1. Importance of Carbon 2. Functional Groups 3. Physical Properties 4. Types of Formulas 5. Isomers...
Transcript of 1. Importance of Carbon 2. Functional Groups 3. Physical Properties 4. Types of Formulas 5. Isomers...

Introduction to Organic Chemistry
1. Importance of Carbon2. Functional Groups3. Physical Properties4. Types of Formulas5. Isomers6. The IUPAC System

Functional Groups

115. (a) List two characteristics of a homologous series. (1) 115. (a) one general formula/same general formula;
differ by CH2;similar chemical properties;gradual change in physical properties; 1
Award [1] for any two of the above characteristics.
State two characteristics of a homologous series. 68. (a) same general formula;
successive members differ by CH2, Do not allow elements or just “they”.
similar chemical properties; Allow same/constant
gradual change in physical properties;◦ Do not allow change periodically.
same functional group; 2◦ Award [1] each for any two.
Key Terms

A functional group is an atom or combination of atoms which gives an organic molecule its distinctive and characteristic chemistry.
The term “functional group” is linked to the concept of a homologous series.
A homologous series is a group of molecules with the same general formula and the same functional group.
They have similar physical and chemical properties (albeit with trends e.g. increasing boiling point with increasing carbon chain length).
Key Terms

Functional groups have:1. The same functional group
2. The same general formula, but successive members differ by –CH2
3. The same chemical properties
4. Their physical properties change gradually
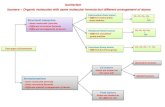
Characteristics of homologous series/classes

Homologous series and the Functional Group that makes them special
Homologous series
Prefix or Suffix
Examples Functional Groups
Alcohols -olEthanol
CH3CH2OH R –OH
Aldehydes -alEthanalCH3CHO
Carboxylic Acid -anoic acid Ethanoic acidCH3COOH

A functional group is a specific atom or group of atoms that confers a particular chemical property on a molecule, e.g the -OH group in ethanol
Each one of the following homologous series/class has a characteristic functional group.
Functional Groups
1. Alkane2. Alkene3. Alkyne4. Alcohol5. Aldehyde6. Ketone7. Carboxylic acid
8. Halogenoalkane9. Amine 10.Amide 11.Ester 12.Ether13.Nitrile 14.Arenes

Class with characteristic
Functional Groups

Alkanes, Alkenes, and Alkyne

Aldehyde & Carboxylic Acid
Aldehyde functional group; carboxyl is the functional group

Alcohol, Aldehyde, and Ketone
Functional Group: hydroxyl aldehyde carbonyl

Please identify the functional group and write
down the name of the corresponding homologous
series
Functional Groups

Aldehyde (functional group and class)

Functional group: cyanoClass: Nitrile

Alkane

Functional Group: carbonylClass: Ketone

Halogenoalkane(Haloalkanes)

Functional Group: esterClass: ester

Functional Group: amidoClass: amide

Functional Group: carboxylClass: carboxylic acid

Functional Group: aminoClass: Amine

Alkene

Functional Group: hydroxylClass: alcohol

Identification of a functional group in a compound

Again!!!Please identify the
functional group and write down the name of the
corresponding homologous series
Functional Groups

Functional Group: hydroxylClass: alcohol

Functional group: cyanoClass: Nitrile

Function group: carbonylClass: ketone

Aldehyde (class and functional group)

Ester (class and functional group)

Ester (class and functional group)

Halogenoalkane(Haloalkanes)

Alkene

Alkane

Functional Group: amidoClass: amide

Functional Group: carboxylClass: carboxylic acid

Functional Group: aminoClass: amine

Functional Group: carbonylClass: Ketone

Aldehyde (class and functional group)


Nomenclature1. Must memorize
prefixes (up to 6 for IB)2. To name, look at the
formula for the hydrocarbon
3. Determine if it is an alkane, alkene, or alkyne
4. Use the prefix for the number of carbons
5. Add ending (ane, ene, yne)
Prefix # of carbon atoms
Meth- 1
Eth- 2
Prop- 3
But- 4
Pent- 5
Hex- 6
Hept- 7
Oct- 8
Non- 9
Dec- 10

Meth-
One Carbon

Eth-
Two Carbons

Prop-
Three Carbons

But-
Four Carbons

Pent-
Five Carbons

Hex-
Six Carbons

Trying Again the Prefixes

Prop-
Three Carbons

Eth-
Two Carbons

Hex-
Six Carbons

But-
Four Carbons

Pent-
Five Carbons

Meth-
One Carbon