Protein structure
-
Upload
food-chemistry -
Category
Food
-
view
321 -
download
2
description
Transcript of Protein structure

Protein Structure and Function
Compiled & Edited by
Dr. Syed Ismail
VN Marathwada Agril. University Parbhani

DEFINITIONS OF PROTEIN
• Proteins are one of the essential building blocks of the human body. • They provide amino acids, which are a nutritional
requirement of the body to produce its own proteins and a variety of nitrogen-based molecules.
• It is common for programs to recommend a minimum of 50 grams of protein per day to maintain healthy levels.
• Proteins vary in structure as well as function. They are constructed from a set of 20 amino acids and have distinct three-dimensional shapes.

General Characteristics of Proteins
• They are the most complex and most diverse in chemical composition, conferring upon the different tissues.
• Protein molecule contains elements of C, H, O,N, S, and P together with traces of Fe, Cu, I, Mn, and Zn.
• It has a molecular weight of 5,000 to 3,000,000
• They are the most important of the biologic substances being the fundamental constituent of cell cytoplasm.
• They supply not only heat and energy but also material for building and repair.
• Unlike carbohydrates and lipids, only small amounts of protein is temporarily stored in the body, and which can be quickly used up upon demand.

FUNCTIONS OF PROTEIN

FUNCTIONS OF PROTEIN
• Antibodies - are specialized proteins involved in defending the body from antigens (foreign invaders). One way antibodies destroy antigens is by immobilizing them so that they can be destroyed by white blood cells.
• Enzymes - are proteins that facilitate biochemical reactions. They are often referred to as catalysts because they speed up chemical reactions. Examples include the enzymes lactase and pepsin. Lactase breaks down the sugar lactose found in milk. Pepsin is a digestive enzyme that works in the stomach to break down proteins in food.
• Hormonal Proteins - are messenger proteins which help to coordinate certain bodily activities. Examples include insulin, Insulin regulates glucose metabolism by controlling the blood-sugar concentration.

FUNCTIONS OF PROTEIN
• Contractile Proteins - are responsible for movement. Examples include actin and myosin. These proteins are involved in muscle contraction and movement.
• Structural Proteins - are fibrous and stringy and provide support. Examples include keratin, collagen, and elastin. Keratins strengthen protective coverings such as hair, quills, feathers, horns, and beaks. Collagens and elastin provide support for connective tissues such as tendons and ligaments.
• Storage Proteins - store amino acids. Examples include ovalbumin and casein. Ovalbumin is found in egg whites and casein is a milk-based protein.
• Transport Proteins - are carrier proteins which move molecules from one place to another around the body. Examples include hemoglobin and cytochromes. Hemoglobin transports oxygen through the blood. Cytochromes operate in the electron transport chain as electron carrier proteins.

Classification of Proteins
Based on Composition:
• Simple proteins – composed of entirely amino acids only.
Ex. Albumin, Globulin
• Complex or Conjugated proteins – made up of amino acids and other organic compounds. The non-amino acid group is termed as the prosthetic group.
Ex. Nucleoproteins, lipoproteins,
glycoproteins, metalloproteins

Classification of Proteins
Based on Axial Ratio: Axial ratio is the ratio of the length to the breath. • Globular proteins – with axial ratio less than 10 but
not below 3 or 4. They are compactly folded and coiled.
Ex. Insulin, plasma albumin, globulin, enzymes • Fibrous proteins – with axial ratio greater than 10.
They are spiral and helical and are cross linked by disulfide and hydrogen bonds.
Ex. Keratin, myosin, elastin, collagen

Globular Proteins
• Globular proteins have their axial ratio less than 10 but not below 3 or 4. They are compactly folded and coiled.
• Examples are insulin, plasma albumin, globulin, enzymes

Fibrous Proteins
• Fibrous proteins are spiral and helical and are cross linked by disulfide and hydrogen bonds
• Examples are keratin, myosin, elastin, collagen

Based on Biological Functions
• Structural proteins: collagen, elastin, keratin, fibroin of silk and webs
• Transport proteins: hemoglobin, myoglobin, lipoproteins • Protective proteins: immunoglobulins, fibrinogen, thrombin,
snake venoms, bacterial toxins • Contractile proteins: actin, myosin, tubulin • Catalytic proteins: enzymes • Regulatory proteins: hormones • Storage proteins: ferritin, hemosiderin, gluten, casein,
ovalbumin • Reception of Stimuli: rhodopsin, membrane receptor
proteins, acethylcholine, insulin • Germicidal proteins: Polymyxin B1, Gramicidin S

Shape = Amino Acid Sequence
• Proteins are made of 20 amino acids linked by peptide bonds
• Polypeptide backbone is the repeating sequence of the N-C-C-N-C-C… in the peptide bond
• The side chain or R group is not part of the backbone or the peptide bond

Composed of a chain of amino acids.
R
|
H2N--C--COOH
|
H
Proteins
20 possible groups

0HCOOCNHCOCNH
COOCNHCOOCNH
23
33
-
--

Polar
Basic Acid
?
Acid Amine
Different Amino Acid Classes
OH
O
H
H2N
C
C
R
Generic
Non-polar
C
C
C
OH
H
H
O
H+N
H
H2N
C NH
C
C
Histidine
H
H2N
C
C
H
H
O
OH
O
C
OH
C
Aspartic acid
C
C
C
OH
H
H
O
HS
H
H2N
Cysteine
OH
H
H
O
H
H
H2N
C
C
C
Alanine

Non-Polar
Amino Acids OH
O
H
H2N
C
C
H
Glycine
Leucine
OH
O
H
H2N
C
C
H3C CH3
H
C H
H
C
Methionine
OH
O
H
H2N
C
C
H3C
H
H
C H
H
C
S Alanine
OH
H
H
O
H
H
H2N
C
C
C
Valine
OH
O
H
H2N
C
C
H3C CH3
H
C
Phenylalanine
OH
H
H
O
H
H2N
C
C
C
Tryptophan
OH
O
H
H2N
C
C
NH
H
C
H Isoleucine
OH
O
H
H2N
C
C
H3C H
H
C H
H3C
C
Proline
OH
O
H
H2N+
C
C H2C
CH2 H2C
Protein Structure

Polar Amino Acids
Cysteine
C
C
C
OH
H
H
O
HS
H
H2N
Serine
OH
O
H
H2N
C
C
HO CH3
H
C
Threonine
OH
O
H
H2N
C
C
H
H
C OH
H
C
H
Tyrosine
HO
H
H
O
H
H2N
C
C
C
OH
Asparagine
H
H2N
C
C
H
H
O
OH
O
C
NH2
C
Glutamine
H
H2N
C
C
H
H
O
OH
O
C
NH2
C
C
H
H

Acidic Amino Acids
Aspartic acid
H
H2N
C
C
H
H
O
OH
O
C
OH
C
Glutamic acid
H
H2N
C
C
H
H
O
OH
O
C
OH
C
C
H
H

Basic Amino Acids
Histidine
C
C
C
OH
H
H
O
H+N
H
H2N
C NH
C
C
Lysine
OH
O
H
H2N
C
C
+H3N
H
H C H
H
C
C
H
H H
H
C
Arginine
OH
O
H
H2N
C
C
H
H C H
H
C
C
H
H
H
N +H2N
NH2
C
Protein Structure

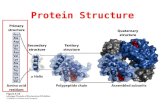
Levels of Protein Organization
• Primary Structure - The sequence of amino acids in the polypeptide chain
• Secondary Structure - The formation of helices and b pleated sheets due to hydrogen bonding between the peptide backbone
• Tertiary Structure - Folding of helices and sheets influenced by R group bonding
• Quaternary Structure - The association of more than one polypeptide into a protein complex influenced by R group bonding

Levels of Protein Organization
Primary Structure
Met-Gly-Ala-Pro-His-Ile-Asp-Glu-Met-Ser-Thr-...
The sequence of amino acids in the primary structure determines the folding
of the molecule.
Protein Structure

Protein Secondary Structure
• The peptide backbone has areas of positive charge and negative charge
• These areas can interact with one another to form hydrogen bonds
• The result of these hydrogen bonds are two types of structures:
helices
b pleated sheets

+ -
Protein Secondary Structure:
Helix
C
O
OH C N
H
H H
C
HO H
C
H
O
C N
H
H H
C
H H
C H N
C
O C
H N
O C
C O C
H N
C H N
C O C
O
C O
C
O
C
H N
H
N
C H N

+ -
Protein Secondary Structure:
Helix
C
O
OH C N
H
H H
C
HO H
C
H
O
C N
H
H H
C
H H
C H N
C
O C
H N
O C
C O C
H N
C H N
C O C
O
C O
C
O
C
H N
H
N
C H N

Protein Secondary Structure:
Helix
R
R R
R
R
R
R
R R
R R
R R
R
R groups stick out from the helix influencing higher levels of protein organization

Yeast Cytochrome C Oxidase
Subunit IV Leader
MLSLRQSIRFFKPATRTLCSSRYLL
R Y
P L
T
C S
R
L
S
T
I
K P
R
F
A
F
M
R Q
L
L
S
S
• This would localise specific classes of amino acids in specific parts of the helix
The order of the amino acids determines the hydrogen
bonding
Neutral Non-polar Polar Basic Acidic

Protein Secondary Structure:
b Pleated Sheet
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O C
H
C C N
O N H
C
O C
H
C C N
O N H
C
O C
H
C C N
O N H
C
O C
H
C C N
O

Protein Secondary Structure:
b Pleated Sheet
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O
C
H
C C
N
O
N H
C
O C
H
C C N
O N H
C
O C
H
C C N
O N H
C
O C
H
C C N
O N H
C
O C
H
C C N
O

Levels of Protein Organization
Tertiary Structure
• Tertiary structure results from the folding of helices and b pleated sheets
• Factors influencing tertiary structure include:
• Hydrophobic interactions
• Hydrogen bonding
• Disulphide bridges
• Ionic bonds

Hydrophobic interactions
Valine
OH
O
H
H2N
C
C
H3C CH3
H
C
Proline
OH
O
H
H2N+
C
C H2C
CH2 H2C
OH
O
H
H2N
C
C
H
Glycine

Hydrogen Bonding
Asparagine
H
H2N
C
C
H
H
O
OH
O
C
NH2
C
Glutamine
H
H2N
C
C
H
H
O
OH
O
C
NH2
C
C
H
H
N-------H (+) (-) O -------C
slightly slightly

Disulphide bridges
Cysteine
C
C
C
OH
H
H
O
HS
H
H2N
Cysteine
C
C
C
OH
H
H
O
HS
H
H2N
Cysteine
C
C
C
OH
H
H
O
S
H
H2N
Cysteine
C
C
C
OH
H
H
O
S
H
H2N

Ionic Bonds
Glutamic acid
H
H2N
C
C
H
H
O
OH
O
C
O-
C
C
H
H Arginine
OH
O
H
H2N
C
C
H
H C H
H
C
C
H
H
H
N +H2N
NH2
C

e.g.G-3-P Dehydrogenase
Tertiary Structure

Levels of Protein Organization
Quaternary Structure
• Quaternary structure results from the interaction of independent polypeptide chains
• Factors influencing quaternary structure include:
• Hydrophobic interactions
• Hydrogen bonding
• The shape and charge distribution on amino acids of associating polypeptides

G-3-P Dehydrogenase
from Bacillus stearothermophilus

Protein structure

Globular and Fibrous
• e.g. haemoglobin
• 3º structure normally folds up in a ball
• hydrophilic R groups point outwards
• Hydrophobic R groups point inwards
• soluble
• metabolic functions
• e.g. collagen
• 2º structure does not fold up, form fibres
• not surrounded by hydrophilic R groups
• insoluble
• structural functions

Haemoglobin
• Haemoglobin is a globular protein with a prosthetic ‘iron’ group
• In adults, hemoglobin is made up of 4
polypeptides (2 polypeptide chains and 2 b polypeptide chains)
• Each polypeptide surrounds a prosthetic ‘haem’ group
• Hydrophobic interactions between side
groups pointing inwards maintain the structure
• Hydrophilic side chains point outwards making it soluble
Fe
b
b

Polypeptide
Backbone

Amino Acids (magic 20)
You need to know these
Hydrophilic Hydrophobic

Protein Folding
• The peptide bond allows for rotation around it and therefore the protein can fold and orient the R groups in favorable positions
• Weak non-covalent interactions will hold the protein in its functional shape – these are weak and will take many to hold the shape

Non-covalent Bonds in Proteins

Globular Proteins
• The side chains will help determine the conformation in an aqueous solution

Hydrogen Bonds in Proteins
• H-bonds form between 1) atoms involved in the peptide bond; 2) peptide bond atoms and R groups; 3) R groups

Protein Folding
• Proteins shape is determined by the sequence of the amino acids
• The final shape is called the conformation and has the lowest free energy possible
• Denaturation is the process of unfolding the protein – Can be done with heat, pH or chemical
compounds
– The chemical compound can be removed and have the protein renature or refold

Refolding
• Molecular chaperones are small proteins that help guide the folding and can help keep the new protein from associating with the wrong partner

Protein Folding
• 2 regular folding patterns have been identified – formed between the bonds of the peptide backbone
• -helix – protein turns like a spiral – fibrous proteins (hair, nails, horns)
• b-sheet – protein folds back on itself as in a ribbon –globular protein

b Sheets
• Core of many proteins is the b sheet
• Form rigid structures with the H-bond
• Can be of 2 types
– Anti-parallel – run in an opposite direction of its neighbor (A)
– Parallel – run in the same direction with longer looping sections between them (B)

Helix
• Formed by a H-bond between every 4th peptide bond – C=O to N-H
• The helix can either coil to the right or the left
• Can also coil around each other – coiled-coil shape – a framework for structural proteins such as nails and skin

Protein Structure

Domains
• A domain is a basic structural unit of a protein structure – distinct from those that make up the conformations
• Part of protein that can fold into a stable structure independently
• Different domains can impart different functions to proteins
• Proteins can have one to many domains depending on protein size

Domains

Useful Proteins
• There are thousands and thousands of different combinations of amino acids that can make up proteins and that would increase if each one had multiple shapes
• Proteins usually have only one useful conformation because otherwise it would not be efficient use of the energy available to the system
• Natural selection has eliminated proteins that do not perform a specific function in the cell

Protein
Families
• Have similarities in amino acid sequence and 3-D structure
• Have similar functions such as breakdown proteins but do it differently

Proteins – Multiple Peptides
• Non-covalent bonds can form interactions between individual polypeptide chains – Binding site – where proteins interact with one
another
– Subunit – each polypeptide chain of large protein
– Dimer – protein made of 2 subunits • Can be same subunit or different subunits

Single Subunit Proteins

Different Subunit Proteins
•Hemoglobin
– 2 globin subunits
– 2 b globin subunits

Protein Assemblies
• Proteins can form very large assemblies
• Can form long chains if the protein has 2 binding sites – link together as a helix or a ring
• Actin fibers in muscles and cytoskeleton – is made from thousands of actin molecules as a helical fiber

Types of Proteins
• Globular Proteins – most of what we have dealt with so far – Compact shape like a ball with irregular
surfaces
– Enzymes are globular
• Fibrous Proteins – usually span a long distance in the cell – 3-D structure is usually long and rod shaped

Important Fibrous Proteins
• Intermediate filaments of the cytoskeleton – Structural scaffold inside the cell
• Keratin in hair, horns and nails
• Extracellular matrix – Bind cells together to make tissues
– Secreted from cells and assemble in long fibers • Collagen – fiber with a glycine every third amino
acid in the protein
• Elastin – unstructured fibers that gives tissue an elastic characteristic

Collagen and Elastin

Stabilizing Cross-Links
• Cross linkages can be between 2 parts of a protein or between 2 subunits
• Disulfide bonds (S-S) form between adjacent -SH groups on the amino acid cysteine

Proteins at Work
• The conformation of a protein gives it a unique function
• To work proteins must interact with other molecules, usually 1 or a few molecules from the thousands to 1 protein
• Ligand – the molecule that a protein can bind
• Binding site – part of the protein that interacts with the ligand – Consists of a cavity formed by a specific arrangement
of amino acids

Ligand Binding

Formation of Binding Site
• The binding site forms when amino acids from within the protein come together in the folding
• The remaining sequences may play a role in regulating the protein’s activity

Antibody Family
• A family of proteins that can be created to bind to almost any molecule
• Antibodies (immunoglobulins) are made in response to a foreign molecule ie. bacteria, virus, pollen… called the antigen
• Bind together tightly and therefore inactivates the antigen or marks it for destruction

Antibodies
• Y-shaped molecules with 2 binding sites at the upper ends of the Y
• The loops of polypeptides on the end of the binding site are what imparts the recognition of the antigen
• Changes in the sequence of the loops make the antibody recognize different antigens - specificity

Antibodies