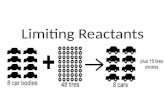
(STOY-KEE-AHM-EH-TREE). Stoichiometry is the part of chemistry that studies amounts of reactants and...
-
Upload
jeanette-venning -
Category
Documents
-
view
217 -
download
0
Transcript of (STOY-KEE-AHM-EH-TREE). Stoichiometry is the part of chemistry that studies amounts of reactants and...

(STOY-KEE-AHM-EH-TREE)
CHAPTER 12
STOICHIOMETRY

What is Stoichiometry?•Stoichiometry is the part of chemistry that studies amounts of reactants and products that are involved in reactions.
•Chemists use a balanced chemical equation as a basis to calculate how much reactant is used or product that is produced in a reaction.
•A balanced chemical equation can be interpreted in terms of different quantities, including number of atoms, molecules, or moles.

•In a balanced chemical equation, the coefficient in an equation represents not only numbers of individual molecules but also numbers of moles.
•The basis for stoichiometry is the law of conservation of mass, which states the mass of the reactants will equal the mass of the products.

Balanced Equation is neededfor Stoichiometry
2 Cr + 1 Mm + 3 Ch 1 Cr2MmCh3

What Do the Coefficients Mean?Balance the equation.
____N2 + ____H2 ____NH3
This equation can be looked at with ________ molecule of N2 reacts with __________ molecules of H2 to produce two ________________ of NH3.
1 3 2
onethree
molecules

Or
1 N2 + 3 H2 2 NH3
This equation can be looked at as one mole of N2 reacts with three ____________ of H2 to produce _________ moles of NH3.
molestwo

Mole RatioA mole ratio is a _______________ factor derived from the ______________ of a balanced chemical equation interpreted in terms of ______________.
conversioncoefficients
moles
FindGiven

Balance the equation and write all six mole ratios.
____Fe + ____O2 ____Fe2O34 3 2
4 Fe3 O2
4 Fe2 Fe2O3
2 Fe2O3
3 O2
4 Fe3 O2
3 O2
2 Fe2O3
2 Fe2O3
4 Fe

*Steps
1.Balance the equation
2.Identify given and finish line
3.Use mole ratio and/or molar mass to solve
4.Check sig. figs. and round if needed

*Mole to Mole
Conversions

How many moles of CO2 will be produced by the complete reaction of
2.00 mol of glucose (C6H12O6)?
C6H12O6 + O2 CO2 +H2O
1 666
2.00 mol C6H12O6 6
mol CO2
1 mol
C6H12O6
= 12.0 mol CO2

How many moles of ammonia (NH3) can be produced from 8.50 mol of hydrogen reacting with nitrogen?
H2 + N2 NH3
8.50 mol H2 2 mol NH3
3 mol H2
= 5.67 mol NH3
3 21

*Mole Mass
Conversions

What mass of hydrogen can be produced by reacting 6.000 mol of Al
with HCl?
Al + HCl AlCl3 + H2 2 326
6.000 mol Al 3 mol H2 2.02 g H2
2 mol Al 1 mol H2
= 18.18 g H2

What mass of AlCl3 can be produced by the reaction of 4.00 mol of HCl
with Al?
Al + HCl AlCl3 + H2 2 326
4.00 mol HCl 2 mol AlCl3 133.33 g AlCl3
6 mol HCl 1 mol AlCl3
= 178 g AlCl3

How many moles of water can be produced by burning 325 g of octane
(C8H18)?
C8H18 + O2 CO2 + H2O 2 18
16
25
325 g C8H18 1 mol C8H18 18 mol
H2O
114.26 g C8H18 2 mol C8H18
= 25.6 mol H2O

How many moles of CO2 can be produced by using up 145 g of
oxygen?
C8H18 + O2 CO2 + H2O 2 18
16
25
145 g O2 1 mol O2 16 mol CO2
32.00 g O2 25 mol O2
= 2.90 mol CO2

*Mass to Mass
Conversions

What mass of carbon dioxide is produced by the complete
combustion of 100.0 g of pentane (C5H12)?
C5H12 + O2 CO2 + H2O 1 658
100.0 g C5H12 1 mol C5H12 5 mol CO2 44.01
g CO2
72.17 g C5H12 1 mol C5H12 1 mol
CO2
= 304.9 g CO2

How many grams of HNO3 are required to produce 8.75 g of
dinitrogen monoxide (N2O) according to the following equation?Zn + HNO3 Zn(NO3)2 + N2O
+ 5 H2O 4 141
0
8.75 g N2O 1 mol N2O 10 mol HNO3 63.02 g
HNO3
44.02 g N2O 1 mol N2O 1 mol
HNO3
= 125 g HNO3

How many grams of NH3 are produced by the reaction of 5.40 g of
hydrogen with excess nitrogen?
N2 + H2 NH3 1 23
5.40 g H2 1 mol H2 2 mol NH3 17.04 g NH3
2.02 g H2 3 mol H2 1 mol NH3
= 30.4 g NH3