Sections 7.1 – 7.3 Electron Spin, Orbital Energies and Electron Configurations
description
Transcript of Sections 7.1 – 7.3 Electron Spin, Orbital Energies and Electron Configurations

Sections 7.1 – 7.3 Electron Spin, Orbital Energies
and Electron Configurations

Atomic Electronic Structure
In these sections…
a. Electron Spin and Magnetism
b.Energies of Orbitals
c. Electron Configurations of Atoms

Electron Spin: Electrons exhibit a magnetic field
We think of them as spinning.
They can spin only two ways: think of it as left or right
Spin quantum number: ms can be +1/2 or -1/2

Magnetic Properties come from additive effects ofelectron spins.
Diamagnetic: all electrons are pairedParamagnetic: 1 or more unpaired electronsFerromagnetic (real magnets): unpaired electrons all lined up in the same direction

Pauli Exclusion Principle
• No two electrons in an atom can have the same 4 quantum numbers
• n, ℓ, mℓ define an orbital
• Therefore: an orbital can hold only two electrons, with opposite spins because ms can only be +1/2 or -1/2

Pauli Exclusion Principle
What’s allowed?

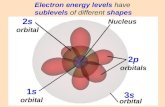
Orbital Energies
Why? With a single electron, energy depends only on how far from the nucleus.With multiple electrons, e-e- repulsions also play a role and differ depending on orbital shape.
Single Electron Atoms Multi-electron Atoms

For most atoms: Energy increases as n increases: 1 < 2 < 3 < 4 … Energy increases as subshells progress: s < p < d < f
Single Electron Atoms Multi-electron Atoms

Atomic Electron ConfigurationsAn atom has lots of electrons and lots of orbitals.
Which orbitals do the electrons occupy?

Atomic Electron Configurations
An atom has lots of electrons and lots of orbitals.
Which orbitals do the electrons occupy?
Electrons fill the lowest energy orbitals first.
Electron Configuration: a listing of how many electrons occupy each orbital.

Electron Configurations
General Rule: electrons fill lowest energy orbitals first
Sodium, Na as an example
Na has 11 electrons.Fill 2 electrons per orbital till you run out
A box represents an orbital.An arrow represents an electron.

Electron Configurations:Three Notation Types
2. spdf (or spectroscopic) notation:
List subshells and how many electrons they contain:
1s22s22p63s1
3. Noble gas notation: short
[Ne]3s1
Where [Ne] = 1s22s22p6
1.

Electron Configurations and thePeriodic Table
Examples using Electron Configuration Simulation
• Periodic Blocks
• Hund’s Rule (using the p block)
• n value increases as you move down table
• Anomalies: Cr and Cu

Electron Configurations and thePeriodic Table: Periodic Blocks

Electron Configurations and the Periodic Table II

Periodic Table and the Order of Filling
In what order are subshells filled?

Hund’s Rule: Subshells are filled to give the maximum number of unpaired electrons

Using Periodic Blocks: C

Using Periodic Blocks: Cl

Noble Gas Notation: Mg

Noble Gas Notation: Mg

Diamagnetic vs. Paramagnetic Elements

d-Block Elements: Fe

Two Anomalies: Cr and Cu