The Bisphosphonate YM529 Inhibits Osteolytic and ... · The Bisphosphonate YM529 Inhibits...
Transcript of The Bisphosphonate YM529 Inhibits Osteolytic and ... · The Bisphosphonate YM529 Inhibits...

The Bisphosphonate YM529 Inhibits Osteolytic and Osteoblastic
Changes and CXCR-4–Induced Invasion in Prostate Cancer
Sotaro Miwa,1Atsushi Mizokami,
1Evan T. Keller,
2Russell Taichman,
3
Jian Zhang,2and Mikio Namiki
1
1Department of Urology, Kanazawa University, Kanazawa, Japan and 2Department of Urology, School of Medicine, and 3Department ofPeriodontics, Prevention and Geriatrics, School of Dentistry, University of Michigan, Ann Arbor, Michigan
Abstract
Bisphosphonates are useful for the treatment of prostatecancer bone metastasis. However, the role of bisphosphonateon the development of the osteoblastic component of prostatecancer bone metastases is not defined. In the present study, thethird-generation bisphosphonate, YM529 (minodoronate), wastested for its effects on the osteolytic PC-3 and novelosteoblastic LNCaP-SF cell lines. YM529 inhibited bothosteolytic and osteoblastic changes in an intratibial tumorinjection murine model. In vitro , YM529 inhibited both theproliferation and the invasion of both prostate cancer celllines. The stromal cell–derived factor-1 (or CXCL12)/CXCR-4pathway is believed to play an important role in thedevelopment of prostate cancer bone metastases. Thus, wedetermined if YM529 affected this pathway. YM529 suppressedCXCR-4 expression in PC-3 and LNCaP-SF in vitro and in vivoand this was associated with decreased in vitro invasion. Theseresults suggest that YM529 may inhibit cancer cell invasioninto the bone matrix by repressing the expression of CXCR-4in bone metastasis lesions. (Cancer Res 2005; 65(19): 8818-25)
Introduction
Prostate cancer has a great predilection for metastasizing tobone resulting in great morbidity to prostate cancer patients (1, 2).In contrast to myeloma and breast cancer, which show osteolyticchanges in bone, a high percentage of patients with prostate cancershow osteoblastic changes in their bones. Roland proposed thehypothesis that osteolysis is an essential component of theprogression of every primary or metastatic tumor in bone (3). Insupport of this proposal, several studies have shown that patientswith advanced prostate cancer express elevated levels of osteolyticbone resorption markers in their urine and blood (4, 5). These dataindicate that although the radiographic appearance of prostatecancer may be osteoblastic a significant feature of prostate cancermetastatic bone disease is osteolysis (6). These observationssuggest that the use of compounds that inhibit osteolysis couldhave a therapeutic role for the treatment to metastatic bonedisease in prostate cancer patients.Bisphosphonates are potent inhibitors of bone resorption and
have been used successfully in osteoporosis and other acceleratedbone turnover diseases. Bisphosphonates have a high affinityfor bone minerals and can quickly accumulate in bone tissue.Bisphosphonates that contain nitrogen atoms in their structural
formula (N-bisphosphonates) have been reported to block themevalonate pathway by inhibiting the biosynthesis of isoprenoids,such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate,resulting in apoptosis of osteoclasts (7). In addition to effects onbone tissue, bisphosphonates have been reported to have directantitumor effects (8–10). The antitumor activities of bisphospho-nates include induction of tumor apoptosis, inhibition of tumor cellproliferation, decreased tumor cell adhesion, and decreasedinvasion into bone. These observations suggest that bisphospho-nates not only act on osteoclast-mediated bone resorption but alsomay affect the invasive behavior of metastatic prostate cancer cellsin bone. Our group has shown that YM529, a newly developedthird-generation N-bisphosphonate that has a strong anti–boneresorption effect, has direct antiproliferative and apoptotic effectson prostate cancer cells in vitro .4
The chemokine receptor CXCR-4 and its ligand, stromal cell–derived factor-1 (SDF-1 or CXCL12), are known be expressed inprostate cancer cells (11–16). The SDF-1/CXCR-4 pathway has beenshown to participate in the proliferation, differentiation, and meta-stasis of many cancers, including prostate cancer (11, 12, 17–19).Accordingly, we hypothesized that bisphosphonates may inhibitprostate cancer cell invasion through the SDF-1/CXCR-4 pathway.We tested this hypothesis by using YM529 to determine if it had aneffect on the development of intraosseous prostate cancer lesionsand if this was associated with regulation of the SDF-1/CXCR-4invasion pathway.
Materials and Methods
Chemicals. YM529 [1-hydroxy-2-(imidazo-[1,2-a]pyridin-3-yl) ethylidenebisphosphonate acid monohydrate] was supplied from Astellas Pharma, Inc.
(Tokyo, Japan). The bisphosphonate was dissolved in saline (pH 7) and used
for various assays described below.Cell lines and culture. Normal primary human osteoblasts (NHOst
Clonetic Cambrex BioScience, Walkersville, MD) were maintained in
Osteoblast Growth Medium Bullet kit (Cambrex BioScience). PC-3 cells
(American Type Culture Collection, Manassas, VA) were cultured in DMEMsupplemented with 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA)
and 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO). LNCaP
parental cells (American Type Culture Collection) and an androgen-
independent LNCaP cell line (LNCaP-SF) that was established afterlong-term subculturing of the parental LNCaP cells in DMEM and 5%
charcoal-stripped FBS were also used. LNCaP-SF cells express androgen
receptor at the same level of parental LNCaP cells and secreteprostate-specific antigen (PSA), which is induced by androgen, but growth
of LNCaP-SF cells is inhibited by androgen (data not shown). Each cell
line was maintained at 37jC in a humidified atmosphere with 5% CO2.
Animal study. Severe combined immunodeficient (SCID) mice weremaintained in a barrier facility and allowed free access to food and water.Requests for reprints: Atsushi Mizokami, Department of Urology, Kanazawa
University, 13-14 Takaramachi, Kanazawa-city, Japan 920-8640. Phone: 81-76-265-2393;Fax: 81-76-222-6726; E-mail: [email protected].
I2005 American Association for Cancer Research.doi:10.1158/0008-5472.CAN-05-0540 4 Submitted for publication.
Cancer Res 2005; 65: (19). October 1, 2005 8818 www.aacrjournals.org
Research Article
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from

Subconfluent PC-3 and LNCaP-SF cell lines were harvested from culture andresuspended to 2 � 107 cells/mL in DMEM. The cells were combined with
an equal volume of Matrigel (Collaborative Biomedical Products, Bedford,
MA) such that 5 � 105 cells were prepared for injection into the left tibia of
anesthetized (pentobarbital at 50 mg/kg body weight i.p.) 5-week-old intactor castrated male SCID mice for PC-3 and LNCaP-SF, respectively.
Intratibial injections were done using a 29-gauge, 0.5-inch needle inserted
through the tibial plateau of the flexed knee as described previously (20).
After 1 week, YM529 in saline (pH 7.0) or saline as a control was injected i.p.(0.1 or 0.3 mg/kg) and each body weight was monitored at weekly inter-
vals. Mice injected with the PC-3 cells were sacrificed and radiographed at
42 days following intratibial injection. Mice injected with LNCaP-SF cells
were sacrificed at 105 days following intratibial injection. Mice that did notrecover from anesthesia or died before these sacrifice times were excluded
from the analysis. This protocol was approved by the Institutional Animal
Care and Use Committee of the Graduate School of Medical Science,Kanazawa University.
Immunohistochemistry. After sacrifice, the tibias were removed and
fixed in 10% buffered formalin for 24 hours and decalcified in 10% EDTA
solution gently stirred for 2 weeks at room temperature. Section from the
formalin-fixed, paraffin-embedded tissues were cut to 4 Am and were
deparaffinized and rehydrated. Following rehydration, the sections were
stained with either H&E for the purpose of detecting prostate cancer cells in
bone or tartrate-resistant acid phosphatase (TRAP) for detecting osteo-
clasts. TRAP staining was done by using TRACP & ALP Double-Stain kit
(Takara, Tokyo, Japan) as directed by the manufacturer. Following TRAP
staining, counter staining with hematoxylin solution was done. Osteoclasts
were determined as TRAP-positive staining multinuclear cells (>3 nuclei)
using light microscope. Osteoclast perimeter (osteoclast number per
millimeter of bone) was compared between each control group and
YM529-treated groups. Four tibias of each group were analyzed in this
manner. Indirect immunohistochemistry was done for CXCR-4 protein
detection. Antigen retrieval was done by incubating the section in Target
retrieval solution (DAKO, Carpinteria, CA) for 20 minutes at 95jC. Afterblocking endogenous peroxidase, the sections were incubated with rabbit
CXCR-4 polyclonal antibody (Biovision, Mountain View, CA) overnight at
4jC. Finally, the antigens were stained using DAKO Envision kit (DAKO).Cell proliferation. PC-3 (2.5 � 104 cells/mL) and LNCaP-SF (5 � 104
cells/mL) were allowed to adhere overnight in six-well plates. At this time,
cells were treated for an additional 24, 48, and 72 hours with various doses
between 0 and 50 Amol/L YM529. Cell proliferation was enumerated intriplicate on a hemocytometer.
Invasion assay. Invasion of prostate cancer cells was assayed using BD
Biocoat Matrigel invasion chamber (BD Biosciences, San Jose, CA), which
consists of an 8 Am pore size polyethylene terephthalate membrane that hasbeen overlaid with Matrigel. Each prostate cancer cell line was cultured to
subconfluence. The cells were washed with PBS and harvested by
trypsinization. PC-3 and LNCaP-SF were suspended at 5 � 104 and 4 �105 cells/mL, respectively. Before preparing the cell suspension, the driedlayer of Matrigel matrix was rehydrated with DMEM for 2 hours at 37jC.After the solution was removed, 0.75 mL of each condition medium together
with 100 ng/mL recombinant human SDF-1a (Biovision) was added to thelower chamber of each well to induce invasion. The cells suspended in 0.5
mL culture mediumwere added to the upper chambers. Following treatment
with YM529 (0-50 Amol/L), the cells were incubated for an additional 22
hours for PC-3 and 70 hours for the LNCaP-SF cells in a humidifiedatmosphere with 5% CO2 at 37jC. Noncoated membrane inserts were also
prepared to serve as controls. After removing noninvading cells, the upper
chambers were removed and the invading cells were fixed and stained using
Diff-Quik kit (Sysmex, Kobe, Japan). To evaluate the role of the SDF-1/CXCR-4 pathway in prostate cancer invasion, 0 or 200 ng/mL SDF-1 and 0 or 20 Ag/mL CXCR-4 blocking antibody (12G 5, R&D Systems, Minneapolis, MN) were
added to the upper chamber and 0 or 200 ng/mL SDF-1 were added to thelower chamber. Each experiment was done in triplicate.
Reverse transcription-PCR and real-time quantitative PCR. Each
prostate cancer cell line and human osteoblast were cultured to
subconfluence, washed in PBS, and seeded at 2 � 106 cells per dish.
Twenty-four hours later, YM529 was added (0-50 Amol/L) for 48 hours toPC-3 and for 72 hours to LNCaP-SF. To human osteoblast, YM529 was
added at 0 to 30 Amol/L for 48 hours. After addition of YM529, each cell
line was harvested and washed with PBS. Total RNA extraction from cell
cultures was done using Isogen (Nippon Gene, Tokyo, Japan). To makecDNA, total RNA (1-2 Ag) was subjected to reverse transcription using
ThermoScript RT (Invitrogen). Reverse transcription-PCR (RT-PCR) was
done using TAKARA Ex Taq Hot Start Version (Takara Bio, Inc., Shiga,
Japan). The sense and antisense primers used were as follows: glyceralde-hyde-3-phosphate dehydrogenase (GAPDH), 5V-GACCACAGTCCATGCCATCAand 3V-TCCACCACCCTGTTGCTGTA; CXCR-4, 5V-GGCAGCAGGTAG-CAAAGTGA and 3V-TGATGACAAAGAGGAGGTCGG; and SDF-1, 5V-TGAT-GACAAAGAGGAGGTCGG and 3V-GGTCTAGCGGAAAGTCCT. RT-PCRconditions were as follows: GAPDH, 94jC for 1 minute followed by 18
cycles of the following varying conditions: 94jC for 15 seconds, 60jC for 45
seconds, and 72jC for 45 seconds; CXCR-4, 94jC for 1 minute followed by28 cycles of the following varying conditions: 94jC for 15 seconds, 55jC for
45 seconds, and 72jC for 45 seconds; and SDF-1, 94jC for 1 minute
followed by 18 cycles of the following varying conditions: 94jC for 15
seconds, 60jC for 45 seconds, and 72jC for 45 seconds. RT-PCR productswere electrophoresed on an 1.5% agarose gel and visualized by ethidium
bromide staining under UV light. To quantify CXCR-4 expression, real-time
quantitative PCR was done with 2 AL cDNA, 2 AL primer, and 4 AL Master
Mix (LightCycler FastStart DNA MasterPLUS SYBR Green I, Roche Diag-nostics, Alameda, CA) in a final volume of 20 AL. The primers used were
same as above. LightCycler System (Roche Diagnostics) was used as
follows: 94jC for 10 minutes followed by 45 cycles of the following varyingconditions: 95jC for 15 seconds, 55jC for 20 seconds, and 72jC for 20
seconds. GAPDH expression was used as a normalization control.
Experiments were done in triplicate.
Western blot analysis. Each prostate cancer cell line was cultured tosubconfluence and washed in PBS and seeded at 2 � 106 cells per dish.
YM529 was added (0-50 Amol/L) for 48 hours to PC-3 and for 72 hours to
LNCaP-SF. Each cell line was harvested, washed with PBS, and lysed
by radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl (pH 8.0),150 mmol/L NaCl, 0.1% Triton X-100, 0.01 mg/mL aprotinin, 0.05 mg/mL
phenylmethylsulfonyl fluoride]. The nuclei and cellular debris were removed
by centrifugation at 15,000 � g for 20 minutes at 4jC. Normalized lysates(50 Ag) in Laemmli buffer were electrophoresed on a 10% polyacrylamide
gel under reducing conditions and proteins were transferred to poly-
vinylidene difluoride membranes. For CXCR-4 protein (43 kDa) detection,
the membranes were blocked by casein PBS and 0.05% Tween 20 for 1 hourat room temperature and incubated with rabbit CXCR-4 polyclonal
antibody overnight at 4jC. Immunoreactive bands were visualized by using
horseradish peroxidase–conjugated secondary antibody and the enhanced
chemiluminescence system (SuperSignal West Pico ChemiluminescentSubstrate, Pierce, Rockford, IL). As positive control, anti-GAPDH rabbit
polyclonal antibody (Trevigen, Gaithersburg, MD) was used for detection of
GAPDH protein (38 kDa). Experiments were done in triplicate.
Statistical analysis. Student’s t test was used to determine the statisticalsignificance of differences of proliferation assay and invasion assay between
the control and others and differences in osteoclast perimeter among the
control groups and the treatment groups. m2 test was used to determine thesignificance of differences of radiographic change between treated groups
and control groups in vivo . A probability value P < 0.05 was considered to be
statistically significant.
Results
Radiographic results of intratibial injection of prostatecancer cell lines. Our first goal was to determine the effects ofprostate cancer cells on bone in our animal models. Accordingly,we did intratibial injection of the prostate cancer cell lines. For PC-3 cells, animals were sacrificed on day 42 after intratibial injectionat which time serum, radiographs, and tissues were obtained.Initially, we injected 15 mice with PC-3 cells; however, 2 mice did
YM529 Inhibits CXCR-4–Induced Prostate Cancer Invasion
www.aacrjournals.org 8819 Cancer Res 2005; 65: (19). October 1, 2005
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from

not recover from anesthesia and 1 mouse died of unknown causebefore the termination of the study. There were no grosslyobservable pathologic lesions or tumor identified in these animalsand they were excluded from further analysis. The PC-3 cellsinduced osteolytic lesions that developed in 10 of the 12 tibias ofthe vehicle-treated mice (Fig. 1A ; Table 1). Histologic analysisshowed that the two tibiae that failed to show any osteolyticchanges did not have tumor cells present in them.LNCaP-SF cells were injected directly into the tibia of castrated
SCID male mice (n = 16 per group). The LNCaP-SF cells are slowerto develop into tumors than the PC-3; therefore, the animals weresacrificed at 105 days after tumor injection. Radiographic evidenceof osteoblastic lesions (Fig. 1B ; Table 1) was observed in 13 of 16animals. Histologic analysis revealed that the three tibiae that didnot form osteoblastic changes did not have tumor cells in thetibiae. These findings suggest that the LNCaP-SF is a useful modelto show osteoblastic bone changes that occur in prostate cancerand therefore represents a valuable tool to model prostate cancerbone metastasis.Effects of YM529 on intratibial tumors. The effects of YM529
were examined using the administration of two doses [0.1 and0.3 mg/kg/wk (or vehicle control)] on the growth of both PC-3 andLNCaP-SF cell lines.
Fifteen animals for each YM529 dose group were injected withtumor cells, but one mouse from each treatment group did notrecover from anesthesia at the time of intratibial injection andthese mice were excluded from further analysis. Mice injectedwith PC-3 cells were sacrificed on day 42. There was nodifference in body weights among the vehicle-treated groups andthe YM529-treated groups (data not shown). Radiographsrevealed that in the group treated with 0.1 mg/kg/wk dose ofYM529 only 2 of the 14 tibiae developed osteolytic lesions andnone of the 0.3 mg/kg/wk dose treatment group (0 of 14 tibiae)developed radiographically detectable osteolytic lesions (Table 1).The radiographic data show that YM529 significantly inhibitedthe osteolytic changes that were observed in the vehicle-treatedgroup (Fig. 1A ; Table 1). In the vehicle-treated group, themajority of the intramedullary space was replaced by PC-3 cellsand there was extensive osteolysis of trabecular and corticalbone. In the YM529-treated groups, the intramedullary space wasalso replaced by PC-3 cells, but trabecular and cortical bone wasnot destroyed (Fig. 2A). We observed a large number of TRAP-positive osteoclasts at the tumor-bone interface in the vehicle-treated group and only a few TRAP-positive osteoclasts wereobserved in the tumor-bone interface of the YM529-treatedgroups (Fig. 2A). Quantification of osteoclast perimeter (numberof osteoclasts per millimeter bone) revealed that there weresignificantly less osteoclasts in YM529-treated groups than in thevehicle-treated group (Table 2). These data suggest that YM529reduces PC-3-induced osteolysis through inhibiting the PC-3-induced increase of osteoclasts.For LNCaP-SF, there were 16 mice injected per treatment
group. One mouse in the 0.3 mg/kg group did not recover fromanesthesia. One mouse in the 0.1 mg/kg/wk group and two micein the 0.3 mg/kg/wk group died of unknown causes before theend of the study. Necropsy did not reveal any gross lesions ortumor and these mice were excluded from the remaininganalyses. On day 105 after intratibial injection, mice weresacrificed. There was no difference in body weights among thevehicle-treated groups and the YM529-treated groups (data notshown). In the group treated with the lower dose (0.1 mg/kg/wk),7 of 15 tibias developed osteoblastic lesions (Fig. 2B). In the grouptreated with a 0.3 mg/kg/wk dose, 6 of 13 tibias developedosteoblastic lesions. In the vehicle-treated animals, histologyrevealed that the LNCaP-SF cells grew throughout the marrowcavity and increased bone production was identified (Fig. 2B).
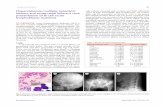
Figure 1. YM529 diminishes osteolytic and osteoblastic bone changes inducedby prostate cancer cells. A, representative radiographs of SCID mouse tibia42 days after intratibial injection of PC-3 cells. Left, nonimplanted tibia side;middle, vehicle-treated tibia; right, PC-3-implanted tibia of mouse treated withYM529 (0.1 mg/kg/wk). B, representative radiographs of castrated SCID mousetibia implanted with LNCaP-SF at day 105. Left, nonimplanted tibia side; middle,vehicle-treated tibia; right, LNCaP-SF-implanted tibia of mouse treated withYM529 (0.1 mg/kg/wk).
Table 1. Radiographic analysis of tibia
PC-3 day 42 osteolytic change (+)
Control 10/12 (83%) P = 0.0004
0.1 mg/kg 2/14 (14%) P < 0.0001
0.3 mg/kg 0/14
LNCaP-SF day 105 osteoblastic change (+)
Control 13/16 (81%) P = 0.044
0.1 mg/kg 7/15 (47%) P = 0.048
0.3 mg/kg 6/13 (46%)
NOTE: m2 test.
Cancer Research
Cancer Res 2005; 65: (19). October 1, 2005 8820 www.aacrjournals.org
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from

YM529 treatment of mice injected with LNCaP-SF cells blockedthe tumor-induced bone changes, although tumor cells werepresent throughout the marrow cavity (Fig. 2B). TRAP stainingshowed that there were fewer osteoclasts present in the osteo-blastic bone compared with osteolytic bone in the vehicle-treatedanimals. Furthermore, TRAP-positive osteoclasts were very rarein the YM529-treated groups as opposed to the vehicle-treatedgroups (Fig. 2G and H). These histologic observations wereconfirmed by measurement of osteoclast perimeter (number ofosteoclasts per millimeter bone). Specifically, the osteoclastperimeter was lower in the osteoblastic bone from the LNCaP-SF-injected mice compared with the osteoclast perimeter in theosteoclastic bone of the PC-3-injected mice (Table 2). Further-more, YM529 decreased the osteoclast perimeter in bone injectedwith either tumor type. Taken together, these data show thatYM529 is an effective agent to block both prostate cancer–mediated osteolytic and osteoblastic changes.YM529 decreases prostate cancer cell growth in vitro . To
further explore the role of the mechanism by which YM529inhibited tumor size in vivo, in vitro investigations were done. Weinitially evaluated for a direct effect of YM529 on prostate cancergrowth in vitro . YM529 diminished cell numbers of both PC-3 andLNCaP-SF cells in a dose-dependent fashion (Fig. 3A and B).Especially, >10 Amol/L YM529 caused significant inhibition in PC-3and >3 Amol/L YM529 caused significant inhibition in LNCaP-SFafter z24 hours of exposure (P < 0.05-0.001).YM529 inhibits prostate cancer cell invasion. In addition to
changes in cell growth, modulation of other metastatic phenotypes,
such as invasive ability, may affect the progression of prostatecancer growth in bone. Accordingly, we evaluated the ability ofYM529 to modulate prostate cancer cell invasion. Both PC-3 andLNCaP-SF cells are able to spontaneously invade through Matrigel-coated chambers (Fig. 4). YM529 started to inhibit PC-3 invasion at1 Amol/L and continued to inhibit it in a dose-responsive fashion(Fig. 4A). Similarly, YM529 decreased the LNCaP-SF invasion butrequired a slightly higher dose (3 Amol/L) than the PC-3 cells to seethe initial inhibition (Fig. 4B).Stromal cell–derived factor-1 promotes invasion of prostate
cancer cells. We next wanted to explore the possibility that YM529modulated the SDF-1/CXCR-4 pathway, which has been implicatedin tumor invasion. We first verified that SDF-1 enhanced invasionin the in vitro assays. When placed only in the lower well of theinvasion chamber, SDF-1 stimulated invasion of both prostatecancer cell lines, but the effect was eliminated when SDF-1 wasadded to both upper and lower wells of the invasion chamber orwhen anti-CXCR-4-neutralizing antibody was added to the upperchambers (Fig. 4C and D). Based on these results, we hypothesizedthat YM529 may affect SDF-1/CXCR-4–mediated tumor metastasisin bone.YM529 inhibited CXCR-4 expression on prostate cancer
cells. Because SDF-1 enhanced prostate cancer cell invasion andYM529 blocked invasion, we next determined if YM529modulated the SDF-1/CXCR-4 axis. We initially identified usingRT-PCR and real-time RT-PCR that both PC-3 and LNCaP-SFexpressed CXCR-4 mRNA and protein (Fig. 5A and B). YM529decreased CXCR-4 expression in a dose-dependent fashion
Figure 2. YM529 decreases prostatecancer–induced osteoclast numbers. A,PC-3 at 42 days. B, LNCaP-SF cells at105 days. Top, H&E staining was done asdescribed in Materials and Methods todetect tumor in the marrow; bottom,TRAP staining was done as described inMaterials and Methods to detectosteoclasts (arrows ), which weredetermined as TRAP-positive stainingmultinuclear cells (>3 nuclei). T, tumorcells. Magnification, �200.
Table 2. Histomorphometric quantification of osteoclast at bone/tumor interface
PC-3 Osteoclasts (number/mm) LNCaP-SF osteoclasts (number/mm)
Control 13.8 F 2.06 P = 0.0060 Control 4.25 F 0.63 P = 0.0049
0.1 mg/kg 4.5 F 0.86 P = 0.0017 1 mg/kg 1.0 F 0.41 P = 0.00160.3 mg/kg 1.8 F 0.85 P = 0.064 0.3 mg/kg 0.5 F 0.29 P = 0.36
NOTE: Student’s t test.
YM529 Inhibits CXCR-4–Induced Prostate Cancer Invasion
www.aacrjournals.org 8821 Cancer Res 2005; 65: (19). October 1, 2005
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from

(Fig. 5A and B). As a control, we assessed both CXCR-4 and SDF-1expression in mature primary human osteoblasts. The osteoblastsdid not express CXCR-4 mRNA (data not shown) but expressedSDF-1 mRNA (Fig. 5C). YM529 did not alter SDF-1 levels in the
osteoblasts (Fig. 5C). Based on these findings, we hypothesizedthat YM529 decreased CXCR-4 expression in prostate cancer cellsbut not SDF-1 expression from osteoblasts.YM529 inhibited CXCR-4 expression in PC-3 and LNCaP-SF
in bone marrow. To provide further support for the possibilitythat YM529 alters the CXCR-4/SDF-1 axis in prostate cancer bonemetastases, we evaluated CXCR-4 expression in the intratibialtumors from the in vivo studies. Using immunohistochemistry, weidentified strong CXCR-4 expression in PC-3 and LNCaP-SF cellsin the tibia (Fig. 6A and B). YM529 markedly decreased CXCR-4expression in both prostate cancer cell lines within the tibia(Fig. 6C and D). These results confirm that YM529 can modulatethe CXCR-4/SDF-1 axis in intraosseous prostate cancer tumorsin vivo .
Discussion
In this study, we show four major findings. Firstly, we identifieda novel osteoblastic prostate cancer model, LNCaP-SF. Secondly,we identified that a new third-generation bisphosphonate, YM529,which, in addition to being effective against prostate cancer–induced osteolytic changes, was also effective against prostatecancer–induced osteoblastic changes in vivo . Thirdly, YM529inhibited proliferation and invasion ability of prostate cancer cellsin vitro assay. Finally, YM529 suppressed CXCR-4 expressionin vitro and in vivo , suggesting that YM529 achieved its ability toinhibit prostate cancer bone lesions, in part, through inhibiting theCXCR-4/SDF-1 axis.We established the LNCaP-SF, primarily for the purpose of
studying androgen-independent cancer, by culturing parentalLNCaP in androgen-deplete environment for 6 months. WhenLNCaP was transplanted in SCID mouse tibia, it showedosteolytic change (21). In contrast, we observed that LNCaP-SFshowed osteoblastic change 4 months after transplantation. It is
Figure 3. YM529 decreases PC-3 and LNCaP-SF cell proliferation in vitro .PC-3 and LNCaP-SF cells were treated with YM529 (0-50 Amol/L) as describedin Materials and Methods. Points, mean percent of control (n = 3); bars, SD.
Figure 4. YM529 decreases and SDF-1 promotesPC-3 and LNCaP-SF cell invasion in vitro. A and B,effect of YM529 on PC-3 and LNCaP-SF cellinvasion in Matrigel-coated chambers. PC-3 (A)and LNCaP-SF (B) cells were treated with YM529(0-50 Amol/L) and invasion assays in vitro wascarried out as described in Materials and Methods.C and D, effect of CXCR-4/SDF-1 pathway onPC-3 (C) and LNCaP-SF (D ) invasion inMatrigel-coated chambers. X axis, reagents addedinto upper and lower chamber (upper/lowerchamber); Ab, CXCR-4 antibody. Columns, mean(n = 3); bars, SD. *, P < 0.05; **, P < 0.01; ***,P < 0.001, Student’s t test.
Cancer Research
Cancer Res 2005; 65: (19). October 1, 2005 8822 www.aacrjournals.org
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from

not clear at this time why LNCaP-SF caused osteoblastic change.It has been shown that prostate cancer cells secrete severalosteoblastic factors, including bone morphogenetic protein (22),transforming growth factor-h (23), insulin-like growth factor (24),platelet-derived growth factor-BB (25), endothelin-1 (26), fibro-blast growth factor (27), urokinase plasminogen activator (28),and PSA (29). We examined the expression of PSA in LNCaP-SFcells. They express PSA; however, the expression level in LNCaP-SF is lower than that of LNCaP cells in the absence of androgen(data not shown) and thus most likely does not contribute to theosteoblastic activity of LNCaP-SF. Unfortunately, the parentalLNCaP cells do not readily form intratibial tumors, making it anextremely challenging tumor cell line to use for in vivo studies;thus, we could not do a direct in vivo comparison betweenLNCaP cells and LNCaP-SF cells.Currently, there are no optimal models of prostate cancer bone
metastases. Several prostate cancer osteoblastic cell lines or xeno-grafts currently exist, including LUCaP-23.1 (21), LAPC-9 (30), MDAPCa2b (29), and C4-2B (31). However, these are associated withtechnical difficulties, such as a requirement to passage the tumorsin vivo to maintain them (i.e., LUCaP-23.1 and LAPC-9) or a lowfrequency of development of osteoblastic tumors (MDA PCa2b)or mixed osteoblastic and osteolytic tumors (C4-2B). Yonou et al.succeeded in establishment of osteoblastic metastasis to humanadult bone transplanted s.c. into nonobese diabetic/SCID miceusing LNCaP (32). However, this method is complicated and
expensive. In contrast to these challenges, the LNCaP-SF cells canbe passed in vitro and the cells have a high probability (81%) ofdeveloping osteoblastic lesions. Moreover, because LNCaP-SF cellswere established in vitro , their characteristics should be moresimilar to the parental LNCaP cells than C4-2B, which were derivedfrom LNCaP cells by coinjecting them with bone stromal cellsin vivo . Therefore, LNCaP-SF cells transplanted into the tibia ofSCID mouse is a very convenient model to investigate osteoblasticchange.Bisphosphonates have already been proven to be effective for
the treatment of breast cancer bone metastasis and myeloma,which show osteolytic change with bone pain (33–35). Although ahigh percentage of prostate cancer bone metastasis showsosteoblastic change compared with breast cancer and myeloma,clinical efficacy of bisphosphonates for bone pain caused byprostate cancer bone metastasis has already been shown (36). Ithas been shown that a third-generation bisphosphonate, zoledronicacid, decreases skeletal-related events, such as fracture and bonepain, in men with prostate cancer and thus is an importanttreatment option for prostate cancer bone metastasis (37–39).Furthermore, we reported previously that a third-generationbisphosphonate, incadronate, decreased serum PSA levels in ahormone-refractory prostate cancer patient with bone metastases,suggesting that incadronate inhibits prostate cancer progression(40). One reason these bisphosphonates are effective in prostatecancer is that osteoclastic activity is an important component of
Figure 5. YM529 decreases CXCR-4 expressionin prostate cancer cells in vitro . Twenty-fourhours after PC-3 (A) or LNCaP-SF (B) cells wereseeded, cells were treated with the indicatedconcentrations of YM529 and cultured for 24 or72 hours, respectively. RT-PCR analysis ofCXCR-4 and GAPDH mRNA was done asdescribed in Materials and Methods. CXCR-4mRNA expression levels were quantified usingreal-time quantitative RT-PCR of PC-3. CXCR-4amounts were normalized to GAPDH mRNAexpression. For Western blot analysis, cytosolproteins of PC-3 and LNCaP-SF cells wereextracted as described in Materials and Methodsand loaded on an 8% SDS-polyacrylamide gel forWestern blot analysis. C, effect of YM529 onSDF-1 expression in human osteoblast. RT-PCRwas done as described in Materials and Methods.
YM529 Inhibits CXCR-4–Induced Prostate Cancer Invasion
www.aacrjournals.org 8823 Cancer Res 2005; 65: (19). October 1, 2005
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from

prostate cancer bone metastases even in osteoblastic lesions (41).Many studies have shown that patients with advanced prostatecancer exhibit elevated levels of osteolytic bone resorption markersin urine and blood (4, 5). Additionally, histomorphometric studiesof prostate cancer bone metastases have shown that osteoblasticlesions are actually mixed in nature, with increased activities ofboth osteoblasts and osteoclasts (42). These findings are consistentwith the hypothesis stated by Roland that every primary ormetastatic cancer in bone begins with osteolysis (3). Theseobservations show that osteolysis is an important component ofprostate cancer bone metastasis even if the radiographic appear-ance is primarily osteoblastic and thus suggest that bisphosphonatemay play an important role in therapy of prostate cancer bonemetastases.In our study, YM529 suppressed not only osteolytic change but
also osteoblastic changes. Moreover, YM529 reduced the number ofosteoclasts in the tibia transplanted with PC-3 and LNCaP-SF cells,which cause osteolytic and osteoblastic changes, respectively. Ourobservation that the osteolytic PC-3 cell tumors had three timesmore osteoclasts than the osteoblastic LNCaP-SF tumors suggeststhat PC-3 secretes more substances, such as interleukin-6 orRANKL, which stimulate osteoclast proliferation than LNCaP-SF.Alternatively, LNCaP-SF may secrete more osteoclastic inhibitoryfactors, such as osteoprotegerin (20).In contrast to our results, it was reported that zoledronic acid
did not inhibit osteoblastic change induced by the LAPC-9prostate cancer xenograft in vivo , although it suppressedosteoclast numbers (43). The reasons for these different observa-tions are not clear; however, differences of cell type and cellproliferation ability, cell’s response to bisphosphonate, or differ-ences in direct antitumor effects or effects on osteoblasts betweenthe different bisphosphonates may account for these contrastingobservations.The role that bisphosphonates play directly on tumors, including
induction of tumor cell apoptosis, inhibition of proliferation, altering
tumor cell adhesion, and invasive ability, has been receiving muchattention (9, 10, 44, 45). Bisphosphonates have been shown to havedirect antitumor effects on prostate cancer cells. For example, Coreyet al. reported the apoptotic induction and suppression ofproliferation by zoledronic acid in LNCaP and PC-3 cells (46). Inaddition, bisphosphonates inhibited prostate cancer adhesion tobone in vitro (46). These observations, therefore, suggest thatbisphosphonates not only act on osteoclast-mediated bone resorp-tion but also may affect the invasive behavior of metastatic prostatecancer cells in bone. Our results are consistent with these previousreports and identified that YM529 has a direct antitumor effect,including inhibition of proliferation and invasion.Tumor invasion is one of the key mechanisms through which
prostate cancer proliferates in the limited bone marrow space. Inthe present study, we showed that YM529 inhibited prostatecancer cell invasion. Our in vitro and in vivo data suggest that itmediates this effect, in part, through decreasing the ability ofprostate cancer cells to respond to SDF-1 by decreasing CXCR-4.This is consistent with our previous report that SDF-1 andCXCR-4 confers a bone-specific phenotype on the metastasis ofprostate cancer (11) and the report by Singh et al. that showedthe SDF-1/CXCR-4 pathway modulated migration, invasion, andsecretion of several matrix metalloproteinases (MMP) by prostatecancer (14). SDF-1 activation of CXCR-4 confers invasive abilitythrough several mechanisms. Fernandis et al. reported that SDF-1 activates several proinvasive factors, including focal adhesionkinase, RAFTK/Pyk2, phosphatidylinositol 3-kinase, Cb1, SHP2,MMP-2, and MMP-9, in breast cancer (18). Consistent with ourresults, Denoyelle et al. showed that zoledronic acid inhibitsthe chemotactic effect induced by SDF-1 both by reducing cellmotility through inhibiting RhoA and by decreasing CXCR-4expression (47).Clinically, bisphosphonates provide effective relief for bone pain
(38, 48). Tumor burden and its effects on bone remodeling as wellas tumor-mediated cytokine production may contribute to pain.Recently, Oh et al. published that CXC chemokines produced painvia direct action on chemokine receptor expressed by nociceptorneurons (49). Therefore, the observations that YM529 candecrease CXCR-4 expression and inhibit osteoclastogenic boneremodeling suggest that YM529 may be effective for decreasingbone pain.In summary, our work suggests that following models: YM529
decreases CXCR-4 expression resulting in decreasing the prostatecancer’s invasive ability, which then would result in inhibitingtumor progression. Additionally, YM529 inhibits osteoclastogenesis,which in turn will diminish the release of growth factors from thebone microenvironment and inhibit the prostate cancer–inducedbone remodeling. In conclusion, our results suggest that YM529has dual effect on prostate cancer growth in bone (i.e., a directantitumor effect and an indirect effect on the bone microenviron-ment). Our results suggest that YM529 may be useful for treatmentof prostate cancer bone metastasis.
Acknowledgments
Received 2/18/2005; revised 6/1/2005; accepted 7/20/2005.Grant support: Japan Society for the Promotion of Science grant 15390488
(M. Namiki) and National Cancer Institute grant P01 CA093900.The costs of publication of this article were defrayed in part by the payment of page
charges. This article must therefore be hereby marked advertisement in accordancewith 18 U.S.C. Section 1734 solely to indicate this fact.
We thank Yukari Kawabuchi, Saeko Fuji, and Atsuko Kojima for their technicalassistant.
Figure 6. YM529 decreases CXCR-4 expression in prostate cancer cells inmice tibiae in vivo . Immunohistochemistry was done to detect CXCR-4expression as described in Materials and Methods. Vehicle-treated groupof PC-3 cells at 42 days (A), YM529-treated group (0.1 mg/kg/wk) of PC-3 at42 days (B), vehicle-treated group of LNCaP-SF at 105 days (C ), andYM529-treated group (0.1 mg/kg/wk) of LNCaP-SF at 105 days (D).Magnification, �400.
Cancer Research
Cancer Res 2005; 65: (19). October 1, 2005 8824 www.aacrjournals.org
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from

References
1. Abrams HL, Spiro R, Goldstein N. Metastases in car-cinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74–85.
2. Koutsilieris M. Osteoblastic metastasis in advancedprostate cancer. Anticancer Res 1993;13:443–9.
3. Roland SI. Calcium studies in ten cases of osteoblasticprostatic metastasis. J Urol 1958;79:339–42.
4. Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE,Delmas PD. Markers of bone turnover for the manage-ment of patients with bone metastases from prostatecancer. Br J Cancer 2000;82:858–64.
5. Sano M, Kushida K, Takahashi M, et al. Urinarypyridinoline and deoxypyridinoline in prostate carcinomapatients with bone metastasis. Br J Cancer 1994;70:701–3.
6. Nielsen OS, Munro AJ, Tannock IF. Bone metastases:pathophysiology and management policy. J Clin Oncol1991;9:509–24.
7. Rogers MJ, Gordon S, Benford HL, et al. Cellular andmolecular mechanisms of action of bisphosphonates.Cancer 2000;88:2961–78.
8. Boissier S, Ferreras M, Peyruchaud O, et al. Bisphosph-onates inhibit breast and prostate carcinoma cellinvasion, an early event in the formation of bonemetastases. Cancer Res 2000;60:2949–54.
9. Green JR. Antitumor effects of bisphosphonates.Cancer 2003;97:840–7.
10. Green JR. Anti-tumor potential of bisphosphonates.Med Klin (Munich) 2000;95:23–8.
11. Taichman RS, Cooper C, Keller ET, Pienta KJ,Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancermetastasis to bone. Cancer Res 2002;62:1832–7.
12. Darash-Yahana M, Pikarsky E, Abramovitch R, et al.Role of high expression levels of CXCR4 in tumorgrowth, vascularization, and metastasis. FASEB J 2004;18:1240–2.
13. Sun YX, Wang J, Shelburne CE, et al. Expression ofCXCR4 and CXCL12 (SDF-1) in human prostate cancers(PCa) in vivo . J Cell Biochem 2003;89:462–73.
14. Singh S, Singh UP, Grizzle WE, Lillard JW, Jr.CXCL12-CXCR4 interactions modulate prostate cancercell migration, metalloproteinase expression and inva-sion. Lab Invest 2004;84:1666–76.
15. Vaday GG, Hua SB, Peehl DM, et al. CXCR4 andCXCL12 (SDF-1) in prostate cancer: inhibitory effects ofhuman single chain Fv antibodies. Clin Cancer Res 2004;10:5630–9.
16. Sun YX, Schneider A, Jung Y, et al. Skeletallocalization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis andgrowth in osseous sites in vivo . J Bone Miner Res 2005;20:318–29.
17. Burger M, Glodek A, Hartmann T, et al. Functionalexpression of CXCR4 (CD184) on small-cell lung cancercells mediates migration, integrin activation, andadhesion to stromal cells. Oncogene 2003;22:8093–101.
18. Fernandis AZ, Prasad A, Band H, Klosel R, GanjuRK. Regulation of CXCR4-mediated chemotaxis and
chemoinvasion of breast cancer cells. Oncogene 2004;23:157–67.
19. Bartolome RA, Galvez BG, Longo N, et al. Stromalcell-derived factor-1a promotes melanoma cell invasionacross basement membranes involving stimulation ofmembrane-type 1 matrix metalloproteinase and RhoGTPase activities. Cancer Res 2004;64:2534–43.
20. Zhang J, Dai J, Qi Y, et al. Osteoprotegerin inhibitsprostate cancer-induced osteoclastogenesis and pre-vents prostate tumor growth in the bone. J Clin Invest2001;107:1235–44.
21. Corey E, Quinn JE, Bladou F, et al. Establishment andcharacterization of osseous prostate cancer models:intra-tibial injection of human prostate cancer cells.Prostate 2002;52:20–33.
22. Bentley H, Hamdy FC, Hart KA, et al. Expression ofbone morphogenetic proteins in human prostaticadenocarcinoma and benign prostatic hyperplasia.Br J Cancer 1992;66:1159–63.
23. Yonou H, Aoyagi Y, Kanomata N, et al. Prostate-specific antigen induces osteoplastic changes by anautonomous mechanism. Biochem Biophys Res Com-mun 2001;289:1082–7.
24. Doherty A, Smith G, Banks L, Christmas T, Epstein RJ.Correlation of the osteoblastic phenotype with prostate-specific antigen expression in metastatic prostatecancer: implications for paracrine growth. J Pathol1999;188:278–81.
25. Yi B, Williams PJ, Niewolna M, Wang Y, Yoneda T.Tumor-derived platelet-derived growth factor-BB playsa critical role in osteosclerotic bone metastasis in ananimal model of human breast cancer. Cancer Res 2002;62:917–23.
26. Nelson JB, Hedican SP, George DJ, et al. Identifica-tion of endothelin-1 in the pathophysiology ofmetastatic adenocarcinoma of the prostate. Nat Med1995;1:944–9.
27. Guise TA, Mundy GR. Cancer and bone. Endocr Rev1998;19:18–54.
28. Goltzman D. Mechanisms of the development ofosteoblastic metastases. Cancer 1997;80:1581–7.
29. Yang J, Fizazi K, Peleg S, et al. Prostate cancer cellsinduce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res 2001;61:5652–9.
30. Lee Y, Schwarz E, Davies M, et al. Differences in thecytokine profiles associated with prostate cancer cellinduced osteoblastic and osteolytic lesions in bone.J Orthop Res 2003;21:62–72.
31. Lin DL, Tarnowski CP, Zhang J, et al. Bone metastaticLNCaP-derivative C4-2B prostate cancer cell linemineralizes in vitro . Prostate 2001;47:212–21.
32. Yonou H, Yokose T, Kamijo T, et al. Establishment ofa novel species- and tissue-specific metastasis model ofhuman prostate cancer in humanized non-obesediabetic/severe combined immunodeficient miceengrafted with human adult lung and bone. CancerRes 2001;61:2177–82.
33. Hillner BE, Ingle JN, Berenson JR, et al. AmericanSociety of Clinical Oncology guideline on the role ofbisphosphonates in breast cancer. American Society of
Clinical Oncology Bisphosphonates Expert Panel. J ClinOncol 2000;18:1378–91.
34. Berenson JR, Hillner BE, Kyle RA, et al. AmericanSociety of Clinical Oncology clinical practice guidelines:the role of bisphosphonates in multiple myeloma. J ClinOncol 2002;20:3719–36.
35. Heidenreich A, Ohlmann C, Body JJ. Ibandronate inmetastatic bone pain. Semin Oncol 2004;31:67–72.
36. Heidenreich A, Hofmann R, Engelmann UH. The useof bisphosphonate for the palliative treatment of painfulbone metastasis due to hormone refractory prostatecancer. J Urol 2001;165:136–40.
37. Parker CC. Re: Long-term efficacy of zoledronic acidfor the prevention of skeletal complications in patientswith metastatic hormone-refractory prostate cancer.J Natl Cancer Inst 2004;96:1480–1.
38. Saad F, Gleason DM, Murray R, et al. A randomized,placebo-controlled trial of zoledronic acid in patientswith hormone-refractory metastatic prostate carcino-ma. J Natl Cancer Inst 2002;94:1458–68.
39. Saad F, Gleason DM, Murray R, et al. Long-termefficacy of zoledronic acid for the prevention of skeletalcomplications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004;96:879–82.
40. Asahi H, Mizokami A, Maeda Y, Komatsu K, KoshidaK, Namiki M. Bisphosphonate therapy for hormonerefractory prostate cancer with bone metastasis. J Urol2003;169:281–2.
41. Keller ET, Brown J. Prostate cancer bone metastasespromote both osteolytic and osteoblastic activity. J CellBiochem 2004;91:718–29.
42. Keller ET. The role of osteoclastic activity inprostate cancer skeletal. Drugs Today (Barc) 2002;38:91–102.
43. Lee YP, Schwarz EM, Davies M, et al. Use ofzoledronate to treat osteoblastic versus osteolytic lesionsin a severe-combined-immunodeficient mouse model.Cancer Res 2002;62:5564–70.
44. Green JR. Bisphosphonates in cancer therapy. CurrOpin Oncol 2002;14:609–15.
45. Green JR, Clezardin P. Mechanisms of bisphospho-nate effects on osteoclasts, tumor cell growth, andmetastasis. Am J Clin Oncol 2002;25:S3–9.
46. Corey E, Brown LG, Quinn JE, et al. Zoledronic acidexhibits inhibitory effects on osteoblastic and osteolyticmetastases of prostate cancer. Clin Cancer Res 2003;9:295–306.
47. Denoyelle C, Hong L, Vannier JP, Soria J, Soria C.New insights into the actions of bisphosphonatezoledronic acid in breast cancer cells by dual RhoA-dependent and -independent effects. Br J Cancer 2003;88:1631–40.
48. Saad F, Karakiewicz P, Perrotte P. The role ofbisphosphonates in hormone-refractory prostate cancer.World J Urol 2005;23:14–8.
49. Oh JW, Drabik K, Kutsch O, Choi C, Tousson A,Benveniste EN. CXC chemokine receptor 4 expressionand function in human astroglioma cells. J Immunol2001;166:2695–704.
YM529 Inhibits CXCR-4–Induced Prostate Cancer Invasion
www.aacrjournals.org 8825 Cancer Res 2005; 65: (19). October 1, 2005
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from

2005;65:8818-8825. Cancer Res Sotaro Miwa, Atsushi Mizokami, Evan T. Keller, et al. Prostate Cancer
Induced Invasion in−Osteoblastic Changes and CXCR-4 The Bisphosphonate YM529 Inhibits Osteolytic and
Updated version
http://cancerres.aacrjournals.org/content/65/19/8818
Access the most recent version of this article at:
Cited articles
http://cancerres.aacrjournals.org/content/65/19/8818.full#ref-list-1
This article cites 48 articles, 13 of which you can access for free at:
Citing articles
http://cancerres.aacrjournals.org/content/65/19/8818.full#related-urls
This article has been cited by 12 HighWire-hosted articles. Access the articles at:
E-mail alerts related to this article or journal.Sign up to receive free email-alerts
Subscriptions
Reprints and
To order reprints of this article or to subscribe to the journal, contact the AACR Publications
Permissions
Rightslink site. (CCC)Click on "Request Permissions" which will take you to the Copyright Clearance Center's
.http://cancerres.aacrjournals.org/content/65/19/8818To request permission to re-use all or part of this article, use this link
Research. on April 13, 2021. © 2005 American Association for Cancercancerres.aacrjournals.org Downloaded from