Isomers lesson
-
Upload
mrglosterscience -
Category
Education
-
view
1.961 -
download
1
Transcript of Isomers lesson

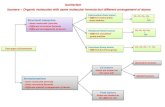
Structural, Geometric, Enantiomer
Isomers

Isomers
Compounds that have the same molecular formula but difference structure and different properties
3 types:
Structural isomers
Geometric isomers
Enantiomers


Structural Isomer
same KIND and AMOUNT of atoms(molecular formula) but the atoms have different connectivity
Structural isomers usually have different physical and chemical properties

Structural Isomer

Structural Isomer

Structural Isomer

Structural Isomer

Structural Isomer
How many different structures can you make from C5H12?

Geometric Isomers
SAME molecular formula and the SAME connectivity but different spatial arrangements
Due to inflexibility of a bond, usually either:
double bonds (cis and trans formation)
bonds that can’t rotate (i.e. in a ring formation)

Geometric Isomers

Geometric Isomer
Why is example 1 not a pair of geometric isomers?
Hint: Look at example 2 & 3 and compare to example 1. What is the minimum requirement for a pair of molecules to be considered geometric isomers?

Geometric Isomer
Answer: Each carbon of the double bond must have two different substituent groups
So if example 1 is not a pair of geometric isomers, what are they classified as?

Geometric Isomer
Another type of geometric isomer is found in ring structures and doesn’t involve double bonds
Due to the inflexible nature of the bonds that make up the ring
Note: The ring lies on a flat plane (e.g. your paper) and the OH groups are perpendicular to the plane (e.g. up and down)

Enantiomer
Molecules that are mirror images of each other
Note: left and right hands are a pair of enantiomers (mirror images not identical)
What other body parts that are enantiomers?

Enantiomer
Activity: Build these molecule
pairs Compare them to
determine whether they are enantiomers

Enantiomer

Enantiomer
How are the lower pairs of molecules different from the upper pairs?
What condition do you think is necessary for a pair of molecules to be enantiomers?
X

Enantiomer
Requirement: Enantiomers can only occur when each of the four groups attached to the central carbon atom are all different.
The central carbon is known as a chiral carbon and the molecule is chiral.

Chiral Molecules
Asymmetric carbon: carbon with 4 different groups bonded to it
No plane of symmetry Mirror image is non-superimposable

Achiral Molecule
lacks chiral properties has a plane of symmetry

Practice: Identify the chiral carbons
Double bond to
carbon = achiral

Application: Amino acids
Amino acids are building blocks of proteins
Some amino acids can exist as enantiomers because of their chiralty
Switching an enantiomer in a biological system can have detrimental effects

Application: Enzymes
Enzymes are always chiral. Their binding sites are in a specific orientation that
fits only one form of an enantiomer. Binding sites won’t fit if the wrong enantiomer is
present.

Racemic mixture
A mixture that contains equal quantities of both enantiomers
Enantiomers can interconvert in vivo

Racemic mixture
Story of thalidomide (1960’s) A racemic drug given to
pregnant women to combat morning sickness
One of the enantiomers caused birth defects (teratogen) and death
http://www.thalidomide.ca/the-canadian-tragedy/

Racemic mixture
What’s in Advil (ibuprofen)? Production results in a racemic mixture One of the enantiomers is effective as an
anti-inflammatory Takes about 30 minutes for the inactive
enantiomer to be converted
http://www.brookscole.com/chemistry_d/templates/student_resources/0534389996_mcmurry/CHEM_A_WORK/chapter9.htm
The World of Chemistry, 4 ed. 2007. Thomson Brooks/Cole. Joesten, Castellion, & Hogg.

Steps to identifying isomers
Do the molecules have the same chemical formula? If the formula is different, they are NOT
isomers but completely different molecules Are the molecules identical? If they are, they are NOT isomers.
Are all the atoms connected in the same way to other atoms? If not, they are STRUCTURAL isomers
Look for an inflexible bond (double bond or ring) for geometric isomers
Look for mirror images for enantiomershttp://2.bp.blogspot.com/_K91FA3B4cpM/SJ_NUmBWWGI/AAAAAAAAACY/UzL8H8Q4oag/s400/ist2_1744503_frustration.jpg

Summary
Isomer Same Different Requirements
Structural
Geometric
Enantiomer

Summary
Isomer Same Different Requirements
StructuralMolecular
formulaConnectivity
Geometric
Enantiomer

Summary
Isomer Same Different Requirements
StructuralMolecular
formulaConnectivity
GeometricMolecular formula &
connectivity
Spatial arrangement
Double bondDifferent substituent
groups on each carbon
Enantiomer

Summary
Isomer Same Different Requirements
StructuralMolecular
formulaConnectivity
GeometricMolecular formula &
connectivity
Spatial arrangement
Double bondDifferent substituent
groups on each carbon
EnantiomerMolecular formula &
connectivity
Spatial arrangement
Chiral moleculesMirror image

Practice: Identify the chiral carbon

Practice: Identify the chiral carbon
**
*
* **

structural isomers
structural isomers
geometric
isomers
Practice: Identify the chiral carbons

structural isomers
structural isomers
geometric
isomers
**
* *
**
* *
*
**
* *
*
Practice: Identify the chiral carbons