Too Much Pancreas Pathology
description
Transcript of Too Much Pancreas Pathology

Too Much Pancreas Pathology

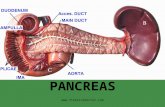
Normal Pancreas• 15 cm long, 60-140g• Shape is compared to letter J turned sideways, with loop of J
around the duodenum• Divided into head (right of left border of superior mesenteric
vein; contains uncinate process), body (between left border of superior mesenteric vein and left border of aorta) and tail
• A retroperitoneal organ, lies within duodenal curve, close to superior mesenteric artery and portal vein
• Anterior body of pancreas touches posterior wall of stomach; posterior of pancreas touches aorta, splenic vein and left kidney
• Pancreatic tail extends to the splenic hilum• Has large functional reserve of cells

Normal Pancreas

Exocrine Pancreas• Pancreatic enzymes: trypsin, chymotrypsin, aminopeptidases, elastase,
amylases, lipase, phospholipases, nucleases• Trypsin: catalyzes activation of the other enzymes• Pancreatic self-digestion is prevented by:
– packaging of most proteins as inactive proenzymes, enzyme sequestration in zymogen granules
– proenzymes activated only by trypsin which is activated only by duodenal enterokinase
– trypsin inhibitors are present in ductal and acinar secretions– intrapancreatic release of trypsin activates enzymes which degrade other
digestive enzymes before they can destroy pancreas– lysosomal hydrolases can degrade zymogen granules to prevent auto
destruction if acinar secretion is impaired– acinar cells themselves are highly resistant to trypsin, chymotrypsin and
phospholipase A2

Endocrine Pancreas• Consists of islets of Langerhans, represents 1% of pancreas (percentage higher at
birth)• Round, compact, highly vascularized with scanty connective tissue; more irregular
outline and trabecular arrangement in posterior head of pancreas with cells producing pancreatic polypeptide
• Size of islets usually 0.1 to 0.2 mm, endodermal origin, one million islets present in pancreas
• Islet composition: beta cells (68%), alpha cells (20%), delta cells (10%), D1 cells (2%), Enterochromaffin cells (rare)
• Alpha cells: produce glucagon; peripheral dense and round on EM• Beta cells: produce insulin and islet cell amyloid polypeptide (amylin), crystalline
appearance on EM with surrounding halo• Delta cells: produce somatostatin (represses release of insulin and glucagon), large
pale granules on EM• D1 cells: produce HPP, stimulates GI fluid secretion and excess causes secretory
diarrhea• Enterochromaffin cells: synthesize serotonin, excess produces carcinoid syndrome

Congenital Abnormalities• Agenesis• Annular pancreas
– Incidence 1 per 7000– Head of pancreas circles duodenum as a collar and may constrict lumen due to failure of
ventral bud to rotate properly– Associated with Down’s syndrome– Associated with pancreatitis, duct obstruction, peptic ulcer
• Ectopic Pancreas– Often in gastric antrum, duodenum, jejunum, Meckel’s diverticulum, gastroesophageal
junction– Case report of pancreatic cyst in anterior mediastinum– Usually incidental findings but may cause ulceration, obstruction, intussusception– Vulnerable to same diseases as normal pancreas (2% of islet cell tumors arise in ectopic
pancreatic tissue), may undergo malignant transformation• Pancreatic Divisum
– 3-10% of population with incomplete fusion of dorsal and ventral pancreatic buds / ducts– Duct of Santorini (accessory duct) and separate pancreatic duct drain into the distal
duodenum.– May predispose to recurrent acute pancreatitis if minor papilla is obstructed

Complete & Partial Pancreatic Divisum

Acute Pancreatitis
• Acute onset of abdominal pain due to enzymatic necrosis and inflammation
• 80% associated with biliary tract disease or alcoholism
• 1/3 to 2/3 of patients have gallstones, but only 5% with gallstones develop pancreatitis
• 75% of gallstone related cases occur in women• 86% of alcohol related cases occur in men• Alcoholism associated: 2/3 of all cases in US

Acute Pancreatitis• Less common causes: • trauma (including post-operative),• infection (mumps, coxsackievirus, Mycoplasma pneumonia,
adenovirus in immunocompromised, AIDS related toxoplasmosis
• Acute ischemia (thromboemboli, vasculitis, shock)• Drugs (thiazides, azathioprine, estrogen, sulfa, frusemide,
methyldopa)• Hyperlipidemia, hyperparathyroidism or other causes of
hypercalcemia, hyperthyroidism• 10% idiopathic

Pathophys of Acute Pancreatitis• Obstruction from gallstones or alcohol associated concretions
increases intraductal pressure, causing enzyme-rich interstitial fluid to accumulate, which causes fat necrosis, which attracts neutrophils that release cytokines and cause interstitial edema, which impairs blood flow and causes ischemia and acinar cell injury
• Acinar cell injury also caused by infections, drugs, trauma, shock, premature release of proenzymes and lysosomal hydrolases
• Obstruction or alcohol cause proenzymes to be delivered in an intracellular compartment with lysosomal hydrolases, which may activate them prematurely
• Alcohol may also reactivate chronic pancreatitis due to secretion of protein-rich pancreatic fluid, which causes deposition of protein plugs, causing obstruction of small pancreatic ducts

Acute Pancreatitis - Clinical• Symptoms: abdominal pain, high white blood count, DIC, ARDS, diffuse fat
necrosis, peripheral vascular collapse, acute tubular necrosis, shock (blood loss, electrolyte disturbances, endotoxemia, release of cytokines), hypocalcemia, hyperglycemia
• DD: acute abdomen (appendicitis, perforated peptic ulcer, acute cholecystitis with rupture, occlusion of mesenteric vessels with bowel infarction)
• Diagnosis: elevated amylase (also seen in duodenal ulcer, volvulus, gangrenous cholecystitis, ruptured abdominal aortic aneurysm, mesenteric thrombosis), elevated lipase, elevated CRP – Xray (pancreas large and inflamed)
• Outcome: 5% die of shock during first week; overall mortality is 20% (10% if swollen/edematous) vs. 50% if hemorrhagic/necrotic
• Acute respiratory distress syndrome or acute renal failure are poor prognostic factors
• Gross: swollen, edematous or hemorrhagic/necrotic, yellow nodules represent fat necrosis in pancreas, mesenteric and peritoneal fat; may spread to colon and cause ileus, stenosis, perforation, fistulas


Chronic Pancreatitis• Men, 40+, often alcoholics; biliary disease usually not a factor in
chronic pancreatitis• Repeated attacks of inflammation with loss of parenchyma and
replacement with fibrosis• Causes variable pain and symptoms of pancreatic insufficiency
(malabsorption, steatorrhoea, diabetes)• May coexist with pancreatic carcinoma or intraductal papillary
mucinous neoplasm (IPMN)• Attacks precipitated by alcohol, overeating, opiates, other drugs• Other risk factors: hypercalcemia, hyperparathyroidism,
hyperlipoproteinemia, pancreas divisum (seen in 12%), pancreatic neoplasm or IPMN, cystic fibrosis but no known risk factor in 30%
• Also associated with mumps, polyarteritis nodosa, sarcoidosis, malakoplakia, primary sclerosing cholangitis, HIV (mild changes)

Other Subtypes of Chronic Pancreatitis
• Autoimmune pancreatitis (IgG, mass formed by dense periductal infiltrate compressing the bile duct)
• Herpes simplex (parenchymal necrosis, hemorrhage, minimal fat necrosis, many multinucleated giant cells with ground-glass appearance
• Very rare: CMV and eosinophilic pancreatitis

Chronic Pancreatitis - Clinical• Diagnosis: mildly elevated amylase during attacks; CT scan shows
calcifications; weight loss, intractable abdominal pain, hypoalbuminaemia and associated oedema due to pancreatic insufficiency
• Treatment: pancreatic duct drainage, Whipple resection (relieves pain in 50% of patients with pain)
• Gross: hard, dilated ducts, visible calcified concretions (protein plugs), pseudocysts; 5% have obstruction due to tumor or stones
• Micro: loss of acini with relative sparing of islets, irregularly distributed periductal fibrosis, obstruction of pancreatic ducts of all sizes; chronic inflammation (including mast cells) around lobules and ducts; dilated ducts with concretions; ductal epithelium is atrophic, hyperplastic or undergoes squamous metaplasia; islets may become sclerotic and disappear; may have islet cell proliferation with invasive-like pattern

Fibrosis due to Chronic Pancreatitis

Congenital Cysts
• Polycystic disease affecting liver and kidney• Von Hippel Lindau syndrome (rare autosomal
dominant mutation of VHL TS gene – found in inbred feuding hillbillies made angry by phaeochromocytomas)
• Pancreatic cystic dysplasia • Cysts of Cystic Fibrosis
The very angry Hatfield clan in 1897

Acquired Cysts & Tumours

Serous Cystadenoma
• Serous cystadenomas are characterized by a circumscribed, sponge-like gross appearance with a central stellate scarm Microscopically: characterized by clear glycogen-rich ells

Mucinous Cystic Neoplasm• Almost always women, mean age 45 (young), if malignant then
better prognosis than ductal adenocarcinoma• Present with abdominal pain or mass• Can be benign, borderline or malignant• Metastases usually restricted to abdominal cavity; metastases to
ovary may simulate primary ovarian tumors• Gross: large (mean 10 cm); usually in body/tail, multilocular
(occasionally unilocular) megacysts that don’t communicate with ductal system unless fistula are present; cyst wall is papillary, trabecular or thickened; has mucoid/watery cyst contents
• Micro: lined by tall mucin-producing cells, often forming papillae; supported by ovarian-type stroma; endocrine cells often scattered among columnar lining cells.

Mucinous Cystic Neoplasm (LP)

Mucinous Cystic Neoplasm (HP)

Solid Pseudopapillary Neoplasm
• Rare, benign or low grade malignant forming cystic structures of epithelial origin containing hemorrhagic debris and usually surrounded by necrotic-hemorrhagic tissue

Intraductal papillary mucinous neoplasm (IPMN)
• Affects mostly older males (> 60 YO)• Papillary proliferation of ductal epithelium of the
head with excessive thick mucin production• Somewhat indolent; 30% associated with invasive
carcinoma, which is often colloid carcinoma• Gross: May present as multilocular cystic masses• Micro: complex papillary fronds of mucin-producing
epithelial cells; ductal fibrosis, acinar atrophy but well preserved islets; associated with chronic pancreatitis

IPMN
• (IPMN) grossly involves the main pancreatic ducts

IPMN
• The tail of the pancreas contained an intraductal papillary mucinous neoplasm (IPMN) which may be a a precursor to invasive carcinoma

MCN v IPMN
MCN versus IPMNF >> M M > F40-50 60-70Tail HeadNot in duct In ductOvarian stroma No ovarian type stroma

Exocrine Cancers - Ductal Adenocarcinoma• Ductal adenocarcinoma (85% of pancreatic cancers)• Risk factors: Family Hx, smoking, alcohol abuse, obesity, beta-naphthylamine or benzidine
exposure, familial relapsing pancreatitis, oldness (uncommon < 40 YO)• Uncertain risk factors: chronic pancreatitis, diabetes, maleness (M/F = 1.6:1), high
consumption of fructose sweetened drinks, high meat/fat – low vegetable diet• Familial syndromes: hereditary nonpolyposis colorectal carcinoma (Lynch Syndrome),
familial atypical mole-melanoma syndrome, Peutz-Jeghers syndrome, hereditary pancreatitis
• Clinical: 60% of tumors are in head, 15% in body, 5% in tail, 20% diffusely involve pancreas• Symptoms: pain, weight loss, anorexia, malaise, weakness• Trousseau sign: migratory thrombophlebitis, in 10% due to tumor or tumor necrosis
producing platelet-aggregating factors and procoagulants; causes arterial and venous thrombi, including pulmonary and portal thromboemboli
• Metastases: liver, lung, peritoneum, adrenal, bone, distal nodes; supraclavicular node metastases may be presenting symptom, metastases to ovary may simulate primary mucinous ovarian tumors
• Treatment (curative): distal pancreatectomy for body/tail tumors, Whipple resection (subtotal pancreaticoduodenectomy) for head/periampullary tumors
• Most (85%) tumors are not amenable to curable surgery• Prognosis: 5 year survival 2-4%, 90% die within 1 year

Ductal Adenocarcinoma

Ductal Adenocarcinoma
Infiltrating pancreatic adenocarcinoma – abnormal nuclei, mitotic figures – the usual cancer stuff. Metastasises to peripancreatic lymph nodes.

Adenosquamous carcinoma
• You thought ductal adenocarcinoma was bad - worse prognosis (mean survival 6 months)
• Malignant squamoid and glandular components• Rare, M/F = 2:1, mean age 65• Aspirates show extensive necrosis with dense
blue globules• Clinical: upper abdominal distension, dyspepsia,
jaundice, and significant weight loss over a period of months

Adenosquamous carcinoma
• adenosquamous carcinoma of the pancreas characterized by clear cells and rhabdoid cells (eosinic)

Colloid Carcinoma
• Tumors composed predominantly (80%+) of scanty malignant epithelial cells floating in nodular extracellular mucin lakes with muconodular invasive component
• Mean age 61; M=F, usually in head of pancreas• May have originated from high grade IPMN• 5 year survival 57%

Endocrine Carcinomas• 3-5% of pancreatic neoplasms, 80% occur in MEN1 patients, usually age 50-60• “Islet cell tumors” is inaccurate, as they are believed to arise from pluripotential
ductal cells that have the capacity to differentiate along neuroendocrine lines• Most are malignant; no TNM staging exists for these tumors• Most tumors are functional and secrete multiple hormones, hCG more common
in malignant tumors. Usually produce glucagon, insulin or pancreatic polypeptide; tendency towards multiple tumors in same patient, often microscopic, which often produce different hormones; associated with nesidioblastosis in 30% of cases (B-cell hyperplasia causing hypoglycemia and hyperinsulinemia)
• Usually occurs in adults in body/tail • Tumors are either syndromic or not; non-syndromic may produce hormones so
are still functional• Tumors are hypervascular and circumscribed• Usually slow growing, metastases to nodes, liver, bone

Pancreatic Endocrine Carcinoma
pink (resemble spleen, lymph node), no well defined capsule, variable fibrous tissue, calcium, bone, cysts