Steam Injected GT Exercise2
-
Upload
yinka-akinkunmi -
Category
Documents
-
view
219 -
download
0
Transcript of Steam Injected GT Exercise2
-
7/31/2019 Steam Injected GT Exercise2
1/9
-
7/31/2019 Steam Injected GT Exercise2
2/9
SOLUTION
1. Simple gas turbine without steam injection
The electrical power output for the gas turbine is:
PGT= G * (PT* m PC)
where
( )43 hhmP gasT =
( )12 hhmP AIRC =
We start with the compressor:
)(32711082.0
)27315(1)( 4.1
4.0
1
2112 orKC
p
pTTT a
SK
=
+=
=
This gives us that t2 = 15+327 C = 342C
The enthalpies for air in point 1 and 2 are (from table at x=0)
h1 = (15C, x=0) = 15 kJ/kg
h2 = (342C, x =0) = 349.6 kJ/kg (interpolated in the table)
The compressor thus consumes
Pc = 30*(349.6-15) kJ/s = 10 038 kW
For the turbine we have to take the fuel flow into consideration as well as the gascontent in flue gas for the enthalpies. We need thus to calculate the specific fuelconsumption . From HEAT BALANCE OVER THE COMBUSTOR:
3,3
,2,3
52.15 DHhLHV
hh
AIR
AIRAIR
=
Point 3 is at 800C in our case. The LHV for light oil is 42.3 MJ/kg.Looking in the enthalpy table for air and flue gas from light oil we find that
h3,AIR = (800C, x=0) = 856 kJ/kgDH3 = (800C) = 76 kJ/kg
-
7/31/2019 Steam Injected GT Exercise2
3/9
The specific fuel consumption becomes:
0126.07652.1585642300
6.349856=
=
The fuel flow becomes: mFUEL = 0.0126*30 = 0.38 kg/s
Now we can also calculate the gas content x:
193.00126.1
0126.052.15
1)52.141( ==
++=
x
The enthalpy in point 3 for flue gas becomes:
h3, GAS = h3, AIR + x*DH3 = 856 + 0.193*76 = 870.7 kJ/kg
The last enthalpy needed to calculate the power output is in point 4 (= turbine outlet). Inorder to get this enthalpy we need the temperature.
The temperature is calculated from:
=a
ST
p
pTTT
)(
11
4
3
343
We have given the temperature t3, we need first to calculate the pressure ratio.
The pressure after the compressor, p2 = 10*1.013 = 10.13 bars.
We loose 2% of this pressure in the combustion chamber. Then
p3 = 0.98*10.13 = 9.927
As the gas turbine is open, p4 = p1 and the pressure ratio p3/p4 thus becomes:
p3/p4 = 9.927/1.013 = 9.8The temperature drop in the turbine part thus becomes
)(408)8.9(
1188.0)273800(
33.1
33.043 orKCTT =
+=
-
7/31/2019 Steam Injected GT Exercise2
4/9
The temperature in the turbine outlet is:
t4 = 800 408 = 392C
Now we can find the enthalpy in the turbine outlet:
h4, GAS = h4, AIR + x*DH4
h4,AIR = (392C, x = 0) = 402.5 kJ/kg
DH4 = (392C) = 30.4 kJ/kg
Thus
h4, GAS = 402.5 + 0.193*30.4 = 408.4 kJ/kg
The turbine output becomes:
PT = (30 + 0.38) * (870.7 408.4) kW = 14 045 kW
The electrical power output of the gas turbine is:
PGT = G * (PT* m PC) = 0.98*(14 045*0.99 10 038) kW
PGT = 3789 kW 3.79 MW
Efficiency
236.03.4238.0
79.3=
=
==
LHVm
P
Q
P
Fuel
GT
Fuel
GTGT
GT = 23.6% (it is low because we have a small gas turbine with relativelycombustion temperature )
-
7/31/2019 Steam Injected GT Exercise2
5/9
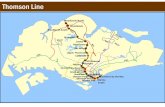
2. Steam-injected gas turbine
G a s T u r b i n e
C y c l e
A i r
F u e l
H e a t R e c o v e r y S t e a m
G e n e r a t o r
S u p e r h
s t e a m
F r e s h w a t e r
The power output for the steam injected gas turbine is calculated the same way asprevious, we just have to consider that we have also steam into the gases, that willexpand in the turbine part.
PGT= G * (PT* m PC)
Reading the hints carefully we could assume that the compressor remains unaffected,i.e. we have the same temperature increase, same airflow and same isentropicefficiency and isentropic exponent. This gives us that
PC = 10 038 kW as in the previous task.
For the turbine output we have to make some new calculations. Steam is introduced inthe combustion chamber, but will not take any active part in the combustionprocess, it just needs heat to increase the temperature from 320C up to 800C;therefore we have to add up some more fuel.
To calculate this NEW fuel flow, we have to make a heat balance over the combustionchamber.
Energy in = Energy out
mair* h2, AIR + mfuel * LHV + msteam * hSteam,320 = (mair+ mfuel ) * h3, GAS + msteam * hSteam,800
-
7/31/2019 Steam Injected GT Exercise2
6/9
The parameters written in bold, which are the steam parameters, have beenadded to the heat balance for the simple gas turbine.
Comparing the balance above with the simple gas turbine (see the Solution guide forgas turbines), it is identical except from the steam parameters!
As the steam is not taking active part into the combustion we can treat it separately as
above. The enthalpy in point 3 considers the enthalpy for flue gas WITHOUT thesteam, i.e. same enthalpy expression as for the simple gas turbine:
h3, GAS = h3, AIR + x*DH3
where x =
++=1
)52.141(x
As the steam flow is very small compared to the airflow, we can neglect the pressureincrease in the combustion chamber when steam is injected. The compressor
determines the pressure in the combustion chamber. The enthalpies for steam are:
hSteam,320 = (320C, 12 bar) = 3087.8 kJ/kg (there is a very little difference ifdetermining this enthalpy for10 bars instead)
hSteam,800 = (800C, 10 bar) = 4155.0 kJ/kg
Now considering that:
= fuel flow (kg/s) / air flow (kg/s) = mfuel /mair
and that msteam /mAIR = 0.05
we obtain:
3,3
320,800,,2,3
52.15
)(05.0
DHhLHV
hhhh
AIR
SteamSteamAIRAIR
+
=
Which is identical to the simple gas turbine calculation except from that we have addedup the steam heating term.
As we are keeping 800C as turbine inlet temperature, h3,AIR and DH3 are the same asin the calculation for the simple gas turbine without the injection. As we also shouldtake into consideration that the compressor remains unchanged we obtain:
0139.07652.1585642300
)8.30874155(05.06.349856=
+
=
-
7/31/2019 Steam Injected GT Exercise2
7/9
Thus the NEW fuel flow becomes:
mFUEL = 30*0.0139 = 0.417 kg/s
We also get a new gas content in the gases (not considering the steam)
213.00139.1
0139.052.15
1)52.141( ==
++=
x
The enthalpy in point 3 for flue gas without steam thus becomes:
h3, GAS = h3, AIR + x*DH3 = 856 + 0.213*76 = 872.2 kJ/kg
According to the hints we could assume that the isentropic efficiency, the isentropicexponent and the outlet pressure remain the same as for the simple gas turbine withoutsteam injection. This means that the temperature drop over the turbine will be the sameas in task no 1.
This gives us that: t4 = 392C.
The enthalpy for flue gas (without steam) in point 4 is:
h4, GAS = 402.5 + 0.213*30.4 = 409.0 kJ/kg
The enthalpy for steam in point 4 is:
hSTEAM, 392 = (1.013 bar, 392C) = 3261 kJ/kg (interpolated in steam table)
The turbine power output is composed by expansion of the flue gas (without steam)and the expansion of the steam:
PT = (mair+ mfuel)*(h3,GAS h4,gas) + mSTEAM*(hSteam, 800 hSteam,392)
The steam flow is: mSTEAM = 0.05* 30 kg/s = 1.5 kg/s
Which gives:
PT = (30+0.417)*(872.2 409.0) + 1.5*(4155-3261) kW = 15 430 kW
The electrical power output becomes:
PGT = G * (PT* m PC) = 0.98*(15 430*0.99 10 038) kW
-
7/31/2019 Steam Injected GT Exercise2
8/9
PGT = 5133 kW 5.13 MW
We have thus increased the power output with 1.34 MW by injecting thesteam
Efficiency
291.03.42417.0
13.5=
=
==
LHVm
P
Q
P
Fuel
GT
Fuel
GTGT
GT = 29.1%
We have thus increased the efficiency with 5.5% by recovering some heatfrom the exhaust to the steam injection
3. Calculation of the stack temperature
Heat balance over the HRSG gives
( )FWSHSteamggPavetot hhmttcm = 41
hSH = hSteam,320 = 3087.8 kJ/kg
hFW = 4.18*35 kJ/kg = 146 kJ/kg
mtot = mair+ mfuel + mSteam
CPAVE = CPG + (msteam/mair)*CPsteam
Assume that stack temperature is around 150C, then the average temperature in theHRSG from inlet to outlet is:
tave = (392+150)/2 = 270C
CPG = (CP diagram for flue gas at 270C, x=0.213) = 1.060 kJ/kg K
Cpsteam = (CP diagram for steam at 270C, 1bar) = 2.0 kJ/kg K
CPAVE = 1.060 + 0.05*2 = 1.16 kJ/kg
Ctg =++
= 27316.1)5.1417.030(
)1468.3087(5.13924
We have made an estimate. If we want a more exact value we iterate once again with anew average temperature: (392+273)/2 = 330CThis gives CPG = 1.075 kJ/kgK and Cpsteam = 2.03 kJ/kg K and thus
-
7/31/2019 Steam Injected GT Exercise2
9/9
CPAVE = 1.177 kJ/kg K and tG4 = 274.5 C
As seen there is still much heat left in the HRSG that can be recovered for use asprocess heat.