The Mole Unit 3 The Mole So far we have been dealing with numbers on the atomic scale… –Atomic...
-
Upload
lee-grace-cunningham -
Category
Documents
-
view
212 -
download
0
Transcript of The Mole Unit 3 The Mole So far we have been dealing with numbers on the atomic scale… –Atomic...

TheTheMoleMole
Unit 3

The MoleThe Mole
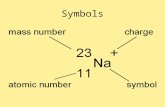
• So far we have been dealing with numbers on the atomic scale…– Atomic Number = # of protons– Mass Number = # of protons + # of
neutrons– Atomic Mass = # of amu in one
average atom

The MoleThe Mole
• This is a really small scale.• Is it logical to try to measure out
chemicals for labs this way?
• NO!
• We need a bigger scale to use for everyday measurements and calculations.

The MoleThe Mole
• We need…
• The MOLE!
• The MOLE is a unit of measure – a unit of “how many”.

The MoleThe Mole
• Just like a dozen eggs tells us “how many” eggs, a mole tells us “how many” particles.
• 1 dozen eggs = 12 eggs
• 1 mole = 6.02 x 1023 particles– Particles can be anything –
atoms, molecules, eggs, etc.

The MoleThe Mole
• This number (6.02 x 1023) is called Avogadro’s Number, in honor of Amedeo Avogadro’s contributions to chemistry.
• Unfortunately for him, his ideas weren’t really recognized as important until after he died.

The MoleThe Mole
• But wait, where did we get this number?
• Scientists have found that there is exactly 1 mole of atoms in the atomic mass of an element when that mass is expressed in grams.
• Huh?

The MoleThe Mole
• For example:
• What is the atomic mass of C-12?
• (Hint – there is only one isotope here, so think mass number)
• Atomic mass = 12 amu

The MoleThe Mole
• Then express that mass in grams 12 g
• In exactly 12 grams of C-12, there is one mole (6.02 x 1023) of atoms.
• This also works for our average atomic masses (from the periodic table).

The MoleThe Mole
• So, in exactly 12.01 g of natural carbon there are 6.02 x 1023 atoms.
• AND, 6.02 x 1023 atoms = 1 mole. So 1 mole of carbon = 12.01 g.
• Hmm…..those sounds suspiciously like conversion factors…
• Time for examples!

The MoleThe Mole
• How many atoms are in 1.00 mole of gold?
1.00 mole Au 6.02 x 1023 atoms
1 mole =
6.02 x 1023 Atoms Au

The MoleThe Mole
• How many molecules are in 2.00 moles of water?
2.00 mole H2O 6.02 x 1023 molecules
1 mole =
1.20 x 1024 MoleculesH2O

The MoleThe Mole
• How many marbles are in 1.00 mole of marbles? (Enough to cover the entire Earth to a depth of 50 miles!)
1.00 mole marbles 6.02 x 1023 marbles
1 mole =
6.02 x 1023 marbles

The MoleThe Mole
• How many grams are in 1.00 mole of neon?
1.00 mole Ne 6.02 x 1023 atoms
1 mole Ne
= 20.2 g Ne
6.02 x 1023 atoms
20.18 g Ne