Treatment of Recurrent Platinum-Refractory Ovarian Cancer
description
Transcript of Treatment of Recurrent Platinum-Refractory Ovarian Cancer

© du Bois 2008© du Bois 2008
Treatment of Recurrent Platinum-Refractory Treatment of Recurrent Platinum-Refractory
Ovarian CancerOvarian Cancer
Andreas du BoisAndreas du BoisDept. Gynecology & Gynecologic OncologyDept. Gynecology & Gynecologic Oncology
Wiesbaden, GermanyWiesbaden, Germany

© du Bois 2008© du Bois 2008
AGO-OVAR Definitions of Platinum Sensitivity*AGO-OVAR Definitions of Platinum Sensitivity*
* A. du Bois et al. 1997
(potentially) platinum-sensitive disease:(potentially) platinum-sensitive disease:
• no prior chemotherapyno prior chemotherapy
• oror response to prior platinum-based Tx or no evidenceresponse to prior platinum-based Tx or no evidence
of disease after prior platinum (CR, PR, NED)of disease after prior platinum (CR, PR, NED)
• andand relapse after treatment free interval > 6 monthsrelapse after treatment free interval > 6 months
platinum-refractory disease:platinum-refractory disease:
• no response to prior platinum-based Tx (NC, PD)no response to prior platinum-based Tx (NC, PD)
• oror PD free interval < 6 months after prior platinum Tx PD free interval < 6 months after prior platinum Tx

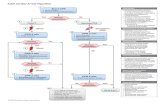
Treatment modalities in Platinum-resistentTreatment modalities in Platinum-resistentRecurrent Ovarian Cancer:Recurrent Ovarian Cancer:
SurgerySurgeryfew selected pts. few selected pts.
(e.g. bowel obstruction)(e.g. bowel obstruction)
Endocrine TXEndocrine TXSelected pts.,Selected pts.,
rather 3rd/4th line ?rather 3rd/4th line ?
Supportive careSupportive careevery pt. as neededevery pt. as needed
RadiotherapyRadiotherapyfew selected pts.few selected pts.
Psy-Soc SupportPsy-Soc Supportevery pt. as neededevery pt. as needed
““new drugs“new drugs“only in clinical trialsonly in clinical trials
non-Pt mono-Txnon-Pt mono-Tx
non-Pt non-Pt combination Txcombination Tx
Pt based therapyPt based therapymainly pt-sensitive ROCmainly pt-sensitive ROC

© AdB & JP 2007
What is the Evidence – 9/2008 ?Randomised Studies in Recurrent OC:
Studies Pts.
• mono- vs. mono chemotherapy 10 2.195
• mono: schedule/dose/application 7 1.614
• mono- vs. endocrine therapy 2 303
• endocrine vs. endocrine therapy 2 106
• combination vs. combination 2 107
• mono vs. combination* 14 3.499
all: 37 7.924
* Including 1 trial with multiple regimens according to testing; most other trials in pts. with platinum sensitive relapse

© AdB 2008
TCA Ovarian Cancer Trial
A prospective randomised controlled trial of tumour chemosensitivity assay directed chemotherapy versus physician’s choice in patients with recurrent platinum-resistant ovarian cancer.
Ian A Cree, on behalf of the TCA Ovarian Cancer Trial Group
Methode:ATP-based Tumor ChemosensitivityAssay (ATP-TCA)
Andreotti et al., Cancer Research 1995; 55: 5276-82

© AdB 2008
Patients - tested drugs/combinations
180 randomised
86 assignedPhysician’s choicechemotherapy
94 assignedAssay-directedchemotherapy
28 received >= 6 cycles50 received < 6 cycles8 treatment never began
30 received >= 6 cycles51 received < 6 cycles13 treatment never began
72 died14 alive at analysis
80 died14 alive at analysis
86 assessed for PFS
94 assessed forPFS
180 randomised
86 assignedPhysician’s choicechemotherapy
94 assignedAssay-directedchemotherapy
28 received >= 6 cycles50 received < 6 cycles8 treatment never began
30 received >= 6 cycles51 received < 6 cycles13 treatment never began
72 died14 alive at analysis
80 died14 alive at analysis
86 assessed for PFS
94 assessed forPFS
cisplatincisplatin gemcitabinegemcitabine cisplatin + gemcitabinecisplatin + gemcitabine doxorubicin (Doxil/Caelyx)doxorubicin (Doxil/Caelyx) paclitaxelpaclitaxel mitoxantronemitoxantrone mitoxantrone + paclitaxelmitoxantrone + paclitaxel topotecan topotecan treosulfantreosulfan treosulfan + gemcitabinetreosulfan + gemcitabine treosulfan + epirubicintreosulfan + epirubicin cisplatin (x2) + etoposidecisplatin (x2) + etoposide etoposideetoposide
Physicians choice: mainly single agent therapyPhysicians choice: mainly single agent therapy

© AdB 2008
Results: Chemosensitivity-Assay without any Impact on Outcome
Median PFS: HR: 0.80, 95%CI 0.59 - 1.10Physician’s choice: 93 daysAssay-directed: 104 days
= + 11 days (median)
number at risk
PC 38 22TCA 49 33
0.00
0.25
0.50
0.75
1.00
Prop
ortio
n st
ill ev
ent f
ree
0 100 200 300 400 500 600Event free survival in days
Physician's choice TCA-directed
Progression Free Survival
number at risk
PC 38 22TCA 49 33
0.00
0.25
0.50
0.75
1.00
Prop
ortio
n st
ill ev
ent f
ree
0 100 200 300 400 500 600Event free survival in days
Physician's choice TCA-directed
Progression Free Survival
Median OS:HR: 1.01 95%CI 0.7-1.3Physician’s choice: 260 daysAssay-directed: 261 days
= + 1 day (median)
number at risk
PC 65 51 38 21TCA 74 55 36 26
0.00
0.25
0.50
0.75
1.00
Prop
ortio
n su
rviv
ing
0 100 200 300 400 500 600 700 800 900 1000 1500Survival in days
randcode = Physician's choice randcode = TCA-directed
Overall Survival
number at risk
PC 65 51 38 21TCA 74 55 36 26
0.00
0.25
0.50
0.75
1.00
Prop
ortio
n su
rviv
ing
0 100 200 300 400 500 600 700 800 900 1000 1500Survival in days
randcode = Physician's choice randcode = TCA-directed
Overall Survival

© AdB & JP 2007
mono vs. combination chemotherapy in refractory recurrent OC
R
Melphalan 8 mg/m² d1-4 q28
Melphalan 6 mg/m² d1-4 q28 Hexa-MM 120 mg/kg d1-14 q28
Pater JL 1987, Cancer Treat Rep
103 pts.
102 pts.
results: OR 2% vs 3% (combi), PFS and OS n.a.
R
Paclitaxel 175 mg/m² 3h q28
Paclitaxel 150 mg/m²Epirubicin 120 mg/m² q28
Bolis G 1999, Gynecol Oncol
41 pts.
40 pts.
results: OR 17% vs. 34% (combi), PFS n.a. 2-YSR 10% vs. 18% (n.s.)

© AdB & JP 2007
R
Paclitaxel 175 mg/m² 3h q21
Paclitaxel 175 mg/m²Epirubicin 80 mg/m² q21
Buda A 2004, Br J Cancer
106 pts.
≤ 12 mos.
106 pts.
results: OR 47% vs. 37% (combi), PFS 6 vs. 6 mos. OS 14 vs. 12 mos. (n.s.)
R
Topotecan 1.25 mg/m² d1-5 q21
Topotecan 1.0 mg/m² d1-5 Etoposid 50 mg po d 6-12 q21 Sehouli J 2008, JCO
178 pts.
177 pts.
results: OR 36% (TE) vs. 32% (TG) vs. 28 % (Topo) mean PFS 15 vs. 13 vs. 13 months (n.s.)
mean OS 23 vs. 18 vs. 24 months (n.s.)
Topotecan 0.5 - 0.75 mg/m² d1-5 Gemcitabine 800 mg/m² d1 + 600 mg/m² d8 q21
app. 20% refractory41% > 12 Mon.
147 pts.
mono vs. combination chemotherapy in refractory recurrent OC

© AdB & JP 2007
Trabectedin+PLD4.0 mos
PLD3.7 mos
PFS events: 163HR: 0.95 (0.70-1.30)P = 0.7540 by courtesy of BJ Monk et al (Email: [email protected])
mono vs. combination chemotherapy in refractory recurrent OC
R
Doxil/Caelyx (PLD) 50 mg/m² q28
Trabectedin 1.1 mg/m² q 21 +Doxil/Caelyx (PLD) 30 mg/m² q28
BJ Monk et all , ESMO 2008
118 pts.
113 pts.
results: OR 12,2% vs 13,4% (combi; n.s.), PFS/OS n.s.

© du Bois 2008© du Bois 2008
Treatment modalities in Platinum-resistentTreatment modalities in Platinum-resistentRecurrent Ovarian Cancer:Recurrent Ovarian Cancer:
SurgerySurgeryfew selected pts. few selected pts.
(e.g. bowel obstruction)(e.g. bowel obstruction)
Endocrine TxEndocrine TxSelected pts.,Selected pts.,
rather 3rd/4th linerather 3rd/4th line
Supportive careSupportive careevery pt. as neededevery pt. as needed
RadiotherapyRadiotherapyfew selected pts.few selected pts.
Psy-Soc SupportPsy-Soc Supportevery pt. as neededevery pt. as needed
““new drugs“new drugs“only in clinical trialsonly in clinical trials
non-Pt mono-Txnon-Pt mono-Tx
Today, no strong evidence supporting combination Txcombination Tx
outside trials (eg.DOVE)
Pt based therapyPt based therapymainly pt-sensitive ROCmainly pt-sensitive ROC

© AdB & JP 2007
What is the Evidence – 9/2008 ?Randomised Studies in Recurrent OC:
Studies Pts.
• mono- vs. mono chemotherapy 10 2.195
• mono: schedule/dose/application 7 1.614
• mono- vs. endocrine therapy 2 303
• endocrine vs. endocrine therapy 2 106
• combination vs. combination 2 107
• mono vs. combination* 14 3.499
all: 37 7.924
* Including 1 trial with multiple regimens according to testing; most other trials in pts. with platinum sensitive relapse

© AdB & JP 2007
AGO-OVAR 2.3 (Stratum 1, Relapse within 6 mos. after Platinum-Paclitaxel)
R
Treosulfan 7 g/m² iv q21
Topotecan 1,5 mg/m² d1-5 q21
Meier W 2003, ASCO
65 pts.
63 pts.
OR 19.3% (topo) vs 7.0% (p=.0524), OS n.s. (+3,9 mos. for topo),PFS significantly superior after topotecan (median +2 mos.)
Topo (63) median 11.2 Mon.
Treo (65) median 7.3 Mon.
HR = 1.36 [0.93-1.97]
p = 0.1111
0%
25%
50%
75%
100%
0 12 24 36 48 60 72
OS
Topo (63) median 4.2 Mon.
Treo (65) median 2.2 Mon.
HR = 1.44 [1.00-2.07]
p = .0476
0%
25%
50%
75%
100%
0 12 24 36 48 60
PFS

© AdB & JP 2007
R
Paclitaxel 175 mg/m² 3h iv q21
Topotecan 1,5 mg/m² iv d1-5 q21
ten Bokkel Huinink 1997, J Clin Oncol
114 pts.
50% refractory, but „only“ to platinum
112 pts.
results: OR 14% vs. 21% (Topotecan)
median PFS 15 vs. 19 months (n.s.)
median OS 53 vs. 63 months (n.s.)
Cave: patients had no taxans during 1st-line
… but today, most patients had platinum
plus taxan-containing 1st-line therapy!
Therefore, data support mainly Topotecan.
mono vs. mono chemotherapy in recurrent (mostly) refractory OC - RCTs

© AdB & JP 2007
R
Paclitaxel 175 mg/m² 3h iv q21
Caelyx 50 mg/m² iv q28
O’Byrne 2002, ASCO
107 pts.
62% refractory, but „only“ to platinum
106 pts.
results: OR 23% vs 19% (Caelyx; n.s.)
median PFS 4.4 vs. 4.8 months (n.s.)
median OS 13 vs. 11 months (n.s.)
Cave: patients had no taxans during 1st-line
… but today, most patients had platinum
plus taxan-containing 1st-line therapy!
Therefore, data support mainly Caelyx.
mono vs. mono chemotherapy in recurrent (mostly) refractory OC - RCTs

© AdB & JP 2007
R
Topotecan 1,5 mg/m² iv d1-5 q21
Caelyx 50 mg/m² iv q28
Gordon 2001, J Clin Oncol 2004, Gynecol Oncol
235 pts.
55% Pt.-refractory, > 70% prior taxans
239 pts.
Results platinum refractory subgroup: Caelyx (130) Topotecan (124) p-value
PFS (weeks, median) 9,1 13,1 0.733
OS (weeks, median) 36 41 0.455
G3/4 toxicity (all pts.;%)
Neutropenia 12 77 < 0.001Anemia 5 28 < 0.001Thrombocytopenia 1 34 < 0.001Leukopenia 10 50 < 0.001Treatment-related sepsis 0 4 < 0.001
Alopecia (all grades) 16 49 0.007Hand-Foot-Syndrom 23 0 < 0.001Stomatitis 8 0.4 < 0.001
mono vs. mono chemotherapy in recurrent (mostly) refractory OC - RCTs

© AdB & JP 2007
R
Canfosfamid 1000 mg/m² iv d1 q21
Caelyx 50 mg/m² iv q28 orTopotecan 1.5mg/m² iv d1-5 q21
Vergote 2007, ASCO
232 pts.
3rd-line after platinum-taxan and caelyx or topotecan 2nd-line
229 pts.
mono vs. mono chemotherapy in recurrent (mostly) refractory OC - RCTs
N PFS, median (wks)
Canfosfamide 232 10
Control 229 18.8
weeks
N OS, median (wks)
Canfosfamide 232 37
Control 229 58.9
wks
Results: canfosfamide inferior compared to standard arm

© AdB & JP 2007
R
Gemcitabine 1000 mg/m² d1+8 q21
Caelyx 50 mg/m² d1 q28
Mutch, JCO 2007
99 pts.
96 pts.
Results:
mono vs. mono chemotherapy in recurrent (mostly) refractory OC - RCTs
66 pts.
64 pts.
ParameterCAELYX(n=96)
Gemcitabine (n=99)
ORR (pts w/ measurable disease)
median PFS
median OS
8%
3.1 mos.
13.5 mos.
6%
3.6 mos.
12.7 mos.
Toxicity
Neutropenia, grade 3/4 Constipation, grade 2-4 N/V, grade 2-4 HFS, grade 2/3 Mucositis, grade 2/3
18%9%
12%19%*15%*
38%*25%*28%*
--3%
*Statistically significant.

© AdB & JP 2007
Results:
OR 16% vs. 18% (Gem), OR duration 18 vs. 17 (Gem) weeks ; n.s.
QoL advantage for caelyx in 2 of 4 time points (p < 0.05)
R
Gemcitabine 1000 mg/m² d1,8, 15 q28
Caelyx 40 mg/m² d1 q28
Mito-3
G Ferrandina et al JCO 2008
77 pts.
100% platinum-taxan, TFI < 12 mos. (57% < 6 mos.)
76 pts.
mono vs. mono chemotherapy in recurrent (mostly) refractory OC - RCTs

© du Bois 2008© du Bois 2008
Treatment modalities in Platinum-resistentTreatment modalities in Platinum-resistentRecurrent Ovarian Cancer:Recurrent Ovarian Cancer:
SurgerySurgeryfew selected pts. few selected pts.
(e.g. bowel obstruction)(e.g. bowel obstruction)
Endocrine TxEndocrine TxSelected pts.,Selected pts.,
rather 3rd/4th linerather 3rd/4th line
Supportive careSupportive careevery pt. as neededevery pt. as needed
RadiotherapyRadiotherapyfew selected pts.few selected pts.
Psy-Soc SupportPsy-Soc Supportevery pt. as neededevery pt. as needed
““new drugs“new drugs“only in clinical trialsonly in clinical trials
1st choice:1st choice:non-Pt mono-Txnon-Pt mono-Tx• Caelyx Caelyx • Topotecan Topotecan • Gemcitabine
Today, no strong evidence supporting combination Txcombination Tx
outside trials (eg.DOVE)
Pt based therapyPt based therapymainly pt-sensitive ROCmainly pt-sensitive ROC