THE EFFECTS OF ESTRADIOL ON PLASMA …...and medullary bone proliferation are followed by resorption...
Transcript of THE EFFECTS OF ESTRADIOL ON PLASMA …...and medullary bone proliferation are followed by resorption...

-Compo Biochem. Physiol. Vol. 84A, No. I, pp. 107-110, 1986Printed in Great Britain
0300-9629/86 $3.00 + 0.00© 1986 Pergamon Press Ltd
THE EFFECTS OF ESTRADIOL ON PLASMA CALCIUMAND FEMORAL BONE STRUCTURE IN ALLIGATORS
(ALLIGATOR MISSISSIPPIENSIS)
RUTHM. ELSEY*and CAROLES. WINKt*Louisiana Department of Wildlife and Fisheries, Rockefeller Wildlife Refuge, Grand Chenier,
LA 70643, USA. +Department of Anatomy, Louisiana State University Medical Center,1901 Perdido Street, New Orleans, LA 70112, USA. Telephone: (504) 582-5718
(Received 3 September 1985)
Abstract-I. The effects of exogenous estradiol on plasma calcium and femoral bone structure werestudied in young male and female alligators.
2. Males and females responded in the same manner to estrogen treatment.3. Eight days after the initial injection, plasma calcium was significantly greater in experimentals than
in controls.4. No changes in femoral bone structure were observed.
INTRODUCfION
Birds and many reptiles are egg-layers. In birds,pre-ovulatory, estrogen-dependent hypercalcemiaand medullary bone proliferation are followed byresorption of this endosteal bone which can be cor-related with formation of the calcareous eggshell(Simkiss, 1967; Taylor et al., 1971). Estrogen treat-ment elicits hypercalcemia and endosteal bone for-mation in birds (Bloom et al., 1941), and hyper-calcemia without endosteal bone formation in lowerreptiles, such as turtles (Simkiss, 1961; Magliola,1984). Turtles, apparently, have not evolved theskeleton-sparing mechanism of pre-ovulatory boneformation to form eggshells; they resorb structuralbone during eggshell formation (Edgren, 1960;Simkiss, 1961; Suzuki, 1963; Magliola, 1984). It hasbeen shown that breeding female alligators, likeother egg-laying vertebrates, exhibit a pre-ovulatoryhypercalcemia associated with eggshell formation(Lance et aI., 1983). Recent studies in our laboratoryhave indicated that the alligator also resorbs struc-tural bone during eggshell formation (Elsey andWink, 1985). It is not known, however, how theskeleton and plasma calcium levels of alligators re-spond to exogenous estrogen treatment. Alligators,which are closely related to birds in evolution, may,like birds, develop hypercalcemia and form endostealbone in response to estrogen treatment in addition toresorbing structural bone during eggshell formation,as do turtles. Therefore, the purpose of this study wasto determine the effects of estrogen treatment onplasma calcium levels and femoral bone morphologyof the alligator.
MATERIALS AND METHODS
AnimalsTen young alligators (Alligator mississippiensisy (3.5 years
old) were obtained from a commercial alligator farm(Sauros Inc., Bell City, LA). These alligators had beenhatched from artificially incubated alligator eggs collectedfrom Rockefeller Wildlife Refuge (Joanen and McNease,
1977). The animals were sexed by palpating within thecloaca and noting the presence or absence of a penis. Theywere then divided into two groups, with three femalesand two males in each group. They were maintained atRockefeller Wildlife Refuge, Grand Chenier, LA, in con-trolled environmental chambers (Joanen and McNease,1977) and fed a diet of ground nutria (Myocastor coypus).
An initial 5 ml blood sample was taken from each animalby cardiac puncture with a heparinized syringe. One group(three females, two males) was injected with 5 mg estradioldissolved in 1 ml sesame oil (experimental). The other groupof animals (three females, two males) was injected with 1 mlsesame oil only (controls). Injections were given intra-muscularly at three sites (tail and each lower extremity).Every 8 days for the next month, blood samples were takenfrom each animal, and each animal was re-injected withestradiol, or vehicle, as above. Blood samples were immedi-ately centrifuged and the plasma was removed and frozenfor later assay.
At the end of 1 month (after four injections) the animalswere killed by a blow to the head and the spinal cordssevered. Alligators were weighed and body lengths weremeasured. Necropsies were performed, and livers, ovaries,oviducts, testes and penes were removed and weighed.Femora were also removed and frozen for later analysis.Femora were chosen because Ferguson (1982) showed thatthe femur undergoes less remodeling during aging thanother bones sampled (tibia, fibula, humerus, radius, ulna,mandible, vertebrae, frontal, maxilla, palatine, pterygoid,nasal, jugal, ribs and dorsal neck scutes).
Plasma calcium determinationsPlasma calcium levels were determined by atomic
absorption with a 305B Perkin-Elmer atomic absorptionspectrophotometer.
Femur robusticity indexFemora were thawed and placed in an 85°C water bath
for 1 week to remove soft tissue. They were then defattedand dehydrated in a series of alcohols and ethers and finallydried in a vacuum oven as described previously (Wink andFelts, 1980).They were weighed, and lengths were measuredwith vernier calipers. Bone robustness was determined usingthe formula:
bone lengthrobusticity index = 3/ .v (bone weight)
107

r108 RUTHM. ELSEYand CAROLES. WINK
This robusticity or ponderal index was first proposed byRiesenfeld (1972) and has been used to indicate the robust-ness or density ofa bone as a whole (Riesenfeld, 1975, 1981;Simon, 1984). The higher the index number, the less densethe bone; the lower the number, the more robust or denserthe bone.
Femoral morphometries
Sections of bone 2 em long were cut from the middle ofeach dried femoral shaft and embedded in Ward's Bio-plastic. Cross sections of 200 11m thickness were cut fromeach piece using a Bronwill Hard Tissue Cutting Machineand a diamond saw. Microradiographs of the femoral crosssections were made with a Faxitron X-ray machine andKodak high resolution plates (649-0). The microradiographswere processed routinely, projected with a microprojectorand drawn at 20 x magnification. Drawings of bone sectionswere analyzed with a Numonics Electronic Calculator fortotal area, area occupied by bone (mm"), percentage areaoccupied by bone, area occupied by marrow cavity (mrn")and percentage area occupied by marrow cavity. Total areawas obtained by marking around the perimeter of theenlarged drawing of each bone section. Area occupied bybone was obtained by marking around bone in the section(excluding marrow cavity and "holes" or radiolucent areasin the bone). Area of marrow cavity was obtained bymarking around the marrow cavity of each section. Thepercentage areas occupied are obtained using:
area occupied by bonepercentage area occupied by bone = ----'-----
total areaarea of marrow cavity
percentage area of marrow cavity =----:----~total area
Data were analyzed by Student's t-test.
RESULTS
There were no significant differences in bodyweight, body length, liver weight or gonad weightbetween the sexes (male and female control animalsor male and female experimental animals). Therewere no significant differences in body weight, bodylength or gonad weight between experimentals andcontrols (Table I). Oviduct weight was significantlygreater in estrogen-treated females than in controlfemales and liver weight was significantly greater inexperimentals than in controls (Table 1). There wereno significant differences in femur robusticity indices,percentage area occupied by bone or percentage area
of marrow cavity between the groups (Table 2, Fig.I). Figure 2 shows that at the beginning of theexperiment (0 weeks) there were no significantdifferences in plasma calcium levels between experi-mentals .and controls. One week after the initialinjection, plasma calcium levels were significantlygreater (P < 0.02) in experimentals when comparedto controls; after 2 weeks, P < 0.001, after 3 weeks,P < 0.001 and after 4 weeks, P < 0.01.
DISCUSSION
It is well known that in egg-laying vertebrates thedeveloping ovarian follicle secretes estradiol, whichstimulates the liver to synthesize vitellogenin, acalcium-binding lipophosphoprotein (Ho et al.,1982). Similarly, estradiol injections caused synthesisof vitellogenin in a crocodilian, Caiman latirostris,(Van Brunt and Menzies, 1971). In the present studyestrogen treatment induced liver hypertrophy in maleand female alligators as it did in snakes (Dessauerand Fox, 1959) and lizards (Callard et al., 1972;Callard and Klotz, 1973; Hahn, 1967). This liverhypertrophy was probably secondary to vitellogeninsynthesis. Our study also confirms the findings ofForbes (1938), who observed an extreme hypertrophyof the oviducts of female alligators injected withestrogen, and no differences in the penes of estrogen-treated males when compared to those of controlmales. Histological studies (Forbes, 1938) of thegonads from estrogen-treated animals showed hyper-trophy of both ovarian and testicular cortices with nochanges seen in the medullas. Gonad weights in theexperimental alligators of our study were greater(although not significantly) than in the controls,which may have reflected cortical hypertrophy withno increase in the medulla.
Prosser and Suzuki (1968) injected another croco-dilian, Caiman sclerops, with estrogen and observeda gradual hypercalcemic response that did not be-come significant until 3 weeks after treatment wasbegun. Also, there were no morphological changes inthe bones. However, the investigators used hatchlingand young juvenile caimans for their studies andnoted that the immaturity of the animals may havebeen responsible for the low response to estrogen
Group N
Table I. Effects of estrogen on body weight and length, weight of gonads, penis, oviduct, liver and liver weight/body weight (mean ± SD)
Body weight Body length Gonad weight Penis weight Oviduct weight Liver weight Liver weight(kg) (em) (g) (g) (g) (g) Body weight
Experimental 5(Estrogen-trea ted)
7.81 ± 0.85 132.33 ± 3.73 4.70 ± 1.67 5.80 ± 0.85 (2) 204.40 ± 58.10* (3) 136.18 ± 26.80' 1.73 ± 0.23*
Control 7.69 ± 0.79 135.38± 2.79 3.56 ± 1.27 7.45 ± 6.86 (2) 9.83 ± 4.70 (3) 90.7 ± 15.20 l.l7 ± 0.09'Significantly different from controls, P < 0.0 I.(X) = number of animals.
Table 2. Effects of estrogen on femoral robusticity index, total area of femoral microradiographs,and percentage area occupied by marrow cavity and bone (mean ± SD)
Mid-shaft cross section x 20
Robusticity Total Area % MarrowGroup N index rnm! cavity % Bone
Experimental 5 4.07 ± 0.13 282.25 ± 14.25 6.04 ± 2.03 91.40 ± 1.76(estrogen-treated)
Control 4.00 ± 0.13 293.45 ± 27.46 5.43±2.15 91.48 ± 2.49

Effects of estrogen on alligators 109
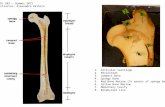
Fig. 1. Microradiograph (4 x magnification) of femoral cross sections from (A) estrogen-treated femaleand (B) control female. Note similarity of marrow cavities and endosteal resorption areas.
treatment. Budy et at. (1952) also attributed lack ofendosteal bone formation, after estrogen treatment,to age in newborn rats. In the present study, we usedolder (3.5 years old) sexually immature alligators, andthe hypercalcemic response was much more rapid (at8 days). Eight days was the earliest time we sampledblood after the initial injection; there may have beena significant difference in calcium levels betweenexperimentals and controls before 8 days. Suzuki andProsser (1968) reported significant differences inserum calcium between estrogen-treated adult lizardsand controls after 5 days.
110
" t',co 90 ,l <,,E , ' --------l~ 70 l:2 lu
111(":c50u
<~ II'" 30< II~ IIL
,I i ! i if10
o 2
WEEKS
Fig. 2. Response of plasma calcium levels in Alligatormississippiensis after estradiol injections. Hatched line repre-sents experimental (estradiol-injected) alligators, solid linerepresents control alligators (in each group N = 5). Experi-mentals were significantly different from controls by I week(P < 0.02); after 2 and 3 weeks (P < 0.001); and after 4
weeks (P < 0.01).
We could see no evidence of medullary boneproliferation in the experimental alligators. Marrowcavity areas were the same in both groups and no"extra" bone filled in resorption areas of the endo-steal bone. It is unlikely that there was medullarybone proliferation in the femur at any other site thanthe one we sampled at midshaft, since the robusticityindices of the femora did not differ between thegroups.
In conclusion, it appears that the alligator, likelower reptiles, such as turtles, lizards and snakes,responds to estrogen treatment with hypercalcemia,and, unlike the bird, has not evolved the skeleton-sparing mechanism of estrogen-dependent, pre-ovulatory medullary bone proliferation for the for-mation of egg shells.
Acknowledgements-The authors would like to thank TedJoanen and Larry McNease of the Louisiana Department ofWildlife and Fisheries for assistance in maintenance andsampling of the alligators. We are grateful to Robert Perkinsfor the alligators used in this project. Appreciation isextended to Dr Valentine Lance for his advice and sug-gestions throughout this project and to Deborah Wilson forhelp with the calcium assays. This project was funded in partby the Louisiana Department of Wildlife and Fisheries.
REFERENCESBloom W., Bloom M. and McLean F. C. (1941)
Calcification and ossification, medullary bone changes inthe reproductive cycle of the female pigeon. Anat. Rec. 81,443-475.
Budy A. M., Urist M. R. and McLean F. C. (1952) Theeffect of estrogens on the growth apparatus of the bonesof immature rats. Am. J. Path. 28, 1143-1167.

logenesis in the male, three-toed box turtle (Terrapenecarolina triumquis). Gen. camp. Endocr. 54, 162-170.
Prosser R. L. and Suzuki H. K. (1968) The effects ofestradiol valerate on the serum and bone of hatching andjuvenile caiman crocodiles (Caiman sclerops). Camp. Bio-chem. Physiol. 25, 529-534.
Riesenfeld A. (1972) Metatarsal robusticity in bipedal rats.Am. J. Phys. Anthrop. 36, 229-234.
Riesenfeld A. (1975) Endocrine control of skeletal robust-icity. Acta Anat. 91. 481-499.
Riesenfeld A. (1981) Age changes of bone size and mass intwo strains of senescent rats. Acta Anat. 109, 64-69.
Simkiss K. (1961) The influence of large doses of oestrogensupon the structure of the bones of some reptiles. Nature190, 1217-1218.
Simkiss K. (1967) Calcium in Reproductive Physiology.Rheinhold, New York.
Simon M. R. (1984) The rat as an animal model for thestudy of senile idiopathic osteoporosis. Acta Anat. 119,248-250.
Suzuki H. K. (1963) Studies on the ossesus system of theslider turtle. Ann. NY Acad. Sci. 109, 351-410.
Suzuki H. K. and Prosser R. L. (1968) The effects ofestradiol valerate upon the serum and bone of the lizard,Sceloparus cyanogenys. Proc. Soc. expo Bioi. med. 127,4-7.
Taylor T. G., Simkiss K. and Stringer D. A. (1971) Theskeleton: Its structure and metabolism. In Physiology andBiochemistry oj the Domestic Fowl (Edited by Bell D. J.and Freeman B. M.), Vol. 2, pp. 621-640. AcademicPress, New York.
Van Brunt J. F. and Menzies R. A. (1971) Synthesis ofserum calcium-binding protein lipophosphoprotein com-plex by South American alligators (Caiman latirostris) inresponse to 17f3-estradiol. Fedn Proc. Fedn Am. Sacs expoBioi. 30, 1160.
Wink C. S. and Felts W. J. L. (1980) Effect of castration onthe bone structure of male rats: a model of osteoporosis.Calcif. Tissue Int. 32, 77-82.
110 RUTHM. ELSEYand CAROLES. WINK
J
Callard 1. P., Doolittle J. and Banks W. L. (1972) Recentstudies on the control of the reptilian ovarian cycle. Gen.camp. Endocr. Suppl. 3. 65-75.
Callard 1. P. and Klotz K. L. (1973) Sensitivity ofparameters of estrogen action in the inguanidlizard, Dipsosaurus dorsalis. Gen. camp. Endocr. 21,314-321.
Dessauer H. C. and Fox W. (1959) Changes in ovarianfollicle composition with plasma levels of snakes duringestrus. Am. J. Physiol. 197, 360--366.
Edgren R. A. (1960) A seasonal change in bone density infemale musk turtles, Sternotherus odoratus (Latreille).Camp. Biochem. Physiol. 1, 213-217.
Elsey R. M. and Wink C. S. (1985) Femoral bone as apossible source of calcium for eggshell deposition inAlligator mississippiensis. Anat. Rec. 211, 57A.
Ferguson M. W. J. (1982) The structure and developmentof the palate in Alligator mississippiensis. Doctoral dis-sertation, Queens University, Belfast.
Forbes T. R. (1938) Studies on the reproductive system ofthe alligator. II. The effects of prolonged injections ofoestrone in the immature alligator. J. expo Zool. 78,335-367.
Hahn W. E. (1967) Estradiol-induced vitellogenesis andconcomitant fat mobilization in the lizard Uta stansbur-iana. Camp. Biochem. Physiol. 23, 83-93.
Ho S. M., Kleis S., McPherson R., Heisermann G. J. andCallard 1. P. (1982) Regulation of vitellogenesis in rep-tiles. Herpetologica 38, 40--50.
Joanen T. and McNease L. (1977) Artificial incubation ofalligator eggs and post hatching culture in controlledenvironmental chambers. Proc. world Marie. Meet. 8,483-490.
Lance V., Joanen T. and McNease L. (1983) Selenium,vitamin E, and trace elements in plasma of wild andfarm-reared alligators during the reproductive cycle. Can.J. Zool. 61,1744-1751.
Magliola L. (1984) The effects of estrogen on skeletalcalcium metabolism and on plasma parameters of vitel-