SAMPLE editorial and article enclosed · Novel biomarkers based on the pathophysiology of ACS †...
Transcript of SAMPLE editorial and article enclosed · Novel biomarkers based on the pathophysiology of ACS †...

Editorials• Introducing Biomarkers in Medicine• The new biomarkers in breast cancer: microRNAs and leptins• Targeted diagnostics and therapeutics for individualized patient management Interview• Dr Walter Carney
Reviews• Structural MRI-derived biomarkers for Alzheimer’s disease• Emerging role of the apelin system in cardiovascular homeostasis• Low high-density lipoprotein cholesterol may signal breast cancer risk• Identification of biomarkers in Alzheimer’s disease – from bench to bedside• Biomarkers in neonatal infection• Homocysteine: biomarker or cause of adverse pregnancy outcome?• Biomarker research in multiple sclerosis• Imaging and molecular biomarkers of vulnerable atheromatous plaques• Early detection of acute coronary syndromes by multimarker analysis• Aspects of β-amyloid as a biomarker for Alzheimer’s disease
Technology Report• Future of liquid chromatography-mass spectrometry in metabolic profiling and metabolomic studies for biomarker discovery
Priority Paper Evaluations• Assessing the roles of EGFR copy number and protein expression in NSCL cancer • Early advances of α1-antitrypsin precursor using the proteomics
Perspective• Mucosally restricted antigens as novel immunological targets for antitumor therapy
SAMPLE editorial and article enclosed

Executive summary – Highlights the key issues and provides an accessible snapshot of the most crucial points
Highest production values –Extensive use of textbook-qualityimagery and tabular data
Annotated references – Attention is immediately drawn tothe most important points
Biomarkers in Medicineprovides an important forum
for reporting innovation, offeringperspectives, and commenting ondevelopments in the rapidly evolvingfield of biomarker research. As such,it is a key element of the frameworkupon which to build this nascent discipline.
Scott A Waldman, Thomas Jefferson University, USA
Aims and Scope
Biomarkers in Medicine is a peer-reviewed, rapid publication journal delivering commentary
and analysis on the advances in our understanding of biomarkers and their potential and actual
applications in medicine. The journal facilitates translation of our research knowledge into the
clinic to increase the effectiveness of medical practice.
Biomarkers in Medicine provides a platform for commentary and debate for all professionals
with an interest in the identification of biomarkers, elucidation of their role and formalization
and approval of their application in modern medicine.
The audience for Biomarkers in Medicine includes academic and industrial researchers,
clinicians, pathologists, clinical chemists and regulatory professionals.
In-depth coverage of biomarker research:� Themed sections divide review coverage into therapeutic area and disease state
� Biomarker profiles – highly structured reviews providing a comprehensive overview of aspecific biomarker, coupled with its applications and clinical utility
� Biomarkers in drug discovery and development
� Optimum biomarker selection, validation, and application
� Pharmacokinetic/pharmacodynamic modeling and simulation to improve and refine drug development
� Biomarker application, using pharmacoepidemiology, pharmacogenetics, pharmacogenomics and functional proteomic techniques
� Biomarkers for clinical safety assessment and predicting adverse effects
� Bioanalytical method development and validation
� Disease process and therapeutic intervention assessments
� Impact of biomarkers on medicine including regulatory and ethical issues
Acute coronary syndromes and risk stratification by multimarker analysis – REVIEW
3future science groupfuture science group www.futuremedicine.com
patient who presents early, ED physicians mustwait a few hours before diagnosis and treatmentcan be initiated. Moreover, there are substantialcosts that are incurred for keeping patients in acrowded ED while a definitive diagnosis is beingestablished. Some hospitals have opted to reducetheir costs by instituting an aggressive dischargepolicy. Unfortunately, this will increase the rateof inadvertent discharge of patients who haveAMI or will suffer one in the near term(e.g., 24 h). Missed AMIs are the leading causeof malpractice lawsuits in the ED today [6].
In order to reduce hospital expenses andmaintain diagnostic accuracy, many EDs havedeveloped ‘chest pain’ centers of the rapid rule-out of CVD. The frequency for cardiac bio-marker and ECG testing is increased. If a patienthas a positive result with either test, they areimmediately admitted to an appropriate hospitalbed. If these tests are negative 6–9 h after presen-tation, patients are sent for a stress test or anuclear imaging analysis for evaluation of theircardiac status. Those with negative stress testresults are unlikely to suffer a major cardiac eventwithin the next few days and are medicallycleared for discharge [7].
A patient who presents to the ED with symp-toms or electrocardiographic evidence suggestiveof myocardial ischemia, and has a positive tro-ponin result in serum defines an AMI. Thispatient should be admitted and appropriatelytreated. No further laboratory testing is neededto triage this patient. While troponin is the goldstandard for AMI diagnosis, there are remaining
questions and clinical situations that are notanswered when the results of troponin are nega-tive. Therefore, a biomarker or a panel of tests isneeded that is:
• Reliably increased at the time of ED presenta-tion of a patient with ACS, before troponin isincreased;
• Consistently negative at ED presentation ofpatients who are ultimately ruled out forAMI;
• Predictive of short-term cardiovascular mor-bidity or mortality with a negative troponin atall time points.
Search for novel biomarkers of CVD using proteomics & metabolomicsThe identification of novel biomarkers for CVDdetection is a very active area of research. Theanalytical methodologies and ideal characteristicsof a biomarker have recently been reviewed [8].There are two general approaches towards thesearch for any biomarkers for human diseases. Inthe proteomics and metabolomics approach, tis-sue and/or blood samples from diseased individu-als are compared against either healthy subjects orpatients who have a disorder that mimics the dis-ease in question [9,10]. For example, blood or tis-sues from patients with early myocardial ischemiawould be compared with patients who presentwith noncardiac chest pain. The samples are sub-jected to 2D electrophoresis for separation andthe individual proteins or metabolites are identi-fied by sophisticated mass spectrometric tech-niques. The proteomic/metabolomics approach
Figure 2. Stages in the pathophysiology of acute coronary syndromes.
(A) Coronary artery that is largely free of plaques. (B & C): Progressively increasing stages of plaque formation. (D) A plaque that has formed a lipid core. A thick fibrous cap protects the lipid core from the circulation. (E) A plaque that is vulnerable to rupture, characterized by a thin fibrous cap. (F) A ruptured plaque. The exposure of the lipid core to circulating blood activates the thrombosis cascade and the aggregation of platelets (not shown). Adapted and used with permission from reference [2].
REVIEW – Wu
12 Biomarkers Med. (2007) 1(1) future science groupfuture science group
Bibliography1. Libby P, Theroux P: Pathophysiology of
coronary artery disease. Circulation 111, 3481–3488 (2005).
2. Apple FS, Wu AHB, Mair J et al.: Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin. Chem. 51, 810–824, (2005).
3. Panteghini M, Gerhardt W, Apple FS, Dati F, Ravkilde J, Wu AHB: Quality specifications for cardiac troponin assays. Clin. Chem. Lab. Med. 39, 174–178 (2001).
4. Nomenclature and criteria for diagnosis of ischemic heart disease: report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 59, 607–609 (1979).
5. Joint European Society of Cardiology/American College of Cardiology Committee: Myocardial infarction redefined – a consensus document of the joint European Society of Cardiology/American
College of Cardiology Committee for the redefinition of myocardial infarction. J. Am. Coll. Cardiol. 36, 959–969 (2000).
6. Storrow AB, Gibler WB: Chest pain centers: diagnosis of acute coronary syndromes. Ann. Emerg. Med. 35, 449–461 (2000).
7. Lindahl B, Andren B, Ohlsson J, Venge P, Wallentin L. Risk stratification in unstable coronary artery disease. Additive value of troponin T determinations and pre-discharge exercise tests, FRISK Study Group. Eur. Heart J. 18, 762–770 (1997).
8. Vasan RS: Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 113, 2335–2362 (2006).
9. Arab S, Gramolini AO, Ping P et al.:Cardiovascular proteomics. Tools to develop novel biomarkers and potential applications. J. Am. Coll. Cardiol. 48, 1733–1741 (2006).
10. Vivanco F, Martin-Ventura JL, Duran MC et al.: Quest for novel cardiovascuar biomarkers by proteomic analysis. J. Protome Res. 4, 1181–1191 (2005).
11. Granger CB, Van Eyk JE, Mockrin SC, Anderson NL: National Heart, Lung, and Blood Institute Clinical Proteomics Working Group report. Circulation 109, 1697–1703 (2004).
12. Martin-Ventura JL, Duran MC, Blanco-Colio LM et al.: Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation 100, 2216–2219 (2004).
13. Marshall J, Kupchak P, Zhu W et al.:Processing of serum proteins underlies the mass spectral fingerprinting of myocardial infarction. J. Protome Res. 2, 361–372 (2003).
14. Lindsey ML, Goshorn DK, Comte-Walters S et al.: A multidimensional proteomic approach to identify hypertrophy-associated proteins. Proteomics6, 2225–2235 (2006).
15. Brindle JT, Antti H, Holmes E et al.: Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabolonomics. Nature Med. 8, 1439–1444 (2002).
Executive summary
Pathophysiology of acute coronary syndromes (ACS)
• Rupture of a coronary artery is the most frequent etiology of ACS.• Inflammation, plaque instability and myocardial ischemia are steps that preceed necrosis.
Existing biomarkers for acute myocardial infarction (AMI)
• Creatine kinase and its MB isoenzyme and myoglobin have been replaced with troponin.• B-type natriuretic peptide and N-terminal prohormone brain natriuretic peptide are strong predictors of heart failure and
cardiovascular death.
European Society of Cardiology/American College of Cardiology redefinition of AMI
• AMI is predicated on an increase in the concentration of cardiac troponin in blood.
Clinical need for early biomarkers of ACS
• Troponin is not reliably increased in the first few hours after AMI.• The unmet needs include a marker for early diagnosis and rule out of ACS and risk stratification for short-term morbidity
and mortality.
Novel biomarkers of coronary vascular disease using proteomics and metabolomics
• Proteomics and metabolomics promises to provide new cardiac markers.• There are limitations to the clinical implementation of new tests.
Novel biomarkers based on the pathophysiology of ACS
• Markers of inflammation and plaque instability include C-reactive protein, placental-like growth factor, myeloperoxidase and pregnancy-associated plasma protein A.
• CD40 ligand, whole-blood choline and ischemia-modified albumin indicate plaque rupture, thrombosis and myocardial ischemia.
Need for multimarker testing for risk stratification and myocardial ischemia
• A multimarker approach is needed as individual markers lack clinical specificity.• Algorithms are necessary to interpret multimarker data.
Optimization of multimarker analysis
• The Multimarker Index™ produces a number that acts like a cutoff for a single test.• Classification and regression tree analysis is an algorithm that has different branch and decision limits.• A neural network is free from the assignment of cut-off concentrations and detects patterns of laboratory test results.
ISSN: 1752-0363Published: 6 per year Volume: Number 1 (2007
Please see back page for free trial information
Editorials• Introducing Biomarkers in Medicine• The new biomarkers in breast cancer: microRNAs and leptins• Targeted diagnostics and therapeutics for individualized patient management Interview• Dr Walter Carney
Reviews• Structural MRI-derived biomarkers for Alzheimer’s disease• Emerging role of the apelin system in cardiovascular homeostasis• Low high-density lipoprotein cholesterol may signal breast cancer risk• Identification of biomarkers in Alzheimer’s disease – from bench to bedside• Biomarkers in neonatal infection• Homocysteine: biomarker or cause of adverse pregnancy outcome?• Biomarker research in multiple sclerosis• Imaging and molecular biomarkers of vulnerable atheromatous plaques• Early detection of acute coronary syndromes by multimarker analysis• Aspects of β-amyloid as a biomarker for Alzheimer’s disease
Technology Report• Future of liquid chromatography-mass spectrometry in metabolic profiling and metabolomic studies for biomarker discovery
Priority Paper Evaluations• Assessing the roles of EGFR copy number and protein expression in NSCL cancer • Early advances of α1-antitrypsin precursor using the proteomics
Perspective• Mucosally restricted antigens as novel immunological targets for antitumor therapy
New in 2007

Editorial Advisory Board
Benowitz NL, University of California San Francisco, USA
Bjornsson T, Wyeth Research, USA
Bustin S, University of London, UK
Diamandis E, Mount Sinai Hospital, Canada
von Eggeling F, Friedrich-Schiller-University Jena, Germany
Ellis I, University of Nottingham, UK
Fernandez-Aviles F, University Hospital Gregorio Marañon, Spain
Flockhart DA, Indiana University School of Medicine, USA
Gardner R, Glasgow Royal Infirmary, UK
Gries J-M, Paramount Biocapital, USA
Hammarström S, Umeå University, Sweden
Holmes E, Imperial College London, UK
Hoon DSB, John Wayne Cancer Institute, USA
Jaffe AS, Mayo Clinic, USA
Kricka L, University of Pennsylvania Medical Center, USA
Lewis M, Celera Diagnostics, USA
Libri V, GlaxoSmithKline, UK
Licinio J, University of Miami, USA
Biomarkers in Medicine features an Editorial Advisory Board drawn from theleading forces in academia and industry. Together with a dedicated in-house editorial team Biomarkers in Medicine provides the scientific community with a unique source of commentary and opinion.
Call for Papers:Biomarkers in Medicine accepts unsolicited manuscripts – if you are interested in submitting an article, or have any queries regarding article submission please contact [email protected]
Senior Editors:Andre Terzic, Mayo Clinic College of Medicine, USAScott A Waldman, Thomas Jefferson University, USA
Advisory Board:
Liotta LA, George Mason University, USA
Lively T, National Cancer Institute, USA
Maimonis P, Decision Biomarkers, USA
McLeod HL, University of North Carolina, USA
Nafziger AN, Ordway Research Institute, Inc., USA
Nicolini A, University of Pisa, Italy
Petricoin EF, George Mason University, USA
Ratain MJ, University of Chicago, USA
Ross JS, Albany Medical College, USA
Schernthaner G, Rudolfstiftung Hospital Vienna, Austria
Sikora K, Hammersmith Hospital, UK
Srivastava S, National Cancer Institute, USA
Tammen H, Digilab BioVisioN, Germany
Temple R, US Food and Drug Administration, USA
Thurin M, National Cancer Institute, USA
Turck C, Max Planck Institute of Psychiatry, Germany
Wilson ID, AstraZeneca, UK
Zetterberg H, Göteborg University, Sweden

EDITORIAL
10.2217/17520363.1.1.xxx © 2007 Future Medicine Ltd ISSN 1752-0363 Biomarkers Med. (2007) 1(1), xxx–xxx1
part of
Targeted diagnostics and therapeutics for individualized patient management
Scott Waldman1† & Andre Terzic2†
†Authors for correspondence1Thomas Jefferson University, Department of Pharmacology and Experimental Therapeutics, Division of Clinical Pharmacology, Department of Medicine, Thomas Jefferson University, 132 South 10th Street, 1170 Main, Philadelphia, PA 19107, USAE-mail: [email protected] Clinic, Divisions of Cardiovascular Diseases & Clinical Pharmacology, Departments of Medicine, Molecular Pharmacology & Experimental Therapeutics and Medical Genetics, Mayo Clinic, 200 First Street, S.W. Rochester, MN 55905, USAE-mail: [email protected]
“Molecular biomarkers represent a path forward from the current
curative model of patient care to preemptive prognostic and
predictive medicine.”
Advances in deconvoluting complex diseaseprocesses, enabling genomic, proteomic, metab-olomic and imaging technologies, high-through-put drug discovery and development platforms,collectively offer unique opportunities in person-alized diagnostic and therapeutic management.This revolution in clinical care is predicated onthe development and refinement of biomarkersenabling disease prevention, diagnosis and treat-ment of patients and populations. Biomarkersare measurable characteristics, amenable to pro-spective evaluation. Indeed, biomarkers providesets of information to detect or define diseaseprogression, or predict and quantify therapeuticor adverse responses. Traditional biomarkersencompass:
• Surrogate physiological measurements such asheart rate, blood pressure and performancestatus;
• Clinical images such as chest x-ray or mam-mograms;
• Individual macromolecules such as prostate-specific antigen or carcinoembryonic antigen.
The decoding of the human genome, coupledwith exponential advances that permit ultrarapidbiomolecular analysis, have produced the nextgeneration of technologies, including genetic andepigenetic profiling; cell enumeration, characteri-zation and isolation; and in vivo imaging of tran-scriptional and translational processes providingbiomarkers with increased disease sensitivity andspecificity for improved patient management. Fur-thermore, application of these technologies in pro-grams elaborating fundamental mechanismsunderlying disease pathophysiology has identifiedmolecules with dimensions that extend wellbeyond simple prognosis and prediction, to mech-anism-based targets for individualized therapy.
Importantly, the ongoing transformation intechnology and discovery has produced amarked evolution along the entire downstreamcontinuum of diagnostic and therapeutic devel-opment to realize the promise and potential ofbiomarkers in individualized and populationmedicine. Technological advances in biomarkerdiscovery have entrained the coevolution ofparadigms addressing biomarker analytic valida-tion, clinical qualification and application [1].This concurrence in the development of valida-tion paradigms for complex biomarkers hasengendered newly recognized issues surroundingapproval and marketing by regulatory agencies.Furthermore, these advances in regulation, theemergence of requirements for robust analyticvalidation and clinical qualification, and theattendant patient- and capital-intensiveresources necessary to support these activities hasdriven the formation of new collaborationsbetween federal agencies, academia, and thepharmaceutical and biotechnology industries tomaximally exploit the full potential of bio-markers in clinical care [2].
“Importantly, the ongoing transformation in technology and discovery has
produced a marked evolution along the entire downstream continuum of
diagnostic and therapeutic development to realize the promise and
potential of biomarkers.”
Clinical utility is predicated upon individualization of patient managementPreventive biomarkers prospectively identify indi-viduals at increased risk for developing pathology[3]. Patients with mutations in the gene encodingthe huntingtin protein are at risk of developingHuntington’s disease, and identification of thesemutations mandates aggressive disease surveillanceand genetic counseling for risk reduction in theirextended family. Diagnostic biomarkers identifythe presence of disease at the earliest stage, beforeclinical manifestation. Blood pressure identifiespatients with hypertension, serum glucose

Targeted diagnostics and therapeutics for individualized patient management – EDITORIAL
2future science groupfuture science group www.futuremedicine.com
identifies patients who might be developing diabe-tes, and fecal occult blood identifies patients whomight harbor occult colon cancer. Prognosticbiomarkers stratify risk of disease progression inpatients undergoing definitive therapy. Gene-mutation profiling in patients with estrogen recep-tor-positive, lymph node-negative breast canceridentifies those at increased risk for developingrecurrent disease [4]. Predictive biomarkers identifypatients who are most likely to respond to specifictherapy. Quantification of the human epidermalgrowth factor receptor (HER)2 expression identi-fies breast cancer patients expressing this receptorwho will respond to treatment with monoclonalantibodies to HER2 [5]. Therapeutic biomarkersquantify responses in patients undergoing treat-ment. Evaluation of minimal residual disease inpatients with chronic myelogenous leukemia bydetection of the Philadelphia chromosome usingpolymerase chain reaction (PCR) quantifies theefficacy of therapy [6]. Finally, biomarkers identifypatients at risk for developing adverse reactions tospecific therapeutics. Individuals with specificmutations in one isoform of the detoxifyingenzyme uridine diphosphate glucurono-syltransferase are slow metabolizers of irinotecanand can develop severe diarrhea and neutropeniain the absence of dose adjustments [7].
Advances in technology rapidly translated to patientsThe science of biomarkers has been driven by theparallel development of rapid analytic high-throughput technologies and conceptual
advances in molecular mechanisms underlyingdisease, which have yielded targets of increasingcomplexity that address individualization ofmedical management. Initially, biomarkersevolved as single molecular elements related tothe presence of disease, such as serum glucoseand diabetes, sedimentation rate and inflamma-tion and prostate-specific antigen in prostatecancer. More recently developed biomarkersexploit technological and mechanistic advances,for example prognostic markers such as low-den-sity lipoprotein in cardiovascular disease, muta-tions in the adenomatous polyposis coli geneassociated with development of colorectal cancer,and the detection of HIV genes employing PCRto assess the risk of developing AIDS. Moreover,linear approaches involving single moleculebiomarkers have progressed, reflecting bio-system-wide insights into pathophysiology, andpanels of markers and their disease-specific alter-ations are quantified and their integrated prog-nostic or predictive value defined. Assessment ofa panel of genes and their associated mutationsin breast tumors stratifies estrogen receptor-posi-tive, lymph node-negative patients for risk ofdisease recurrence [4]. Similarly, evaluation of agene panel and their associated mutations instool can identify patients who may harboroccult colorectal tumors [8]. Beyond specificgroups of biomolecules, the entire transcriptomeor proteome can be assessed, distinguishing dis-eased and normal tissues or disease categorieswith different risk profiles. Distinct patterns ofgene expression in tumors and normal tissues
Figure 1. Biomarkers: translating molecular discoveries into disease management.
Biomarkers emanating from the revolution in enabling technologies and insights in pathophysiology have become integral to individualizedpatient care. In turn, those biomarkers further potentiate the discovery of novel molecular mechanisms underlying disease pathogenesis
Biomarkers
Molecularpathways
Prognosis
Informatics Prevention
Enablingtechnologies
Diagnosisand therapy
Curatedbiorepository
Prediction
Diseasemanagement
Diseasepathophysiology

EDITORIAL – Waldman & Terzic
3 Biomarkers Med. (2007) 1(1) future science groupfuture science group
have been elucidated in breast and colon [9],while profiling the serum proteome employingmass spectrometry distinguishes patients withovarian cancer from those without [10].
Biomarker discovery entrains the continuum of development & regulationThe prolific pace of biomarker discoveryreflects the emergence of technologies, andtheir potential to change patient managementbrings into specific relief associated issues ofdevelopment, regulation and application whichmust coevolve to realize their full clinicalpotential. In that context, these considerationshave raised the need for regulatory oversight ofanalytic validation and clinical qualificationand to systematically transition biomarker dis-covery to clinical care. Indeed, the paradigm inwhich biomarker analyses are conducted byemploying home-brew assays in individual lab-oratories is rapidly evolving. In the near future,biomarkers will require rigorous analytic valida-tion to define performance metrics, includingreproducibility, sensitivity and precision
employing technology platforms that have beencross validated to ensure the highest levels ofreproducibility [11–13]. Additionally, quantita-tive and qualitative relationships betweenbiomarkers and disease management willrequire rigorous clinical qualification, with evi-dence linking a biomarker with biological andclinical end points [1,14]. Moreover, relation-ships describing the clinical utility of biomark-ers will require assessment in prospectiverandomized clinical trials, and subsequentlyvalidated in follow-up trials, to minimize over-estimation of clinical value reflecting bias andchance [5].
“In the near future, biomarkers will require rigorous analytic validation to
define performance metrics.”
Translating discovery into practice: biomarker commercializationBiomarkers influence clinical decision making,substantially impacting healthcare economics. Pre-ventive tests that screen for genetic mutationsidentify patients at risk for developing diseaseswho become new customers to the healthcare sys-tem. Prognostic tests define the risk of diseaserecurrence and identify patients who may not ben-efit from expensive chemotherapy or who requirefurther evaluation employing expensive imagingprocedures. Predictive tests identify patients whocould benefit from expensive biomolecular ther-apy. The profound impact on patient outcomes,and the resulting savings in budget funds, has beenused to justify high prices for biomarkers, tradi-tionally reserved for therapeutics [15]. Their emer-gence as high-cost and, consequently, high-profitproducts is driving the biotechnology communityto launch new companies focusing on biomarkerdevelopment and application. Success in thoseventures depends on the ability of biomarkers toaddress substantial markets and direct decisionmaking regarding expensive, complex or danger-ous therapeutic interventions [15]. Currently themarket stands at US$5billion and is increasing atannual rate of 25%.
Biomarkers at the intersection of practice, business & regulationHistorically, US FDA approval was obtained tomarket test kits that would then be sold to clini-cal laboratories. More recently, biomarkers havebypassed FDA approval and distribution to locallaboratories and, rather, undergo analysis in
Figure 2. Biomarkers at the intersection of science, clinical practice and regulation.
Biomarker discovery reflects the emergence of enabling technologies, and their potential to change patient management brings into specific relief associated issues of development and regulation, which must coevolve to realize their full clinical potential
Dia
gnos
tic a
nd th
erap
eutic
targ
ets
Enab
ling
plat
form
s
Dis
ease
pat
hway
s
Application and com
mercialization
Analytical variation
Clinical qualification
Diagnosis, prediction, prevention,therapy and prognosisIndividualized medicine
Population sciences
Biomarkers
Sci
ence
tech
nolo
gy
Developm
ent
regulation
Clinicalpractice

Targeted diagnostics and therapeutics for individualized patient management – EDITORIAL
4future science groupfuture science group www.futuremedicine.com
central laboratories [15]. Certainly, abrogatingFDA approval permits a shorter and less costlydevelopment timeline from discovery to market-place. However, savings in money and time mayreflect the absence of analytical validation andclinical qualification inherent in approval by theFDA. Indeed, failure to provide definitive valida-tion and qualification of biomarker value hascontributed to the slow integration of the newestgeneration of molecular biomarkers into main-stream patient management paradigms [3,11,14].
“There is recognition that the true value of biomarkers in clinical medicine will only be achieved by developing the compelling evidence basis for their
performance and application.”
In that context, there is recognition that the truevalue of biomarkers in clinical medicine will onlybe achieved by developing the compelling evidencebasis for their performance and application. Today,biomarkers are almost universally marketed as testsconducted in a single central laboratory and theFDA does not regulate the conduct of those tests,their analytic validity or their clinical qualification[12,13]. Rather, individual laboratories are certifiedby Clinical Laboratory Improvement Amend-ments (CLIA) to perform testing on human speci-mens and report patient-specific results. CLIAcertification requires laboratories to adhere to qual-ity control standards, personnel qualifications anddocumentation and validation procedures. Fur-thermore, laboratories performing high-complex-ity testing must enroll in a specialty area thatprovides for proficiency testing. However, there isno specialty area identified for molecular andgenetic testing, and there are no specific qualitycontrols, personnel qualification, or proficiencytesting requirements for these types of biomarkers[13]. The Centers for Medicare and Medicaid Serv-ices (CMS) within the Department of Health andHuman Services is responsible for the quality ofCLIA-approved laboratories. While physicians,patients, and laboratory directors have lobbied forproficiency testing standards for laboratories pro-viding high-complexity molecular and genetic test-ing services, CMS has asserted in the past thatthese issues may not achieve sufficient critical levelto regulate [13]. In the context of the failure of atleast a third of CLIA-certified laboratories per-forming genetic testing to participate in profi-ciency testing and the inverse relationship betweenerrors in diagnostic analyses and proficiency test-ing, the current regulatory position will continue
to be an area in evolution and flux within the diag-nostic and clinical practice communities [13].
Furthermore, while the FDA has authority toregulate molecular tests, in the past it has exer-cised enforcement discretion. More recently,the FDA issued a draft guidance extending reg-ulatory enforcement authority to a subset ofhome-brew molecular tests termed in vitrodiagnostic multivariate index assays [101]. Multi-variate index assays measure multiple analytesin the context of other clinical information andanalyze the data with algorithms or softwareprograms that are often proprietary, resulting inan inability of physicians to interpret resultsdirectly. In the future, in vitro diagnostic multi-variate index assays will most likely requiresome level of FDA review, and some mayrequire full regulatory approval. The FDA posi-tion with respect to an over-arching policyregarding oversight of molecular biomarkers asa class continues to evolve.
Collaborations across communities of practice will drive biomarker integration in patient managementIntegration of biomarkers into clinical para-digms for patient management will require dra-matic changes across the continuum ofdiscovery, development, regulation and utiliza-tion, requiring collaboration across historicallydisparate communities of practice. Biomarkerapplication to optimize disease-free survival forpatients will require comprehensive relationaldatabases of tens of thousands of patients trackedlongitudinally to compare individual molecularprofiles to clinical characteristics of patients andpathologic characteristics of diseases [11]. Tomaximize accessibility, applicability and utility,databases will be constructed utilizing commonstatistical algorithms, universally accepted trialdesigns and standardized analytical platforms.Ultimately, integration of biomarkers intopatient management paradigms will requireengineering of those databases for use by practic-ing community physicians to truly individualizepatient therapies across whole populations, usingthe integrated grid of biomarkers to define dis-ease prognosis, predict responsiveness to specifictargeted therapies and anticipate and avoidpatient-specific adverse reactions [11].
Realization of this envisioned future mandatescollaboration among stakeholders involved inbiomarker development, application and regula-tion. In that context, cultural barriers to this col-laboration are significant. There has been a

EDITORIAL – Waldman & Terzic
5 Biomarkers Med. (2007) 1(1) future science groupfuture science group
historical polarity in missions of academia, indus-try and regulatory agencies. In addition, progressmay be further impeded by issues surroundingpatient confidentiality, privacy rights and the fearof misuse of molecular data for discrimination[11]. Importantly, there is recognition by the vari-ous stakeholders that barriers will be overcomeand progress will be enjoyed through strategicalignment around a common vision. The Phar-maceutical Research and Manufacturers of Amer-ica and the FDA have taken a first step bycreating a consortium for the development ofbiomarkers established under the Foundation forthe NIH, in collaboration with CMS, academicinstitutions and representatives from the privatesector including pharmaceutical, biotechnologyand diagnostics companies [1]. The consortium isopen to institutions in the public or private sec-tors and will manage biomarker programs toensure scientific integrity, appropriate resources,and compliance with relevant statutes. The con-sortium will focus on identifying, validating andqualifying biomarkers that will be integrated intothe clinical application of marketed drugs. Thisrepresents a necessary, first step to realizing thegoal of biomarker-based prognostic and predic-tive individualized medicine through requisitecooperation and collaboration.
Biomarkers in Medicine: the engine facilitating disciplinary evolutionMolecular biomarkers represent a path forwardfrom the current curative model of patient care topreemptive prognostic and predictive medicine.However, their integration into mainstream clini-cal practice is predicated on the evolution ofdevelopment and regulatory paradigms centeredon analytic validation, clinical qualification andthe evidence basis of practice grounded in rigor-ous clinical trial design, analytical methodologiesand statistical evaluation. In conjunction,
regulatory quality control and oversight will con-tinue to evolve in this rapidly developing field.Furthermore, progress will be achieved only in thecontext of collaboration between stakeholders inthe academic, private and governmental sectors, atthe interface of these historically opposing com-munities of practice. To facilitate that exchangeand integration, to provide a dedicated forum fordiscourse on key issues of critical importance, andto place the science in the larger economic, polit-ical and social context, Biomarkers in Medicinewill be the authoritative journal dedicated to thediscovery, development, regulation and utility ofbiomarkers and their integration into clinicalcare. Reflecting the rapid evolution in this fieldof technical and conceptual advances, and thepotential for imposing transformative changes inthe management of individual patients and pop-ulations, Biomarkers in Medicine will deliver crit-ical commentary and analysis on advances in ourunderstanding of biomarkers and their potentialand actual applications in medicine. Further-more, the journal will serve as a conduit for pri-mary research, perspectives, commentary andinterpretive analysis for key advances in thehighly technological underpinnings of the fieldand the fundamental pathophysiological mecha-nisms from which biomarker discovery andapplication emanate. Moreover, the journal willhighlight the ability of these technical andpathophysiological elements to reach beyond thetraditional application of biomarkers as informa-tional tools, extending into therapeutic applica-tions. It is anticipated that the journal will serveas the voice for the nascent discipline of biomar-kers in medicine, contributing to, and in partdriving, the direction, focus and identity of thismultidisciplinary community of practice. Welook forward with excitement and anticipation,on the threshold of a new era in the integrationof science and medicine.
Bibliography1. Wagner J, Williams S, Webster C:
Biomarkers and surrogate endpoints for development and regulatory evaluation of new drugs. Clin. Pharmacol. Ther. 81, 104–107 (2007)
2. Waldman SA, Christensen NB, Moore JE et al.: Clinical pharmacology: the science of therapeutics. Clin. Pharmacol. Ther. 81, 3–6 (2007).
3. Wilson C, Schulz S, Waldman SA: Biomarker development, commercialization, and regulation: individualization of
medicine lost in translation. Clin. Pharmacol. Ther. 81, 153–155 (2007).
4. Paik S, Shak S, Tang G et al.: A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
5. Wilson JF: The rocky road to useful cancer biomarkers. Ann. Intern. Med. 144, 945–948 (2006).
6. Faderl S, Hochhaus A, Hughes T: Monitoring of minimal residual disease in chronic myeloid leukemia. Hematol. Oncol. Clin. North Am. 18, 657–670 (2004).
7. Iyer L, King CD, Whitington PF et al.:Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J. Clin. Invest. 101, 847–854 (1998).
8. Imperiale TF, Ransohoff DF, Itzkowitz SH et al.: Fecal DNA versus fecal occult blood for colorectal-cancer screening in anaverage-risk population. N. Engl. J. Med.351, 2704–2714 (2004).

Targeted diagnostics and therapeutics for individualized patient management – EDITORIAL
6future science groupfuture science group www.futuremedicine.com
9. Sjoblom T, Jones S, Wood LD et al.: The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274 (2006).
10. Petricoin EF, Ardekani AM, Hitt BA et al.:Use of proteomic patterns in serum to identify ovarian cancer. Lancet 359, 572–577 (2002).
11. Dalton WS, Friend SH: Cancer biomarkers – an invitation to the table. Science 312, 1165–1168 (2006).
12. Hudson KL: Genetic testing oversight. Science 313, 1853 (2006).
13. Hudson KL, Murphy JA, Kaufman DJ et al.: Oversight of US genetic testing laboratories. Nat. Biotechnol. 24, 1083–1090 (2006).
14. Williams SA, Slavin DE, Wagner JA et al.:A cost-effectiveness approach to the qualification and acceptance of biomarkers. Nat. Rev. Drug Discov. 5, 897–902 (2006).
15. Licking EF, Longman R: The new diagnostics companies. Start-Up: Windhover’s Rev. Emerging Med. Ventures 12–19 (2006).
Website101. US FDA. Draft guidance for industry,
clinical laboratories, and FDA staff: In vitrodiagnostic multivariate index assays (2006)www.fda.gov/cdrh/oivd/guidance/1610.pdf
Pharmacogenomics, Personalized Medicineand OncologyJournals 2007
Free trials availableContact: [email protected]

REVIEW
10.2217/17520363.1.1.xxx © 2007 Future Medicine Ltd ISSN 1752-0363 Biomarkers Med. (2007) 1(1), xxx–xxx1
part of
Early detection of acute coronary syndromes and risk stratification by multimarker analysisAlan HB WuUniversity of California, Department of Laboratory Medicine, San Francisco, CA, USATel.: +1 415 206 3540;Fax: +1 415 206 3045;E-mail: [email protected]
Keywords: plaque instability, plaque vulnerability, proteomics, myocardial ischemia, myocardial necrosis, multimarker index, neural network, troponin
Cardiac troponin is the standard biomarker for the diagnosis of acute myocardial infarction (AMI) and risk stratification for short-term adverse cardiac events (death, AMI or need for revascularization). Unfortunately, the concentration of troponin in blood is normal in AMI patients who present early after the onset of symptoms. As such, there is active research being conducted in finding early markers of AMI and risk stratification. Despite years of testing dozens of candidates, no single test has had the necessary clinical sensitivity and specificity for this indication. Therefore, many researchers have advocated multimarker testing. There are two approaches that have been taken for discovering new markers. The proteomic approach involves focusing on the differences in the biochemical signatures between the tissues or biological fluids of normal compared with diseased individuals. Specific biochemical targets are not preselected. The pathophysiologic approach involves combining biomarkers that indicate a particular pathway or event known to be involved in the disease process. In both approaches, some bioinformatic algorithm will be necessary in order to combine the information provided by the individual tests. Representative approaches include the Multimarker Index™, classification and regression tree analysis and neural networks.
Cardiovascular disease (CVD) continues to be theleading cause of morbidity and mortality in theUSA and throughout the western world [101].According to the American Heart Association,approximately 64.4 million Americans haveCVD, which accounts for 38.5% of alldeaths [101]. Approximately 13.2 million have cor-onary artery disease, 7.8 million have suffered anacute myocardial infarction (AMI) and6.8 million have suffered angina pectoris [101].CVD also accounts for a large number of individ-uals who present to the emergency department(ED) with chest pain. Approximately eight out ofa total of 95 ED admissions (~28%) are due topatients who present with chest pain or othersymptoms suggestive of CVD (Figure 1). A largefraction of patients have a noncardiac source oftheir symptoms, such as skeletal muscle pain, pul-monary emboli, aortic dissection, blunt trauma tothe chest or emphysema, amongst other factorsand are ruled out for AMI. The rate of discharge isdependent on the aggressiveness of the hospital insending patients home. The remaining patientsare admitted either to a chest-pain center for rapidrule out of AMI, a hospital bed or the coronary-care unit. More than 50% of these patients will beruled out for acute coronary syndromes (ACS)within the ensuing 1–3 days. Of the remainingpatients, there are roughly 1.2 million who sufferAMI and another 800,000 who have unstable
angina. There are also a significant number ofpatients who die of ACS before they appear in theED or shortly after arrival. Usually death is causedby cardiac dysrhythmias induced by the AMI,which occurs before traditional biomarkers arereleased into the circulation.
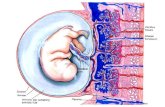
Pathophysiology of ACSAlthough there are many etiologies to AMI, theocclusion of a coronary artery by the erosion orrupture of an atherosclerotic lesion on a coronaryartery is responsible for the majority of cases [1].There are many events that occur leading up tothe rupture of this artery. Figure 2 illustrates thevarious stages of ACS. Stage A shows a clean coro-nary artery [2]. Atherosclerosis (stages B and C)typically begins during early adulthood dependingon diet and lifestyle habits. In a stable plaque(stage D), there is a significant lesion that pro-trudes into the coronary artery and is filled withlipids (shown in yellow). A thick fibrous cap pro-tects this lesion from rupture. Stage E shows thedevelopment of a plaque that is vulnerable to rup-ture under the sheer stress of arterial blood pres-sure. Inflammation and leukocyte infiltrationrelease cytokines and enzymes that degrade thecollagen layers that protect the artery. AMI occursfollowing plaque rupture (F). The release of lipidsand extracellular matrix ‘gruel’ stimulates plateletactivation and thrombus formation.

Acute coronary syndromes and risk stratification by multimarker analysis – REVIEW
2future science groupfuture science group www.futuremedicine.com
Existing biomarkers for acute myocardial infarctionCreatine kinase (CK) and its MB isoenzyme(CK–MB) was the mainstay for the diagnosis ofAMI. CK is found in the heart, striated andsmooth muscle and brain. Measurement ofCK–MB has higher specificity towards cardiacinjury. Troponin is today the preferred test forAMI. Cardiac troponins T (cTnT) and I (cTnI)are found in higher concentrations in the heartthan CK, and are more sensitive toward detect-ing myocardial cell death. Troponin is also morespecific towards heart disease, as the skeletalmuscle troponin isoform is distinct from the car-diac form [3]. Myoglobin is a small protein that isreleased earlier after AMI than troponin orCK–MB. Myoglobin is falsely increased inpatients with skeletal muscle disease and renalfailure and, therefore, the utilization ofmyoglobin has greatly diminished. Before thesemacromolecules can traverse the cell membraneand appear in blood, there must be irreversiblemyocyte necrosis. Moreover, the occlusion of acoronary artery blocks the egress of proteinsdirectly into coronary circulation. Therefore,there is a delay from the onset of plaque ruptureto the appearance of biomarkers into blood, andlevels are normal during their initial ED presen-tation. Passage of cardiac proteins must occurthrough lymphatic drainage, which adds furtherdelay to their clearance.
B-type natriuretic peptide (BNP) and N-termi-nal prohormone brain natriuretic peptide(NT-proBNP) are other widely used cardiacbiomarkers. These peptides originate from theventricles of the heart, are released into the bloodin response to volume overload and myocardialwall stress, and are used for the diagnosis of heartfailure. BNP and NT-proBNP are also released inpatients with myocardial ischemia from ischemia-induced ventricular dysfunction and hypoxia.Increased concentrations of BNP/NT-proBNPare associated with an increased risk for cardio-vascular death, and many studies have begun toincorporate them as part of a multimarker panel.
ESC/ACC redefinition of AMIIn 1979, the WHO defined AMI on the basis offinding two out of three equally-weighted crite-ria: clinical history of an ischemic presentation,unequivocal electrocardiographic (ECG)changes and serial changes in cardiac enzymes inblood [4]. With the WHO criteria, it was possibleto diagnose AMI without finding an increase inCK–MB. With the development of troponin,the European Society of Cardiology (ESC) andthe American College of Cardiology (ACC)redefined the criteria for AMI in 2000 [5]. It isnow predicated on the finding of a rise and fallof a cardiac biomarker with evidence of myocar-dial ischemia (ischemic symptoms, ECGchanges, evidence by cardiac catheterization, orpathologic changes at postmortem). Cardiac tro-ponin was recommended as the preferred markerfor AMI diagnosis, replacing CK–MB. Themotivation for making these changes was basedon the clinical observation that any increase introponin was correlated with an adverse short-term outcome (i.e., cardiac death, AMI or needfor revascularization within 30 days). Approx-imately a third of the cases previously defined asunstable angina had increases in troponin, sug-gesting the presence of irreversible damage andsubsequent cardiovascular risk that is similar tothe rate seen for AMI. The ESC/ACC Commit-tee recommended using a troponin cutoff at theupper 99th percentile of a healthy population,ideally using an assay with an imprecision of lessthan 10% at this cutoff.
Clinical need for early biomarkers of acute coronary syndromes Although troponin is now the gold standardmarker for AMI, this marker is usually normalwhen blood is collected within the first fewhours after symptoms onset. Therefore, for a
Figure 1. Annual visits to the emergency departments in US hospitals and the incidence of acute coronary disease among those visits.
AMI: Acute myocardial infarction; ED: Emergency department; UA: Unstable angina.

REVIEW – Wu
3 Biomarkers Med. (2007) 1(1) future science groupfuture science group
patient who presents early, ED physicians mustwait a few hours before diagnosis and treatmentcan be initiated. Moreover, there are substantialcosts that are incurred for keeping patients in acrowded ED while a definitive diagnosis is beingestablished. Some hospitals have opted to reducetheir costs by instituting an aggressive dischargepolicy. Unfortunately, this will increase the rateof inadvertent discharge of patients who haveAMI or will suffer one in the near term(e.g., 24 h). Missed AMIs are the leading causeof malpractice lawsuits in the ED today [6].
In order to reduce hospital expenses andmaintain diagnostic accuracy, many EDs havedeveloped ‘chest pain’ centers of the rapid rule-out of CVD. The frequency for cardiac bio-marker and ECG testing is increased. If a patienthas a positive result with either test, they areimmediately admitted to an appropriate hospitalbed. If these tests are negative 6–9 h after presen-tation, patients are sent for a stress test or anuclear imaging analysis for evaluation of theircardiac status. Those with negative stress testresults are unlikely to suffer a major cardiac eventwithin the next few days and are medicallycleared for discharge [7].
A patient who presents to the ED with symp-toms or electrocardiographic evidence suggestiveof myocardial ischemia, and has a positive tro-ponin result in serum defines an AMI. Thispatient should be admitted and appropriatelytreated. No further laboratory testing is neededto triage this patient. While troponin is the goldstandard for AMI diagnosis, there are remaining
questions and clinical situations that are notanswered when the results of troponin are nega-tive. Therefore, a biomarker or a panel of tests isneeded that is:
• Reliably increased at the time of ED presenta-tion of a patient with ACS, before troponin isincreased;
• Consistently negative at ED presentation ofpatients who are ultimately ruled out forAMI;
• Predictive of short-term cardiovascular mor-bidity or mortality with a negative troponin atall time points.
Search for novel biomarkers of CVD using proteomics & metabolomicsThe identification of novel biomarkers for CVDdetection is a very active area of research. Theanalytical methodologies and ideal characteristicsof a biomarker have recently been reviewed [8].There are two general approaches towards thesearch for any biomarkers for human diseases. Inthe proteomics and metabolomics approach, tis-sue and/or blood samples from diseased individu-als are compared against either healthy subjects orpatients who have a disorder that mimics the dis-ease in question [9,10]. For example, blood or tis-sues from patients with early myocardial ischemiawould be compared with patients who presentwith noncardiac chest pain. The samples are sub-jected to 2D electrophoresis for separation andthe individual proteins or metabolites are identi-fied by sophisticated mass spectrometric tech-niques. The proteomic/metabolomics approach
Figure 2. Stages in the pathophysiology of acute coronary syndromes.
(A) Coronary artery that is largely free of plaques. (B & C): Progressively increasing stages of plaque formation. (D) A plaque that has formed a lipid core. A thick fibrous cap protects the lipid core from the circulation. (E) A plaque that is vulnerable to rupture, characterized by a thin fibrous cap. (F) A ruptured plaque. The exposure of the lipid core to circulating blood activates the thrombosis cascade and the aggregation of platelets (not shown). Adapted and used with permission from reference [2].

Acute coronary syndromes and risk stratification by multimarker analysis – REVIEW
4future science groupfuture science group www.futuremedicine.com
does not presume any prior knowledge of thepathophysiology for the disease in question. Theproteins or metabolites may not be known orcharacterized for this purpose.
To promote proteomics, The National Heart,Lung and Blood Institute assembled a prote-omics working group specifically targetedtowards new CVD biomarker discovery [11].While proteomics has tremendous potential infinding new markers, the working group identi-fied major challenges. There are many steps andexpenses necessary to bring biomarker discover-ies into a clinical test approved by the US FDAfor use on patients. In vitro diagnostic corpora-tions do not have budgets to conduct the trialsnecessary to gain clearances. Many of the novelbiomarker studies are therefore validated as partof substudies originally designed and funded fornew cardiovascular drugs. Dissemination of newassays may also be limited by patents that havebeen granted on specific proteins and their appli-cation to clinical practice. The NIH has begunfunding multidisciplinary translational researchprojects to bring innovative lab tests from the“research bench to patient’s bedside” [102].
There have been a few studies that used thesetechniques to discover new cardiac biomarkers.Using carotid endarterectomy tissue samples,Martin-Ventura and colleagues used differentialproteomics and reported that heat-shock protein(HSP)-27 was decreased in complicated athero-sclerotic plaques [12]. High levels of HSP-27 maybe cardioprotective by inhibition of cardiovascu-lar inflammation and apoptosis. Marshall andcolleagues examined serum from patients withAMI and compared results against healthycontrols [13]. Mass spectrometric analysis showedthat after AMI, the α-chain of complement C3and fibrinogen are degraded to C3f peptide andfibrinogen peptide A, respectively, which arethen further degraded to smaller components byaminopeptidase to form a distinctive fingerprintpattern. Other investigators have used proteomictechniques to identify early stages of heart fail-ure, which could have a significant impact onmorbidity and mortality rates if early diseasedetection can be linked to therapeutics to slow orprevent disease progression [14].
While proteins are the usual target for manyproteomic disease correlations, lower molecularweight targets may be appropriate for ACS. Thisis because proteolytic enzymes are also releasedinto blood that can degrade cardiac proteins.Thus, some investigators have focused on metab-olomic techniques. Brindle and colleagues used
nuclear magnetic resonance imaging of humanserum to determine the presence and severity ofcoronary heart disease [15]. With a clinical specif-icity of more than 90%, they were able to dis-criminate severe multivessel stenosis (>90%)from patients with normal coronary arteries.Sabatine and colleagues also used metabolomicsto identify novel biomarkers of myocardialischemia [16]. These investigators collected bloodfrom patients before and after exercise stress test-ing and identified 18 patients who had an induc-ible ischemia and 18 patients who were normal.Some 23 metabolites were found at higher con-centrations in ischemic compared withnonischemic cases, including six members of thecitric acid pathway. Kiernan and colleagues useda multiplexed mass spectrometric immunoassaytechnique as a tool for biomarker discovery,identification and verification [17].
The use of proteomic and/or metabolonomictechniques in routine clinical practice appearsto be a few years away. The mass spectrometricinstrumentation is expensive and requires spe-cialized operator training, and is currently notamendable towards routine clinical analysis.This is particularly true for markers of myo-cardial ischemia, as the optimum application ofthese tests is in the ED, and a rapid turnaroundfrom sample collection to analysis, would bedesired. It is very likely that proteomics willenable the identification of a few useful non-redundant targets that can be assembled into anarray of immunoassays. Clinical laboratoryinstrumentation is moving towards multi-marker analysis with the development of gene-chip arrays and multicolored-bead analyzers[18]. When a list of analytes has been identified,it would be appropriate to collaborate or licensesuch technologies with these manufacturers.
Novel biomarkers based on the pathophysiology of ACSIn addition to proteomics/metabolomics, thealternate approach towards biomarker discoveryis to focus on the known processes that partici-pate in the pathophysiology of CVD for the spe-cific development of ACS; it is particularlyimportant to focus on processes that participatein the conversion of stable atherosclerosis to thevulnerable plaque [19].
Markers of inflammation & plaque instabilityMyocardial inflammation stimulates the recruit-ment of cellular and humoral elements into the

REVIEW – Wu
5 Biomarkers Med. (2007) 1(1) future science groupfuture science group
coronary arteries. Their presence is responsiblefor the thinning of the fibrous cap and increasingthe vulnerability of previously stable athero-sclerotic lesions and they are targets forbiomarker discovery.
C-reactive proteinMyocardial inflammation is a key step in the for-mation of unstable coronary artery lesions.Inflammation triggers the release of biochemicalfactors that simultaneously protect the body fromforeign antigens and damages vital organs such asthe heart. C-reactive protein (CRP) was the firstinflammatory marker studied for CVD detection.CRP is an acute phase reactant whose concentra-tion is grossly increased (e.g., >100 mg/l) in theblood of patients with systemic inflammation. Inthe absence of a global inflammatory process,localized myocardial inflammation can occur.Using high-sensitivity (hs) CRP assays, the find-ing of a minor elevation in hsCRP (3 to 10 mg/l)has been associated with increased primary risk forCVD over the ensuing years [20].
While hsCRP is not a useful diagnostic test forearly AMI or rule-out, it has been extensivelystudied as a predictor of secondary disease risk forpatients with a history of ACS or those whopresent with acute symptoms of myocardialischemia. Interpretation of hsCRP test results inthis context, however, is complicated by the factthat AMI will stimulate an increase in CRP, there-fore, an increased cutoff level greater than 10 mg/lmay be necessary for secondary risk stratification.The nonspecificity of hsCRP in the acute settinghas limited its application in the ED. However,hsCRP may be useful as a part of a multimarkerpanel for cardiovascular risk assessment. Sabatineand colleagues examined the combination ofhsCRP, troponin and BNP on patients from twolarge clinical trials [21]. These investigators foundthat the three tests were independent predictorsfor death, AMI or heart failure. Relative to onemarker being abnormal, there was a doubling inthe death rate at 30 days for each additionalmarker that was increased. This study was one ofthe first to use a multimarker approach towardsrisk stratification for CVD. There was no attemptto change the cutoff concentrations of the individ-ual markers or use an algorithm to optimize theinterpretation of results.
Placental-like growth factorPlacental-like growth factor (PlGF) is a memberof a family of vascular endothelial growthfactors, and is thought to be an instigator of
vascular inflammation. It functions to stimulatesmooth muscle growth, recruit monocytes andmacrophages into coronary artery plaques andupgregulate proinflammatory cytokines such astissue necrosis factor α. As with hsCRP, increasedconcentrations of PlGF in the blood is a non-specific indicator of myocardial inflammation.In the c7E3 Fab Anti Platelet Therapy in Unsta-ble REfractory angina (CAPTURE) trial,increased concentrations of PlGF was associatedwith a higher risk for death or nonfatal AMI at30 days (odds ratio [OR]: 3.34; 95% confidenceintervals [CI]: 1.79–6.24) [22]. Using a multivar-iate model, these investigators found PlGF to beindependent from cTnT and soluble CD40(sCD40) ligand (discussed below). However,hsCRP was found to be a dependent variable.
MyeloperoxidaseMyeloperoxidase (MPO) is a heme-containingenzyme that is found in azurophilic granules ofpolymorphonuclear leukocytes. It functions tooxidize chloride to hypochlorous acid, which hasbacteriacidal properties. Increased activities ofMPO are found in the shoulder regions of coro-nary artery plaques, where MPO oxidizes low-density lipoprotein. It also activates matrixmetalloproteinases (MMPs) which can degradethe collagen, the protective layer of the fibrouscap, thereby making it vulnerable to rupture.MPO appears to be a marker of risk stratificationfor ACS. Zhang and colleagues reported an ORof 11.9 (95% CI: 5.5–25.5) [23]. In a multivariateanalysis, Baldus and colleagues showed thatMPO, cTnT, vascular endothelial growth factors,sCD40 ligand and CRP were independentpredictors of 6-month outcomes [24].
Pregnancy-associated plasma protein APregnancy-associated plasma protein A (PAPP-A)and MMP-9 were first identified in the plasma ofpregnant women. PAPP-A is an enzyme thatworks with other metalloproteinases, such asMMP-9 and myeloperoxidase, to degrade thefibrous cap. PAPP-A from pregnant women isbound to the proform of eosinophil major basicprotein (proMBP), while that from the coronaryartery is free PAPP-A [25]. Therefore, it is impor-tant for patients with ACS that the PAPP-A assaysthat recognizes either total or free PAPP-A areused, and not just the PAPP-A bound to proMBP[26]. Like PlGF, high concentrations of PAPP-A areassociated with increased risk for short-term cardi-ovascular risk (OR: 2.44; 95% CI: 1.25–5.89) [27].PAPP-A remained an independent variable when

Acute coronary syndromes and risk stratification by multimarker analysis – REVIEW
6future science groupfuture science group www.futuremedicine.com
combined with troponin, sCD40 ligand, and vas-cular endothelial growth factors. hsCRP droppedout of significance in a stepwise multivariateregression analysis model.
Markers of plaque rupture, thrombosis & myocardial ischemiaThe rupture of a coronary artery plaque initiatesa cascade that stimulates platelet aggregation,thrombus formation, reduced coronary arteryblood flow leading to myocardial ischemia and,finally, myocardial cell death. The total occlu-sion of a coronary artery is an ST-elevation MI(STEMI) and causes a large release of troponin.The non-ST-elevation MI (nSTEMI) results ina partially occluded artery, and generally has lit-tle or no myocardial damage or troponinrelease. However, any coronary artery plaquerupture is a harbinger of adverse cardiac out-comes, and biomarkers are needed to denote thepresence of this event, even if troponin remainsconsistently negative.
CD40 ligandCD40 is a receptor molecule found on mono-cytes, macrophages, endothelial cells and platelets[28]. Ligands for CD40 consist of membrane
bound and sCD40 components. Activation ofplatelets by agonists such as adenosine mono-phosphate, collagen and arachidonic acid resultsin the upregulation and subsequent release of thesoluble form into the circulation. sCD40 ligandcan then activate endothelial cells and stimulatean inflammatory response within a coronaryartery plaque. Patients in the highest quartile ofsCD40 ligands were associated with adverse out-comes at 10 months [29]. Given the role of thesCD40 ligand in platelet activation, blood levelsof sCD40 ligand may also be important in select-ing patients who would benefit most from anti-plaletet drug therapies. Heeschen and colleaguesdemonstrated that patients with increasedsCD40 ligand levels had a lower incidence ofdeath or AMI when treated with the glycoproteinIIb/IIIa inhibitor abciximab than patients withhigh sCD40 ligand levels treated with placebo[30]. There was no difference in morbidity ormortality when abciximab was given to patientswith a low sCD40 ligand level. Although plateletactivation can occur in many noncardiac diseases,a biomarker of platelet activation will likely pro-vide independent information to a panel ofmarkers that address inflammation and plaqueinstability. This is particularly true for nSTEMI,as platelets play a bigger role in subocclusiveblood clots than occlusive clots.
Whole-blood & plasma-blood cholineCholine and phosphatidic acid are released afterthe simulation of phosphlipase D that is found onplatelets, leukocytes and smooth muscles. LikesCD40 ligand, the appearance of choline indi-cates a combination of plaque destabilization andcell activation. This marker is different from theother markers discussed in that it is not a protein,but a low-molecular-weight metabolite. It isunlikely that a traditional immunoassay can bedeveloped to measure whole-blood or serumcholine. The choline that is released fromphospholipase D activation appears in erythro-cytes, but not plasma. Increased whole-bloodcholine is associated with increased short-termcardiovascular death or cardiac arrest risk(OR: 6.0; 95% CI: 1.85–19.49) [31]. An interestingadditional benefit is the observation that choline isreleased into the blood and plasma of patients withsevere life-threatening arrhythmias, presumablydue to tissue ischemia [2]. The measurement ofboth red-cell and plasma choline enables adifferentiation between plaque destabilization(whole-blood choline only) from arrhythmias(both whole-blood and plasma choline).
Figure 3. Relationship between myocardial oxygen supply and demand.
Under normal conditions, the oxygen supply greatly exceeds the demand and there are no ischemic episodes. As an individual ages, the formation of plaques reduces the volume of blood that flows through the coronary artery and the supply of oxygen. Stable angina (also termed ‘demand ischemia’) occurs when the demand temporarily exceeds the supply, such as during exercise, and results in chest pain for the subject. Plaque rupture results in a more dramatic and prolonged drop in blood flow due to the blockage of a coronary artery. Unstable angina is characterized by chest pain at rest and results from oxygen supply deficit for an extended period of time. The injury is initially reversible, but if the artery is not opened within a few hours, the individual has irreversible heart damage and suffers a myocardial infarction. This is characterized by the release of cardiac troponin. MI: Myocardial infarction; SA: Stable angina; UA: Unstable angina.

REVIEW – Wu
7 Biomarkers Med. (2007) 1(1) future science groupfuture science group
Ischemia-modified albuminMyocardial ischemia preceeds irreversible celldeath in patients with AMI. Ischemia resultsfrom a deficit between oxygen delivery anddemand (Figure 3). A transient increase in thedemand for oxygen, such as in stress or exercise,leads to stable angina, and is characterized bychest pain. A prolonged drop in the delivery ofoxygen occurs when there is plaque rupture thatcauses unstable angina and AMI if the blockageis prolonged. Spontaneous reperfusion or thera-peutic revascularization of the coronary artery isnecessary to restore coronary artery oxygen deliv-ery. The presence of ischemia leads to the pro-duction of hydrogen peroxide and other freeradicals. An alteration in the N-terminus of
albumin caused by free radical damages causesthis protein to lose its ability to bind to freeheavy metals. The Ischemia Modified Albu-min™ (IMA) test measures the decrease incobalt binding in patients with ischemia.
There have been many studies that haveexamined the clinical utility of the IMA test forpatient presenting to the ED with chest pain. Ameta analysis showed that when the IMA test isused alone, it had a negative predictive value of91% for ruling out ACS [32]. When this test isused in a multivariate analysis in combinationwith troponin and ECG, the negative predictivevalue increased to 97%. The major disadvantageof the IMA test is the poor clinical specificity.Other etiologies can cause an increase in IMAsuch as stroke and gastrointestinal ischemia.Therefore, it is likely that this test will best beutilized as part of a multimarker panel.
Need for multimarker testing for risk stratification & myocardial ischemiaOf the dozens of biomarkers studied forischemia and risk stratification, only the mostpromising have been described in this review.As troponin is the gold standard for diagnosis ofAMI, most investigators have shown that thenew tests provide independent and supplemen-tal information. Nevertheless, each of thesetests suffers from poor specificity towardsCVD. Currently, there is no single test thatcomplements troponin. Therefore, mostresearchers in this area have advocated amultimarker approach.
At this time, the most logical approach is toselect markers that are linked to the various patho-physiologic events known to occur in the majorityof ACS cases [33]. For example, Figure 4 lists the dis-tinct events that occur and their candidate markersin patients who have STEMI and nSTEMI plaquerupture. Other etiologies for AMI, such as plaqueerosion or vasospasm, may require a different set ofmarkers. The timing of blood specimens is also acritical element to the testing sequence and inter-pretation of multimarker testing. Figure 5 showsthat it may be useful to use markers for inflamma-tion and plaque vulnerability before the onset ofchest pain. When positive, they would indicatehigh risk for future cardiovascular events. Markersof ischemia would have the most value shortly afterthe onset of chest pain, but before there is irrevers-ible damage. BNP could be included as part of abiomarker panel, as this hormone can be releasedduring myocardial ischemia [34]. NT-proBNP hasrecently been shown to be predictive of adverse
Figure 4. Multimarker approach for early diagnosis of acute coronary syndromes based on plaque rupture as the major etiological event.
The known pathophysiological events that occur following rupture are listed in each box. Biomarkers are listed under each event and can be used to note the presence of that event. Other disease processes can also cause the release of these biomarkers into blood. BNP: B-type natriuretic peptide; cTnI: Cardiac troponin I; cTnT: Cardiac troponin T; hsCRP: High-sensitivity C-reactive protein; IL: Interleukin; IMA: Ischemia-modified albumin; MMP: Matrix metalloproteinase; MCP: Monocyte chemotactic protein; MPO: Myeloperoxidase; Ox-LDL: Oxidized low-density lipoprotein; PlGF: Placental-like growth factor; PAI: Plasminogen activator inhibitor; PAPP: Pregnancy-associated plasma protein; sCD40: Soluble CD40 ; sICAM: Soluble intercellular adhesion molecule; uFFA: Unbound free fatty acids; vWF: von Willebrand’s factor; WB: Whole blood.

Acute coronary syndromes and risk stratification by multimarker analysis – REVIEW
8future science groupfuture science group www.futuremedicine.com
events in a group of patients with stable coronaryheart disease [35]. Both markers have been shownto be largely equivalent, and therefore it is unlikelythat both will be needed in a panel [36]. Troponin ismost likely positive 3–9 h after the onset of symp-toms. However, it is necessary to test troponinwith every sample, as the clinical presentation issubjective, inaccurate or unknown.
The increasing interest in multivariate analysishas prompted the US FDA to issue a draft guid-ance document to scientists within the in vitrodiagnostics industry and clinical laboratories,and the FDA staff [103]. This document defines amultivariate index as:
“test systems that employ data derived in part from one or more in vitro assays, and an algorithm that
usually, but not necessarily, runs on software to generate a result that diagnoses a disease or
condition, or is used in the cure, mitigation, treatment or prevention of disease.”
The elements of the algorithm may includeresults of in vitro diagnostic assays and otherlaboratory data and demographic information.The algorithm assigns weights or coefficientsused in calculating an index, score or classifica-tion. It is supposed that the final calculationcannot be independently derived and
Figure 5. Relationship between onset of disease and relative biomarker release.
Coronary artery plaques that are vulnerable to rupture are caused by infiltration of inflammatory elements that release cytokines and enzymes that result in the degradation of the fibrous cap. PAPP-A is a metalloproteinase that is released into blood during the stage of plaque vulnerability. Once a plaque has ruptured, myocardial ischemia is the initial consequence. This can be detected by the IMA test. Prolonged ischemia leads to myocardial necrosis and release of cTn. After 12–24 h, there can be left ventricular dysfunction andmyocardial muscle overloading causing the release of B-type natriuretic peptide. There may also be release of BNP due to myocardial ischemia (dotted line). BNP: B-type natriuretic protein; cTn: Cardiac troponin; IMA: Ischemia-modified albumin; LV: Left ventricular; PAPP: Pregnancy-associated plasma protein.

REVIEW – Wu
9 Biomarkers Med. (2007) 1(1) future science groupfuture science group
confirmed by another laboratory withoutaccess to the proprietary information used inthe algorithm.
Optimization of multimarker analysisClinical laboratory tests have traditionally reliedon a cutoff concentration to differentiatebetween a normal and an abnormal finding.While a stepwise multivariate regression analysiscan determine if the results of one marker areindependent to the results of other markers for aparticular disease indication, the cutoff concen-trations for each test are independentlyoptimized. A higher level of biomedical compu-tations is necessary to determine cutoff concen-trations that are optimized in the context of theother tests in the panel.
Biosite Multimarker Index™Biosite Incorporated (CA, USA) developed anovel approach toward multimarker analysis.Figure 6 plots the concentration of a biomarkeragainst the distribution of nondiseased and dis-eased populations. The marker is negative inmany patients who do not have the disease andpositive for those who do. As with most bio-markers, there is significant overlap of biomarkerconcentration and the two populations. In theMultimarker Index (MMX) model, a transferfunction is created for each marker. The value iseither zero or one below and above the overlapregion, respectively, and will be between these
limits in a linear response for the overlap region(Figure 6). The individual markers are thencombined to form a multimarker index:
Where Ca is the concentration for analyte a, Ia isthe transfer function for analyte a, and has twoparameters, the midpoint and range (or a lowand high threshold), and Wa is the analyteweighting. Each biomarker has a maximum andminimum amount they can contribute with nosingle marker saturating the index. A searchengine is used to select the parameters (mid-point, range and weighting) in the MMX. TheMMX cutoff value is not used during parameteroptimization. The cutoff for the diagnosis of thedisease in question is optimized at each facilityafter the parameters have been established usingstandard receiver operating characteristic curveanalysis. The Multimarker Index has beenapplied to a panel of traditional markers includ-ing myoglobin, CK–MB, troponin and BNP on2172 ED patients who presented for AMI rule-out [37]. These investigators showed that theMMX was superior to the performance of theindividual tests (area under the receiver operat-ing characteristic curve: 0.98 for the MMD vs0.78–0.94 for each marker). Work on an indexfor risk stratification and myocardial ischemia isin progress.
Classification & regression trees analysisAnother approach towards multimarker analysisis the use of classification and regression trees(CART), an empirical statistical algorithm. Inthis model, laboratory data are organized into aflow chart where laboratory test results usingoptimized cut-off concentrations are progres-sively subdivided for groups of patients. The treebegins with one test and branches into two direc-tions based on whether the test is positive or neg-ative. Each daughter branch can be furthersubdivided based on the result of additional test-ing. Different tests can be applied to each daugh-ter branch. This process continues until theregression analysis concludes that no furtherbranching is necessary (or the number of tests inthe panel has been exhausted). Regression analy-sis determines the optimum order of testing inthe classification tree. The tree is plotted againstthe incidence of the particular outcome measuretested. The data do not need to be present para-metrically. As an example, NT-proBNP, cTnT,serum creatinine, estimated glomerular filtrationrate, CRP, hemoglobin, age and gender were
Figure 6. The Biosite MultiMarker™ Index concept.
The distribution of biomarker values between patients without (left curve) and with (right curve) the disease in question, are shown. An indicator function is created in the region where there is overlap between these distributions (middle curve). The indicator function is at a minimum at the biomarker concentration at the beginning of the distribution of patients with the disease. For patients without the disease, the indicator function is a maximum at the biomarker concentration at the end of the distribution of patients without the disease. A linear function is created between these two limits. A transfer function profile is created for each biomarker in the panel.
MMX a(Ia[Ca] Wa)×=

Acute coronary syndromes and risk stratification by multimarker analysis – REVIEW
10future science groupfuture science group www.futuremedicine.com
tested on ED patients for risk stratification usingdeath and intensive care treatment as outcomemeasures [38]. As shown in Figure 7, the treebegins with NT-proBNP testing. A normalNT-proBNP, CRP and age under 80 years wasassociated with the lowest incidence of complica-tions. The highest incidence was associated withhigh NT-proBNP and CRP. Age was importantin subdividing patients with a low NT-proBNP.The other indicators were left out of the CARTmodel. CART analysis is ideally suited for rulesfor clinical decisions and can be applied for bed-side analysis. Specific algorithms can be encodedinto laboratory or hospital information systemsthat routinely report laboratory test results.
Neural networksA neural network is a form of nonlinear artificialintelligence. The network combines data to formpatterns of test results much in the same manneras the human brain is able to process light anddark shapes and colors into visual images. Thenetwork must be trained by correlating
laboratory data with clinical outcomes. Once apattern has emerged, the network is tested byproviding data and determining the accuracy ofits outcome predictions. Unlike the MMX orCART, where a dichotomous cutoff concentra-tion is established for each test independent ofeach other, a neural network does not use indi-vidual cut-offs. An individual test of a panel willlikely have different degrees (or multiples of nor-mal) depending on the disease entity present.The establishment of a neural network for riskstratification is shown in Figure 8. The differentinputs include markers that characterize a differ-ent phase of the pathophysiology of ACS. Theoutcome is risk or no risk, rather than the resultsof individual tests.
The concept of neural networks has beenpreviously tested for biomarkers of ACS. Baxtcombined 40 variables, including three labora-tory tests as a network for the diagnosis of myo-cardial infarction [39]. The clinical sensitivityand specificity was 94.5 and 95.9%, respec-tively. While these results are good, they are not
Figure 7. Classification and regression tree analysis for risk stratification of emergency department patients.
Measurement of NT-proBNP using a cutoff of 3.806 ng/ml separates subjects that have a low incidence of death and cardiac complications (6.4%) from those with a high incidence (35.7%). For patients with a high NT-proBNP, C-reactive protein at a cutoff of 23.6 mg/l further separates this group into a lower (20.8%) and higher (55.6%) incidence of adverse events. For patients with a low NT-proBNP, an age cutoff of 80 years separates low (5.2%) versus intermediate (21.4%) incidence of adverse events. For patients who are both below the NT-proBNP cutoff and ≤80 years, C-reactive protein separates this group into very low (3.8%) and intermediate (12.1%) rates of adverse events. Classification and regression tree analysis defines the order by which tests are used and their specific cutoff concentrations. CRP: C-reactive protein; NT-proBNP: N-terminal prohormone brain natriuretic peptide. Reproduced with permission from [38].

REVIEW – Wu
11 Biomarkers Med. (2007) 1(1) future science groupfuture science group
substantially better than troponin alone andmay not have been the best platform to test thecapabilities of the network. Detection of myo-cardial ischemia and risk stratification willlikely be a better model to demonstrate theenhanced ability of a neural network, as there isno single test that performs as well as troponindoes for AMI diagnosis.
Future perspectiveThe need for novel cardiac markers will con-tinue as the population in the USA and theWestern world increases. Other regions of theworld, such as Asia, have also experienced anincreased incidence of CVD, as their affluence
has improved and their diets and exercise habitshave changed such that they are at higher riskfor CVDs than ever before. A multimarkerapproach may be needed to solve the more diffi-cult problems such as the diagnosis of myo-cardial ischemia and early risk stratification forfuture near-term cardiovascular events. Manu-facturers of in vitro diagnostics equipment andexperts in bioinformatics have begun developinganalytical systems and interpretative algorithmsthat will enable routine multimarker testing.Acceptance by clinicians, regulatory andreimbursement agencies will be decidedly sloweras it represents a shift from the traditionalapproach towards biomarker testing.
Figure 8. Multimarker analysis of cardiac biomarkers using a neural network.
The results of different biomarkers on blood collected at one or two early time points after a patient with chest pain presents to the emergency department are inputted into the neural network program. Based on the inter-relationships between these markers and time points, the network produces a value that can be interpreted as either ischemia or early AMI, thereby putting the patient at high risk for death or MI in the short term, or the presenting symptoms are found to be consistent with noncardiac chest pain and the individual is at low-risk for adverse events. Neural networks do not separate biomarker testing results into negative and positive groups. AMI: Acute myocardial infarction; LV: Left ventricle.

Acute coronary syndromes and risk stratification by multimarker analysis – REVIEW
12future science groupfuture science group www.futuremedicine.com
Bibliography1. Libby P, Theroux P: Pathophysiology of
coronary artery disease. Circulation 111, 3481–3488 (2005).
2. Apple FS, Wu AHB, Mair J et al.: Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin. Chem. 51, 810–824, (2005).
3. Panteghini M, Gerhardt W, Apple FS, Dati F, Ravkilde J, Wu AHB: Quality specifications for cardiac troponin assays. Clin. Chem. Lab. Med. 39, 174–178 (2001).
4. Nomenclature and criteria for diagnosis of ischemic heart disease: report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 59, 607–609 (1979).
5. Joint European Society of Cardiology/American College of Cardiology Committee: Myocardial infarction redefined – a consensus document of the joint European Society of Cardiology/American
College of Cardiology Committee for the redefinition of myocardial infarction. J. Am. Coll. Cardiol. 36, 959–969 (2000).
6. Storrow AB, Gibler WB: Chest pain centers: diagnosis of acute coronary syndromes. Ann. Emerg. Med. 35, 449–461 (2000).
7. Lindahl B, Andren B, Ohlsson J, Venge P, Wallentin L. Risk stratification in unstable coronary artery disease. Additive value of troponin T determinations and pre-discharge exercise tests, FRISK Study Group. Eur. Heart J. 18, 762–770 (1997).
8. Vasan RS: Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 113, 2335–2362 (2006).
9. Arab S, Gramolini AO, Ping P et al.:Cardiovascular proteomics. Tools to develop novel biomarkers and potential applications. J. Am. Coll. Cardiol. 48, 1733–1741 (2006).
10. Vivanco F, Martin-Ventura JL, Duran MC et al.: Quest for novel cardiovascuar biomarkers by proteomic analysis. J. Protome Res. 4, 1181–1191 (2005).
11. Granger CB, Van Eyk JE, Mockrin SC, Anderson NL: National Heart, Lung, and Blood Institute Clinical Proteomics Working Group report. Circulation 109, 1697–1703 (2004).
12. Martin-Ventura JL, Duran MC, Blanco-Colio LM et al.: Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation 100, 2216–2219 (2004).
13. Marshall J, Kupchak P, Zhu W et al.:Processing of serum proteins underlies the mass spectral fingerprinting of myocardial infarction. J. Protome Res. 2, 361–372 (2003).
14. Lindsey ML, Goshorn DK, Comte-Walters S et al.: A multidimensional proteomic approach to identify hypertrophy-associated proteins. Proteomics6, 2225–2235 (2006).
15. Brindle JT, Antti H, Holmes E et al.: Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabolonomics. Nature Med. 8, 1439–1444 (2002).
Executive summary
Pathophysiology of acute coronary syndromes (ACS)
• Rupture of a coronary artery is the most frequent etiology of ACS.• Inflammation, plaque instability and myocardial ischemia are steps that preceed necrosis.
Existing biomarkers for acute myocardial infarction (AMI)
• Creatine kinase and its MB isoenzyme and myoglobin have been replaced with troponin.• B-type natriuretic peptide and N-terminal prohormone brain natriuretic peptide are strong predictors of heart failure and
cardiovascular death.
European Society of Cardiology/American College of Cardiology redefinition of AMI
• AMI is predicated on an increase in the concentration of cardiac troponin in blood.
Clinical need for early biomarkers of ACS
• Troponin is not reliably increased in the first few hours after AMI.• The unmet needs include a marker for early diagnosis and rule out of ACS and risk stratification for short-term morbidity
and mortality.
Novel biomarkers of coronary vascular disease using proteomics and metabolomics
• Proteomics and metabolomics promises to provide new cardiac markers.• There are limitations to the clinical implementation of new tests.
Novel biomarkers based on the pathophysiology of ACS
• Markers of inflammation and plaque instability include C-reactive protein, placental-like growth factor, myeloperoxidase and pregnancy-associated plasma protein A.
• CD40 ligand, whole-blood choline and ischemia-modified albumin indicate plaque rupture, thrombosis and myocardial ischemia.
Need for multimarker testing for risk stratification and myocardial ischemia
• A multimarker approach is needed as individual markers lack clinical specificity.• Algorithms are necessary to interpret multimarker data.
Optimization of multimarker analysis
• The Multimarker Index™ produces a number that acts like a cutoff for a single test.• Classification and regression tree analysis is an algorithm that has different branch and decision limits.• A neural network is free from the assignment of cut-off concentrations and detects patterns of laboratory test results.

REVIEW – Wu
13 Biomarkers Med. (2007) 1(1) future science groupfuture science group
16. Sabatine MS, Liu E, Morrow DA et al.:Metabolomic identification of novel biomakers of myocardial ischemia. Circulation 112, 3868–3875 (2005).
17. Kiernan UA, Nedelkov D, Nelson RW: Multiplexed mass spectrometric immunoassay in biomarker research: a novel approach to the determination of a myocardial infarct. J. Proteome Res. 5, 2928–2934 (2007).
18. Wu AHB: A selected history and future of immunoassay development and applications in clinical chemistry. Clin. Chim. Acta 369, 119–124 (2006).
19. Parikh SV, de Lemos JA: Biomarkers in cardiovascular disease: integrating pathophysiology into clinical practice. Am. J. Med. Sci. 332, 186–197 (2006).
20. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR: Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 247, 1557–1565 (2002).
21. Sabatine MS, Morrow DA, de Lemos JA et al.: Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation105, 1760–1763 (2002).
22. Heeschen C, Dimmeler S, Fichtlscherer S et al.: Prognostic value of placental growth factor in patients with acute chest pain. JAMA 291, 435–441 (2004).
23. Zhang R, Brennan ML, Fu X et al.:Association between myeloperoxidase levels and risk of coronary artery disease. JAMA296, 2136–2142 (2001).
24. Baldus S, Heeschen C, Meinertz T et al.:Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 108, 1440–1448 (2003).
25. Wittfooth S, Qin QP, Lund J et al.:Immunofluorometric point-of-care assays
for the detection of acute coronary syndrome-related noncomplexed pregnancy-associated plasma protein A. Clin. Chem. 52, 1794–1801 (2006).
26. Qin QP, Kokkala S, Lund J et al.:Immunoassays developed for pregnancy-associated plasma protein-A (PAPP-A) in pregnancy may not recognize PAPP-A in acute coronary syndromes. Clin. Chem. 52, 398–404 (2006).
27. Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Simoons ML, Zeiher AM. Pregnancy-associated plasma protein-A levels in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 45, 229–237 (2005).
28. Schonbeck U, Lippy P: CD40 signaling and plaque instability. Circ. Res. 89, 1092–1103 (2001).
29. Varo N, de Lemos JA, Libby P et al.: Soluble CD40L. Risk prediction after acute coronary syndromes. Circulation 108, 1049–1052 (2003).
30. Heeschen C, Dimmeler S, Hamm CW et al.: Soluble CD40 ligand in acute coronary syndromes. N. Engl. J. Med. 248, 1104–1111 (2003).
31. Danne O, Mockel M, Leuders C et al.:Prognostic implications of elevated whole blood choline levels in acute coronary syndromes. Am. J. Cardiol. 91, 1060–1070 (2003).
32. Peacock F, Morris DL, Anwaruddin S et al.:Meta-analysis of ischemia-modified albumin to rule out acute coronary syndromes in the emergency department. Am. Heart J. 152, 253–262 (2006).
33. Morrow DA, Braunwald E: Future of biomakers in acute coronary syndromes. Moving toward a multimarker strategy. Circulation 108, 250–252 (2003).
34. Sabatine MS, Morrow DA, de Lemos JA et al.: Acute changes in circulating natriuretic peptide levels in relation to
myocardial ischemia. J. Am. Coll. Cardiol.44, 1988–1995 (2004).
35. Bibbins-Domingo K, Gupta R, Na B, Wu AHB, Schiller NB, Whooley MA: N-terminal pro-B-type natriuretic peptide and incident heart failure in ambulatory patients with coronary disease: data from the Heart and Soul Study. JAMA 297, 169–176 (2007).
36. Cameron SJ, Green GB, White CN et al.:Assessment of BNP and NT-proBNP in emergency department patients presenting with suspected acute coronary syndromes. Clin. Biochem. 39, 11–18 (2006).
37. Hollander JE, Peacock WF, Shofer FS et al.:A cardiac multimarker index performs better than standard markers to diagnose acute myocardial infarction. Acad. Emerg. Med.12(5 Suppl.1), 33 (2005).
38. Mockel M, Muller R, Vollert JO et al.: Role of N-terminal pro-B-type natriuretic peptide in risk stratification in patients presenting in the emergency room. Clin.Chem. 51, 1624–1631 (2005).
39. Baxt WG, Shofer FS, Sites FD, Hollander JE: A neural computational aid to the diagnosis of acute myocardial infarction. Ann. Emerg. Med. 39, 366–373 (2002).
Websites101. Heart disease and stroke. Statistics – 2004
update. American Heart Association www.americanheart.org/downloadable/heart/1079736729696HDSStats2004UpdateREV3–19–04.pdf
102. Institutional Clinical and Translational Science Award http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-06–002.html
103. Draft guidance for industry, clinical laboratories, and FDA staff on in vitrodiagnostic multivariate index assays www.fda.gov/cdrh/oivd/guidance/1610.pdf

Tomorrow’s Medicine Today...
Future Science Group
The Future Science Group publishes award-winning commentary and analysis frominternational opinion leaders on topical issues, cutting-edge research and emergingdevelopments in healthcare. Publications include the Expert Review series of journalsas well as the insightful and respected Future Medicine titles.
� Dynamic and exciting publications program spanning all major therapeutic areas� Expert authorship providing commentary and forward-looking perspectives� Independent, peer-review by key international opinion leaders � Indexing in all major bibliographic databases and reference sources� Extensive use of high-quality graphic imagery and tabular data� Rapid publication – 8-10 weeks publication from first draft
For a full list of publications please visit our websites or [email protected] for further information.
www.future-science-group.com >> www.future-drugs.com www.futuremedicine.com
� Aging Health � Cardiology � Dermatology� Endocrinology & Metabolism � Gastroenterology & Hepatology� Immunology � Infectious Disease � Lipidology� Microbiology � Medical Devices � Molecular Diagnostics� Nanomedicine � Neurology � Oncology � Obstetrics & Gynecology� Ophthalmology � Pediatrics � Personalized Medicine � Pharmacogenomics� Pharmacoeconomics � Proteomics � Regenerative Medicine � Respiratory Medicine � Rheumatology � Vaccines � Virology � WomenÕ s Health

Contact Us
Future Science Group, Unitec House, 2 Albert Place, London, N3 1QB, UK T: +44 (0)20 8349 2033 F: +44 (0)20 8343 2313 E: [email protected]
www.futuremedicine.com www.future-drugs.com
Personal details Professor Dr Mr Miss Mrs Ms
First name:
Family name:
Job title:
Institution:
Address:
City: Post code: Country:
Telephone: Fax: E-mail:
YES! Please send me a print sample copyof Biomarkers in Medicine
YES! Please send me more details about allthe journals in your:
Oncology Collection
Pharmacogenomics and Personalized Medicine Collection
YES! Please sign me up for a free 30-day online trial to Biomarkers in Medicine
To register for your FREE 30-day onlinetrial to Biomarkers in Medicine simply contact us at [email protected],quoting customer reference BMM07 andwe will email you with username andpassword access to our content free for30 days.
Alternatively to register for a print sample copy or extended institutional trial please complete and return the form below.
+44 (0)20 8349 2033
+44 (0)20 8343 2313
Future Science Group Unitec House 2 Albert Place London, N3 1QB, UK
Free trial access or sample copy