain the trend in acidity of the following acids?16
3.2 Explain differences in basic character of aliphatic amines, amides and aromatic amines
BasicityBasicity may be defined by:
The ability of a substance to accept a proton
The ability to donate an electron pairBasicity is measured in the degree of availability of lone pair for conjugation with acidsRNH2 + H2O RNH3 + OH-18Basicity
The lower the pKb the more basic the compoundCan you account for the trend below?19BasicityAmines are basic because the lone pair on nitrogen is available to form a dative bond with an H+ ion in solutionAll aliphatic primary amines are stronger bases than ammoniaIn terms of basicity: 30 amines > 20 amines > 20 amines
BasepKbNH34.74CH3NH23.36CH3CH2NH23.27CH3CH2CH2NH23.1620Basicity of AminesAs the number of alkyl groups attached increases, the basicity of the amine increases as well
EDGs push electron density onto the nitrogen atom
The N is more electron rich and the lone pair is more available for forming a dative bond with H+
(CH3)3N: >(CH3)2NH >CH3NH2 21Basicity: Aromatic AminesAromatic amines are weaker bases than aliphatic amines
Phenylamine is a weaker base than methylamine
In phenylamine the p orbitals of nitrogen (which contain the lone pair) can overlap with the ring system and become delocalized in the ring system (conjugative effect)
Delocalization means the lone pair is unavailable to accept a proton weaker base than methylamine
BasepKbNH34.74Methylamine3.36Phenylamine9.3822Basicity: Amides
http://www.chemguide.co.uk/organicprops/amides/other.htmlThe lone pair on the N atom is almost parallel to the p orbitals on C and O and overlaps with them as they form the pi bond23Basicity: Amidesthe lone pair on N is unavailable to accept a proton because they are used up in the delocalized electron cloud
Amides show little or no tendency to accept a proton and hence are very weak bases, almost neutral
Amides are not soluble in dilute HCl as simple amines because of lesser availability of lone pair of electron of the N of amides compared with simple amineshttp://www.chemguide.co.uk/organicprops/amides/other.html24
3.3 Explain the acid-base properties of amino acids
Amino Acidscompounds containing an amino group, -NH2, and a carboxylic acid group, -COOH
biologically important amino acids have the amino group attached to the carbon atom next door to the -COOH group.
They are known as2-amino acids. They are also known (slightly confusingly) asalpha-amino acids.
the two simplest of these amino acids are 2-aminoethanoic acid and 2-aminopropanoic acid
http://www.chemguide.co.uk/organicprops/aminoacids/background.html
Amino Acids: PropertiesAmino acids are crystalline solids with surprisingly high melting points (200 - 300C)
It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt
Amino acids have both a basic amine group and an acidic carboxylic acid group
There is an internal transfer of a hydrogen ion from the -COOH group to the -NH2group to give a dipolar ion called a zwitterion
Amino Acids: ZwitterionsZwitterion has no overall electrical chargeSoluble in water (polar) but insoluble in non-polar organic solvents such as hydrocarbonsAmino acids exist in even in the solid state
Q. What accounts for the high melting points of amino acids?
Answer. Instead of the weaker hydrogen bonds and other intermolecular forces that you might have expected, you actually have much stronger ionic attractions between one ion and its neighbours.These ionic attractions take more energy to break and so the amino acids have high melting points for the size of the moleculesZwitterions: Buffer CapacityZwitterions are amphoteric and can behave as a buffer in biological systemsIf some acid (H+) is added to an aqueous solution of amino acids the COO- group of the zwitterion will be protonatedIf some base (OH-) is added to aqueous solution of amino acids the NH3+ group is deprotenatedIn each case the zwitterion helps to maintain the pH
Amino Acids:Optical ActivityApart from glycine the carbon at the centre of the structure has four different groups attached ( In glycine, the "R" group is another hydrogen atom)
Because of these four different groups attached to the same carbon atom, amino acids (apart from glycine) arechiral
Questions?So how much did you learn?Which is more acidic? Why?
More acidicNegative electron inductive effect of the Cl atom pulls electron away from thethe delocalization of the lone pair from O. This will enhance the removal of H from the carboxyl group.32Which is more acidic? Why?
More acidicElectron attracting inductive effect of Cl is stronger at the position, thus inductive effect decreases with distance.33Alcohols are weaker acids than phenol because the OH bond in phenol is greatly weakened as a result of electron delocalization towards the ring. In alcohol the weakening of the OH bond is only due to the electron attracting inductive effect of O.Why are alcohols weaker acids than phenols?34Question.Solubility of organic compounds in dilute sodium bicarbonate (NaHCO3) can reflect strength in acidity of these systems. Suggest why does phenol and aliphatic alcohols not react with NaHCO3, but 2,4,6 trinitrophenol reacts.
Answer:Phenols and aliphatic alcohols which are regarded as weak acids do not dissolve in NaHCO3, but 2, 4, 6-trinitrophenol is an exception, because the 3 NO2 increases the acidity of phenol.
35