REVIEWS IN BASIC AND CLINICAL REVIEWS AND PERSPECTIVES ... · cholelithiasis, and...
Transcript of REVIEWS IN BASIC AND CLINICAL REVIEWS AND PERSPECTIVES ... · cholelithiasis, and...
-
GASTROENTEROLOGY 2013;145:1215–1229
REV
IEW
SAND
PER
SPEC
TIVES
REVIEWS IN BASIC AND CLINICALGASTROENTEROLOGY AND HEPATOLOGYRobert F. Schwabe and John W. Wiley, Section Editors
Pathogenesis, Diagnosis, and Management of CholangiocarcinomaSUMERA RIZVI and GREGORY J. GORES
Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Abbreviations used in this paper: a-SMA, a-smooth muscle actin;CA19-9, carbohydrate antigen 19-9; CAF, cancer-associated fibroblast;CCA, cholangiocarcinoma; CT, computed tomography; CXCR4, chemo-kine (C-X-C motif) receptor 4; dCCA, distal cholangiocarcinoma; ECM,extracellular matrix; EGFP, enhanced green fluorescent protein; EGFR,epidermal growth factor–receptor; EMT, epithelial–mesenchymal tran-sition; ERBB2, v-erb-b2 avian erythroblastic leukemia viral oncogenehomolog 2; ERC, endoscopic retrograde cholangiography; ERK, extra-cellular signal regulated kinase; FGFR, fibroblast growth factor receptor;
Cholangiocarcinomas (CCAs) are hepatobiliary can-cers with features of cholangiocyte differentiation;they can be classified anatomically as intrahepaticCCA (iCCA), perihilar CCA (pCCA), or distal CCA.These subtypes differ not only in their anatomiclocation, but in epidemiology, origin, etiology, path-ogenesis, and treatment. The incidence and mortalityof iCCA has been increasing over the past 3 decades,and only a low percentage of patients survive until 5years after diagnosis. Geographic variations in the inci-dence of CCA are related to variations in risk factors.Changes in oncogene and inflammatory signaling path-ways, as well as genetic and epigenetic alterations andchromosomeaberrations, havebeen shown to contributeto the development of CCA. Furthermore, CCAs aresurrounded by a dense stroma that contains manycancer-associated fibroblasts, which promotes theirprogression. We have gained a better understanding ofthe imaging characteristics of iCCAs and have developedadvanced cytologic techniques to detect pCCAs. Patientswith iCCAs usually are treated surgically, whereas livertransplantation after neoadjuvant chemoradiation is anoption for a subset of patients with pCCAs. We reviewrecent developments in our understanding of the epide-miology and pathogenesis of CCA, along with advancesin classification, diagnosis, and treatment.
Keywords: Cancer-Associated Fibroblasts; Distal Chol-angiocarcinoma; Intrahepatic Cholangiocarcinoma;Molecular Pathogenesis.
holangiocarcinoma (CCA) is the most common
FISH, fluorescence in situ hybridization; HGF, hepatocyte growth factor;HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis Cvirus; iCCA, intrahepatic cholangiocarcinoma; IDH, isocitrate dehydro-genase; IL 6, interleukin 6; KRAS, Kirsten rat sarcoma viral oncogenehomolog; MAPK, mitogen-activated protein kinase; miR, microRNA;MCL1, myeloid cell leukemia sequence 1; MET, met proto-oncogene;MMP, matrix metalloproteinase; MRI, magnetic resonance imaging; OR,odds ratio; pCCA, perihilar cholangiocarcinoma; PDGF, platelet-derivedgrowth factor; PI, phosphatidyl inositol; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; PSC, primary sclerosing cholangitis; STAT, signaltransducer and activator of transcription; TP53, tumor protein 53.
© 2013 by the AGA Institute0016-5085/$36.00
http://dx.doi.org/10.1053/j.gastro.2013.10.013
Cbiliary malignancy and the second most commonhepatic malignancy after hepatocellular carcinoma (HCC).1
CCAs are epithelial tumors with features of cholangiocytedifferentiation. Intrahepatic cholangiocarcinomas (iCCAs)are located within the hepatic parenchyma. The second-order bile ducts serve as the point of separation betweeniCCAs and perihilar CCAs (pCCAs) or distal CCAs(dCCAs)—the cystic duct is the anatomic boundary betweenthese latter 2 subtypes (Figure 1A).2 The Bismuth–Corletteclassification stratifies perihilar tumors on the basis ofbiliary involvement. This classification recently wasextended to also take into account arterial and venous
encasement.3 pCCA is the most common type of CCA. In alarge series of patients with bile duct cancer, 8% had iCCA,50% had pCCA, and 42% had distal CCA.4 CCA has a poorprognosis; patients have a median survival of 24 monthsafter diagnosis. The only curative treatment option is sur-gery, for early stage disease.5
Epidemiology
Cholangiocarcinoma accounts for 3% of all gastro-
intestinal tumors. Over the past 3 decades, the overallincidence of CCA appears to have increased.6 The percent-age of patients who survive 5 years after diagnosis has notincreased during this time period, remaining at 10%.7,8
In the United States, Hispanics and Asians have thehighest incidence of CCA (2.8 per 100,000 and 3.3 per100,000, respectively), whereas African Americans have thelowest incidence of CCA (2.1 per 100,000). African Amer-icans also have lower age-adjusted mortality ratescompared with whites (1.4 per 100,000 vs 1.7 per 100,000).Men have a slightly higher incidence of CCA and mortalityfrom cancer than women.7 With the exception of patientswith primary sclerosing cholangitis (PSC), a diagnosis ofCCA is uncommon before age 40 years.
http://dx.doi.org/10.1053/j.gastro.2013.10.013
-
1216 RIZVI AND GORES GASTROENTEROLOGY Vol. 145, No. 6
REV
IEWS
AND
PER
SPEC
TIVES
Globally, hepatobiliary malignancies account for 13% ofcancer-related deaths; 10%–20% of these are attributable toCCA. The mean age at diagnosis of CCA is 50 years. Theglobal incidence of iCCA varies widely, from rates of 113per 100,000 in Thailand to 0.1 per 100,000 in Australia.9,10
Differences in the prevalence of genetic and other riskfactors presumably account for this extensive variation.
Epidemiologic studieshave indicated that the age-adjustedmortality rate for iCCA is increasing, whereas the mortalityrate from pCCA and dCCA could be decreasing.9–14 A studyof a World Health Organization database reported a sub-stantial global increase in iCCA mortality, with a decreasingtrend in mortality from pCCA plus dCCA.15 Although thisobserved increase in the incidence of CCA over the past 30years has been recorded as an increase in iCCA, it could resultfrom misclassification of perihilar tumors as iCCAs.16 Ac-cording to the US Surveillance, Epidemiology, and End Re-sults database, the age-adjusted incidence rate for iCCAincreased from 0.59 per 100,000 in 1990 to 0.91 per 100,000in 2001. It subsequently decreased to 0.6 per 100,000by 2007.Conversely, the incidence rate for pCCAplus dCCA remainedaround 0.8 per 100,000 until 2001, and then graduallyincreased to 0.97 per 100,000 by 2007. Perihilar tumors werecoded as iCCAs before 2001 and subsequently were coded aspCCAs after implementation of the third edition of the In-ternational Classification of Disease for Oncology. This up-date likely influenced the aforementioned changes inincidence rates of both CCA subtypes. Similar trends in theincidence of CCA subtypes were noted in the UnitedKingdom after the change to the third edition of the Inter-national Classification of Disease for Oncology in 2008.6,16
Risk Factors
There are several established risk factors for CCA,
and most cases are sporadic.6,8,17 Geographic variations inincidence rates of CCA are related in part to variations inrisk factors. For example, in Southeast Asia, which has oneof the highest incidence rates of CCA, infection with thehepatobiliary flukes Opisthorchis viverrini and Clonorchissinensis has been associated with the development of CCA.Both parasites cause chronic inflammation and areconsidered carcinogens.8,18 Hepatolithiasis is another riskfactor for CCA (mainly iCCA) in Asian countries.8 Chronicbiliary inflammation secondary to calculi has been pro-posed to increase the risk of malignancy. Moreover,infestation with hepatobiliary flukes has been shown to bemore common in patients with hepatolithiasis.8,19 Theincidence and prevalence of CCA in patients with bile duct(choledochal) cysts are also higher in Asian than inWestern countries.20,21 Choledochal cystic diseases,including Caroli’s disease, are rare congenital abnormal-ities of the pancreatic and biliary ducts. Choledochal cystscan be intrahepatic or extrahepatic, and are diagnosed inpatients at an average age of 32 years old.8,17 Thorotrast, apreviously used contrast agent that is now banned, wasfound to increase risk for CCA by 300-fold in a Japanesestudy.22
In the West, PSC is the most common predisposingcondition for CCA. Among patients with PSC, the annualrisk of development of CCA is 0.5%–1.5%, with a lifetimeprevalence of 5%–10%17; CCA is diagnosed within 2 yearsof PSC in most of these patients. A number of potentialrisk factors for CCA in patients with PSC have beenstudied, including smoking and alcohol, although defin-itive data are lacking.8
Hepatitis B virus (HBV) or hepatitis C virus (HCV) infec-tion and cirrhosis have been proposed as potential etiologiesof iCCA.23–25 A recentmeta-analysis of 11 studies found thatcirrhosis, HBV, and HCV were major risk factors for iCCA,with odds ratios (ORs) of 22.92, 5.1, and 4.8, respectively.26 Acase-control study from Korea found a significant associa-tion between HBV (OR, 2.3) and CCA, but not HCV andCCA. Cirrhosis also was found to be a significant risk factorfor CCA, with an OR of 13.6. HCV and cirrhosis were asso-ciated with iCCA in aUS case-control study. Comparedwithcontrols, patients with iCCA had a higher prevalence ofanti-HCV antibodies, with an OR of 7.9.24
CCA development has been associated with other riskfactors, including inflammatory bowel disease indepen-dent of PSC, alcohol, smoking, fatty liver disease, diabetes,cholelithiasis, and choledocholithiasis.8,27–29 Additionalstudies have associated variants of genes that regulateDNA repair, inflammation, and carcinogen metabolismwith CCA development.8 Further studies are necessary toverify these potential associations.
Cells of Origin
iCCA is a histologically diverse hepatobiliary ma-
lignancy considered to develop from biliary epithelial cellsor hepatic progenitor cells (Figure 1B). A recently pro-posed classification of iCCAs subdivided these tumorsinto the conventional, bile ductular, or intraductalneoplasm type, or rare variants (combined hepatocellularCCA, undifferentiated type, squamous/adenosquamoustype). The conventional type includes small-duct or pe-ripheral type and large-duct or perihilar type.30 Neural celladhesion molecule, a marker of hepatic progenitor cells,has been detected in the bile ductular and combined he-patocellular CCA types, so these might have originatedfrom hepatic progenitor cells.30–32
Distal and pCCA have been proposed to arise from thebiliary epithelium and peribiliary glands.33 Extrahepaticbile ducts and large intrahepatic bile ducts are lined bymucin-producing cuboidal cholangiocytes. A recent studyshowed that mucin-producing iCCAs and hilar CCAs hadgene expression and immunohistochemical profiles similarto those of the cylindric, mucin-producing cholangiocytesthat line hilar and intrahepatic large bile ducts.34
A model in which iCCAs arise from transdifferentiationand subsequent neoplastic conversion of normal hepato-cytes into malignant cholangiocytes has been proposed.Fan et al35 showed in mice that overexpression of Notch1and AKT resulted in the development of invasive cys-tadenocarcinomas via conversion of hepatocytes into
-
Figure 1. Anatomic localization of CCA and cells of origin in CCA. (A)Anatomic localization of CCA. CCA is divided into 3 subtypes, basedon anatomic location. Modified with permission from Elsevier andRazumilava et al.17 (B) Cells of origin in CCA.
December 2013 CHOLANGIOCARCINOMA 1217
REV
IEW
SAND
PER
SPEC
TIVES
cholangiocyte precursors of iCCA.35 Sekiya and Suzuki36
also showed that in mice, Notch-mediated conversion ofhepatocytes into biliary cells leads to macronodularcirrhosis and iCCAs. Therefore, iCCAs may not have asingle lineage, but instead derive from different cells oforigin. In support of this theory, a recent study showedthat transformed hepatocytes, hepatoblasts, and hepaticprogenitor cells can give rise to a broad spectrum of livertumors, ranging from CCA to HCC.37 These studiesindicate that multiple cell types, rather than only chol-angiocytes, transform and develop into CCAs. Additionalanimal models of CCA and lineage tracing studies arenecessary to help identify the cells of origin for CCA.
Inflammation
CCAs frequently arises under conditions of
inflammation, which is believed to contribute to patho-genesis. A variety of cytokines, growth factors, tyrosinekinases, and bile acids can contribute to alterations inproliferation, apoptosis, senescence, and cell-cycle regula-tion required for cholangiocarcinogenesis.5 Inflammatory
cytokines activate inducible nitric oxide synthase, leadingto excess nitric oxide with resultant single-stranded, dou-ble-stranded, and oxidative DNA lesions, as well as inhi-bition of DNA repair enzymes.38 Interleukin (IL)-6, aninflammatory mediator secreted by CCA and stromal in-flammatory cells, can function in an autocrine or para-crine manner to promote cell survival and providemitogenic signals.39,40 Myeloid cell leukemia sequence 1(MCL1) is an anti-apoptotic BCL2 family member thatmediates tumor necrosis factor–related resistance toapoptosis-inducing ligands in CCAs.41 IL-6 increases theexpression of MCL1 via constitutive activation of signaltransducer and activator of transcription (STAT) signalingand protein kinase B (Akt).40,42 MCL1 transcription isactivated by IL-6 via a p38 mitogen-activated protein ki-nase (MAPK)-dependent pathway.43 IL-6 binds to thegp130 receptor, leading to its subsequent dimerizationand activation of the gp130-associated janus kinases,including janus kinase 1 and janus kinase 2, which leadsto STAT3 activation.44,45 Epigenetic silencing of suppressorof cytokine signaling 3 results in sustained IL-6 signaling viaSTAT3.46 Inflammatory signaling pathways thereforeappear to promote the development of CCA by causingDNA damage and blocking the apoptosis normallyinduced by the DNA damage response. These cytokinesalso promote cell proliferation. The combination of DNAdamage, evasion of apoptosis, and cell proliferation are allcomponents of cell transformation.
Epidermal growth factor–receptor (EGFR) signalingalso contributes to cholangiocarcinogenesis and CCAprogression. Activation of EGFR leads to activation ofextracellular-signal regulated kinases (ERKs) 1 and 2 (alsoknown as p44/42 MAPK). EGFR inhibitors decreaseexpression of cyclooxygenase-2 by CCA cells.47 V-erb-b2avian erythroblastic leukemia viral oncogene homolog 2(ERBB2) is another member of the EGFR family thatcontributes to CCA development. In mice, overexpressionof ERBB2 led to formation of tumors along the biliaryepithelium.48 Hepatocyte growth factor (hepapoietin A;scatter factor) (HGF) is a stromal paracrine mediator thatregulates tumor invasiveness and metastasis.49–51 Activa-tion of MET, the receptor for HGF, up-regulates severalsignaling pathways, including those involving phosphati-dylinositol-4,5-bisphosphate 3-kinase (PI3K)–AKT,STAT3, and MAPK.52 CCAs express higher levels of METand HGF than nontumor tissues.53,54 MET overexpressionwas associated with activation of members of the EGFRfamily, particularly of ERBB2.54,55
Cholestasis also contributes to the development ofCCA, and bile acids have important roles in this process,activating growth factors that mediate proliferation. Bileacids activate EGFR and increase expression ofcyclooxygenase-2 via a MAPK cascade.56 In addition to bileacids, cyclooxygenase-2 overexpression is induced by oxy-sterols and inducible nitric oxide synthase.57 Oxysterolsare overlooked in the pathogenesis of CCA.58 Theseoxidative degradation products of cholesterol are abun-dant in bile. They are endogenous ligands for the
-
1218 RIZVI AND GORES GASTROENTEROLOGY Vol. 145, No. 6
REV
IEWS
AND
PER
SPEC
TIVES
hedgehog signaling pathway59—a developmental pathwayimplicated in CCA progression.60
Genetics
A few studies have assessed the roles of genetic factors,
such as chromosome aberrations or genetic and epigeneticalterations in tumor suppressor genes and oncogenes, in thepathogenesis of human CCA. However, these studies haveproducednodefinitive results because they analyzed a limitednumber of genes in combined CCA specimens, withoutseparate analyses of different subtypes.61 A comparative ge-nomics hybridization analysis of 32 CCA samples from pa-tients (7 iCCA, 13 pCCA, and 12 dCCA) showed that they allcontained gains at 16q, 17p, 17q, 19p, and 19q, whichincluded regions encoding ERBB2, mitogen-activated pro-tein kinase kinase 2 (MEK2), and platelet-derived growthfactor- beta (PDGFB).62 Ameta-analysis of 5 studies that usedcomparative genomics hybridization to analyze 98 iCCAsfound copynumber losses at 1p, 4q, 8p, 9p, 17p, and18q, andgains at 1q, 5p, 7p, 8q, 17q, and 20q.61 In this meta-analysis,there was considerable variation among the 4 studies thatwere performed in Asia63–66 and the 1 study from Europe.67
This variation could have resulted from differences inethnicity and etiologic associations among the studies.
Whole-exome sequencing analyses of 8 liver fluke-relatedCCAs identified 206 somatic mutations in 187 genes.68 Thefrequency of these mutations was validated in an addi-tional 46 liver fluke-related CCAs. Mutations frequentlywere detected in oncogenes and tumor suppressor genessuch as those encoding tumor protein 53 (TP53) (muta-tions in 44.4% of CCAs), Kirsten rat sarcoma viral oncogenehomolog (KRAS) (16.7%), and SMAD family member 4(16.7%). Mutations also were found in myeloid/lymphoidor mixed-lineage leukemia 3 (MLL3) (14.8% of cases), ringfinger protein 43 (RNF43) (9.3%), paternally expressed 3(PEG3) (5.6%), and roundabout, axon guidance receptor,homolog 2 (ROBO2) (9.3%). These genes are involved indeactivation of histone modifiers, activation of G-proteins,and loss of genomic stability.68 This study, performed inAsia, has been the only whole-exome sequence analysis ofCCAs. Further whole-genome sequencing studies areneeded to evaluate CCAs from Western patients.
A recent study comprising single-nucleotide poly-morphism array, gene expression profile, and mutationanalyses of 149 iCCAs identified inflammation and prolif-eration classes of this tumor.45 Several copy number alter-ations were identified, including losses at 3p, 4q, 6q, 9p, 9q,13q, 14q, 8p, 17p, and 21q, and gains at 1q and 7p.45 Fea-tures of the inflammation class included activation of in-flammatory pathways, overexpression of cytokines, andactivation of STAT3. The proliferation class was character-ized by activation of oncogene signaling pathways involvingRAS, MAPK, andMET. Activating mutations in KRAS havebeen detected frequently in CCAs.69–71 At least 2 studieshave reported a higher incidence of activating mutations inKRAS in pCCAs compared with iCCAs.71,72 In one cohort,the incidence of these mutations was 53% in pCCAs
compared with 17% in iCCAs.71 In a transcriptome profileanalysis of 104 CCAs and 59 matched nontumor samples(controls), patients could be categorized based on overallsurvival time, early recurrence, and presence or absence ofKRAS mutations; a detailed class comparison identified 4subclasses of patients. Those with CCAs with alteredexpression of genes that regulate proteasome activity; withdysregulation of ERBB2; and with overexpression of EGFR,MET, and Ki67 had the worst outcomes.71
Inactivation of TP53, which regulates the cell cycle, isone of the most common genetic abnormalities in cancercells and also has been detected during chol-angiocarcinogenesis. A review of 10 studies, comprising229 patients with CCA from Europe, Asia, and the UnitedStates, reported TP53 mutations in 21% of CCAs.73 Mu-tations in other genes, including EGFR, neuroblastomaRAS viral (v-ras) oncogene homolog (NRAS), PI3K, andAPC, have been less frequently described.44
There has been growing interest in the effects of somaticmutations in genes encoding isocitrate dehydrogenases(IDH) 1 and 2. IDH1 and IDH2 mutations frequently havebeen detected in gliomas, but rarely have been observed inother solid tumors. IDHmutations were detected in 22% ofCCA specimens—more frequently in iCCAs (28%) thanpCCAs and dCCAs (7%).74 Recurrent mutations in IDH1were observed in a subset of biliary tract tumor samples in arecent broad-based mutation profile analysis of gastroin-testinal tumors.75 A subsequent analysis of 62 CCAsdetected IDH1mutations in only iCCAs.75 IDH1 and IDH2mutations were associated significantly with increasedlevels of p53 and DNA hypermethylation.76 Epigeneticchanges associated with IDHmutations likely mediate theironcogenic effects. The product of the enzymatic activity ofmutant IDH1 and IDH2 is 2-hydroxyglutarate (Figure 2A).This metabolite therefore might serve as a biomarker forIDH1 and IDH2mutations, and for a subset of patientswhomight be treated with IDH inhibitors77–79 (Figure 2B).
A number of epigenetic alterations, such as promoterhypermethylation and microRNA dysregulation, havebeen associated with the development of CCA. However,whole-epigenome analysis has not been conducted andmicroRNA (miR) profiling is possible with only smallnumbers of tumor samples.80,81 Promoter hyper-methylation has been reported to silence tumor suppres-sor genes including CDKN2 (observed in 17%–83% ofCCAs), suppressor of cytokine signaling 3 (in 62%46), Ras as-sociation (RalGDS/AF-6) domain family member 1(RASSF1A) (in 31%–69%), and APC (in 27%–47%).45,61
Gene fusions, such as the BCR-ABL gene in chronicmyeloid leukemia, are drivermutations in cancer,whichplaya role in certain cancers.82 Fibroblast growth factor receptor(FGFR) fusions are active kinases. A recent study identifiednovel FGFR2 gene fusions in CCA.82 Cells with these FGFRfusions were susceptible to FGFR inhibitors, signifying thatFGFR kinase inhibition may be a valid therapeutic strategyin CCA patients harboring these gene fusions.82
miRs are noncoding RNAs that function in post-transcriptional regulation of gene expression. A cluster
-
Figure 2. IDH mutations. (A)Function of wild-type andmutant IDH (mIDH). Wild-typeenzymes catalyze a reactionthat converts isocitrate toa-ketoglutarate and reducesNADP to NADPH. The mutantenzymes acquire a neomorphicactivity that converts the normalmetabolite a-KG to 2-HG, andconsumption rather than pro-ductionofNADPH.2-HG leads toinhibitionof certaindioxygenases,which has been postulated toresult in cancer-promotingevents. (B) Potential of personal-ized medicine for CCA, usingmIDH inhibitors, as an example.a-KG, a-ketoglutarate; 2-HG,2-hydroxyglutarate; NADPH,nicotinamide adenine dinucleo-tide phosphate.
December 2013 CHOLANGIOCARCINOMA 1219
REV
IEW
SAND
PER
SPEC
TIVES
of 38 miRs was expressed differentially in 27 iCCA sam-ples, compared with nontumor tissues. miR21 is overex-pressed in CCAs and could have oncogenic effects, partlyby inhibiting programmed cell death 4 and tissue inhibi-tor of matrix metalloproteinase (MMP)3.83 miR21 alsowas found to regulate phosphatase and tensin homologdeleted on chromosome ten–dependent activation of PI3Ksignaling in CCAs, to affect chemosensitivity.84 miR200Cprevents the epithelial–mesenchymal transition (EMT);changes in its level might be used as a prognostic factor.80
Further studies are needed to determine how alterations inmiRs contribute to the development of CCA, and howthese changes might be used to determine patients’prognoses.
Developmental Pathways
The Notch signaling pathway regulates embryonic
development and proliferation of the biliary tree.85 Notsurprisingly, therefore, Notch dysregulation also hasbeen implicated in cholangiocarcinogenesis. Two recentstudies in mice have shown that Notch activation isrequired for conversion of normal adult hepatocytes tobiliary cells that are precursors of iCCA.35,36 Over-expression of intracellular domain of the Notch 1 re-ceptor in liver cells of mice resulted in formation ofiCCAs.86 In this model, an inhibitor of g-secretase, anenzyme necessary for Notch signaling, suppressed tumorformation.
-
1220 RIZVI AND GORES GASTROENTEROLOGY Vol. 145, No. 6
REV
IEWS
AND
PER
SPEC
TIVES
Another evolutionary conserved, developmentalpathway is the Hedgehog signaling pathway. Hedgehogsignaling is deregulated in many types of tumors,including CCAs. Inhibition of hedgehog signaling withcyclopamine impedes CCA cell migration, proliferation,and invasion.87,88 Hedgehog signaling also has beenimplicated in survival signaling by myofibroblast-derivedCCAs. PDGF-b protects CCA cells and promotes tumorsurvival in mice with CCAs, but cyclopamine reverses theseeffects.60
Wnt signaling also is required for intrahepatic bile ductdevelopment and proliferation.89 Wnt-inducible signalingpathway protein 1v is overexpressed in stroma nestsaround CCAs, and levels of Wnt-inducible signalingpathway protein 1v are associated with reduced survivaltimes of patients. Wnt-inducible signaling pathway pro-tein 1v stimulated the invasive activity of CCA cell lines byactivating MAPK1 and MAPK3.90
Tumor Microenvironment
Carcinogenesis in CCA includes alterations in the
stroma, recruitment of fibroblasts, remodeling of theextracellular matrix (ECM), changing patterns of immunecell migration, and promotion of angiogenesis(Figure 3A).91 iCCAs and pCCAs are characterized by adense and reactive desmoplastic stroma (Figure 3B) thatcontains many a-smooth muscle actin (a-SMA)–positivemyofibroblasts, also known as cancer-associated fibro-blasts (CAFs). The tumor stroma surrounds the malignantducts and glands and comprises most of the tumormass.92,93 The stroma promotes tumor progression viareciprocal communication between the stromal cells andcancer cells.92
The precise origin of CAFs is unclear, although severalcell types, including hepatic stellate cells, portal fibro-blasts, and bone marrow–derived precursor cells, havebeen proposed as candidates.92,94–96 The EMT also hasbeen proposed to produce CAFs.93 During tumorigen-esis, the EMT is characterized by the presence of tumorcells that express mesenchymal markers such as vimen-tin, tenascin, fibronectin, and the zinc finger proteinSnail.92 Immunohistochemical studies have shown theexpression of these markers by human CCA celllines.97–99 In mice, xenograft tumors grown fromenhanced green fluorescent protein (EGFP)-expressinghuman CCA cells were found to be surrounded andinfiltrated by a-SMA–expressing CAFs. Interestingly,EGFP was not co-expressed with a-SMA, indicating thatthe EMT does not produce CAFs in CCAs.100 Based oncombined evidence, a-SMA–expressing CAFs appear tobe a heterogeneous population of cells that originatefrom several cell lineages, but not from epithelial cancercells.
CAFs produce factors that stimulate ECM production,leading to a fibrogenic response (Figure 3C).92 Factorsproduced by CAFs include transforming growth factor-b,PDGF isomers, connective tissue growth factor, and
insulin-like growth factor binding proteins.92 PDGF-mediated interactions between CAFs and tumor cellshave been observed, such as recruitment of CAFs byPDGF-D secreted by CCA cells.60,100,101 PDGF-D stimu-lates CAF migration via its receptor platelet-derivedgrowth factor receptor (PDGFR), which is highlyexpressed on CAFs, and activation of small Rho guanosinetriphosphatases and the JNK signaling pathway.100
Activated CAFs also secrete paracrine factors that pro-mote initiation and progression of cancer. These includematricellular proteins, growth factors, chemokines, andECM proteases. Periostin is a matricellular protein that isoverexpressed by CAFs compared with normal fibroblasts;its presence correlates with shorter survival times of pa-tients. Knockdown of the periostin receptor, the a5 sub-unit of integrin, with small interfering RNA, reducedstimulation of tumor proliferation and invasion by peri-ostin.102 The ECM that surrounds pancreatic tumors alsohas been shown to overexpress periostin, which promotestumor invasiveness.103 Tenascin-C, another ECM proteinproduced by CAFs, also promotes tumor migration andinvasiveness.92 In CCA cell lines, HGF promoted inva-siveness and motility by inducing phosphorylation of Aktand ERK 1/2.104 Similarly, stromal cell–derived factor-1,through activation of its receptor chemokine (C-X-Cmotif) receptor 4 (CXCR4), induced CCA cell invasion viaERK 1/2 and Akt.105,106 This process was disrupted by theCXCR4 inhibitor AMD3100.106
ECM degradation and remodeling is required for tumorprogression. MMPs degrade and remodel the ECM duringfibrogenesis and carcinogenesis. MMP1, MMP2, MMP3,and MMP9 are strongly expressed in CCAs and are asso-ciated with invasive tumors.107,108 Fibroblast activationprotein is a stromal protein; its high expression by CAFshas been associated with tumors with an aggressivephenotype.109
The exact mechanisms by which tumor and stromacommunicate are not clear. However, the importance ofthe desmoplastic stroma in CCA progression indicatesthat it could be a new therapeutic target, perhaps via se-lective targeting of CAFs.110
Animal Models
Animal models are essential for the development of
new therapeutic strategies and diagnostic tools.111 Animalmodels of CCA (Table 1) include mice with xenografttumors,43,112–119 mice with genetic changes that lead toCCA formation,86,120–124 rats with orthotopic tu-mors,125,126 and animals that develop CCAs after exposureto carcinogens.55,127–129 Although these models offer anopportunity to bridge the chasm between in vitro findingsand clinical applicability, they have limitations. The tumormicroenvironment is an important feature in CCA devel-opment. It sometimes can be a challenge to study in-teractions between cancer cells and the stroma in micewith xenograft tumors because the tumor is not growingin the same microenvironment as it does in human beings.
-
Figure 3. Microenvironment of cholangiocarc-inoma. (A) Components of the tumor microenvi-ronment in CCA. (B) Micrograph of a stromalCCA. (C) Factors secreted by cancer-associatedfibroblasts. CTGF, connective tissue growth fac-tor; SDF-1, stromal cell–derived factor 1; TGF-b,transforming growth factor-b.
December 2013 CHOLANGIOCARCINOMA 1221
REV
IEW
SAND
PER
SPEC
TIVES
-
Table 1. Animal Models of Cholangiocarcinoma
Experimental approach Key features Study
Mice with xenograft tumorsInjection of 3 � 106 Mz-ChA-1 cells Tumor development in 3 weeks Fava et al112Injection of 5 � 106 Sk-ChA-1 cells � intratumoral tamoxifen injections Significantly decreased CCA development with intratumoral
tamoxifen injectionsPawar et al113
Injection of 2 � 106 QBC939 cells � magnetic nanoparticle injections via tail vein CCA tumor growth inhibition with magnetic nanoparticles Tang et al114Injection of IL-6 overexpressed Mz-ChA-1 stable cell line (Mz-ChA-IL6) vs
control vector Mz-ChA-1 cell lineOverexpression of IL-6 increased growth of xenograft tumors Meng et al43
Injection of miR26a overexpressed CCLP1 cell line vs scramble controlCCLP1 cell line
Overexpression of miR26a increased growth of xenograft tumors Zhang et al115
Injection of miR494 overexpressed stable HuCCT1 cell line vs control vectorHuCCT1 cell line
Overexpression of miR494 increased growth of xenograft tumors Olaru et al116
Injection of stable QBC939 cell line transfected with Slug siRNA vs controlvector QBC939 cell line
Slug silencing suppressed growth of xenograft tumors Zhang et al117
Injection of CypA silenced stable M139 cell line vs control vector M139 cell line CypA silencing decreased growth of xenograft tumors Obchoei et al118
Injection of stable QBC939 cell line transfected with Beclin-1 siRNA vscontrol vector QBC939 cell line
Beclin-1 silencing decreased growth of xenograft tumors Hou et al119
Genetically engineered mouse modelsLiver-specific inactivation of SMAD4 and PTEN Tumor formation in all animals at 4–7 months of age Xu et al120
Chronic carbon tetrachloride exposure in TP53-deficient mice Development of tumors with dense peritumoral fibrosis and other histologicand genetic features of human iCCA
Farazi et al121
Liver-specific inactivation of macrophage stimulating factor 1 and 2 Tumor development (HCC or CCA) in all mice by 6 months of age Song et al122
Liver-specific ablation of WW45, a homolog of Drosophila Salvador andadaptor for the Hippo kinase
Development of tumors with mixed histologic features of HCC and CCA Lee et al123
Liver-specific activation of KRAS and deletion of TP53 Development of stroma-rich tumors; shortened time to tumor developmentand increased metastasis with the combination of KRAS activation and TP53 deletion
O’Dell et al124
Overexpression of intracellular domain of Notch1 in livers of transgenic mice Formation of tumors with features characteristic of iCCA Zender et al86
Orthotopic rat modelsInoculation of BDEneu cells into bile duct of isogenic rats Rapid (21–26 days) development of cholangiocarcinoma characterized by biliary
obstruction and gross peritoneal metastasis; origin of tumor stroma andtumor tissue from same species (rat)
Sirica et al125
Three-dimensional organotypic culture model of CCA in rats Stromal microenvironment, gene expression profile, and pathophysiologiccharacteristics that mimic desmoplastic iCCA in vivo
Campbell et al126
Carcinogen-induced modelsFuran-induced cholangiocarcinogenesis in rat liver Formation of mucin-producing CCA tumors; overexpression of C-NEU and MET Radaeva et al55
Chronic administration of thioacetamide in lean rats and rats withfaulty leptin receptors
Increased development and growth of CCA tumors inlean rats treated with thioacetamide
Fava et al127
Administration of DEN � LMBDL to induce chronic cholestasisand CCA development
Increased CCA progression in mice with LMBDL given DEN compared withmice without LMBDL given DEN
Yang et al128
Inoculation with O viverrini and administration ofdimethylnitrosamine in hamsters
Development of pus and tumor in liver starting at 20 weeks after O viverriniinfection/DEN administration; all hamsters in experimental groupwere dead by 28 weeks
Plengsuriyakarnet al129
BDEneu, highly malignant cholangiocarcinoma cell line; CCLP1, cholangiocarcinoma cell line; C-NEU, rat homologue of human ERBB2; CypA, cyclophilin A; DEN, diethylnitrosamine; HuCCT1, cholangiocarcinomacell line; KRAS, Kirsten rat sarcoma viral oncogene homolog; LMBDL, left and median bile duct ligation; M139, cholangiocarcinoma cell line; Mz-ChA-1, cholangiocarcinoma cell line; PTEN, phosphatase andtensin homolog deleted on chromosome ten; QBC939, human hilar bile duct carcinoma cell line; Sk-ChA-1, cholangiocarcinoma cell line; siRNA, small interfering RNA; SMAD4, SMAD family member 4.
1222
RIZVI
ANDGORES
GASTR
OEN
TEROLO
GYVol.1
45,N
o.6
REVIEWSAND
PERSPECTIVES
-
December 2013 CHOLANGIOCARCINOMA 1223
REV
IEW
SAND
PER
SPEC
TIVES
A model described by Sirica et al,125 in which rat CCAcells were injected into rat biliary trees, is unique inthat the stroma and epithelial cells were derived from thesame species. These animals allow for investigations oftumor–stroma interactions that more closely resemblethose of patients. Although transgenic models do allowfor study of the tumor microenvironment, they tend to betechnically challenging and expensive. Animals with ge-netic alterations that lead to production of CCAs thatresemble human tumors are needed.
Diagnosis and Management
It can be a challenge to diagnose CCA because of
its paucicellular nature, anatomic location, and silentclinical character. Diagnosis requires a high index of sus-picion and a multidisciplinary approach that involvesclinical, laboratory, endoscopic, and radiographic analyses.
iCCA
iCCA is divided into mass-forming, periductal
infiltrating, and intraductal growth types.130 The clinicalmanifestations of iCCA include nonspecific symptomssuch as abdominal pain, cachexia, malaise, fatigue, andnight sweats.2 iCCA frequently presents as an intrahepaticmass lesion; imaging modalities including computed to-mography (CT) and magnetic resonance imaging (MRI)aid in the diagnosis. The use of contrast enhancementimproves the sensitivity of MRI for detection of iCCAbecause these tumors typically have progressive uptake ofcontrast during the venous phase. HCCs, on the otherhand, are characterized by rapid contrast uptake duringthe arterial phase, followed by a delayed venous washoutphase.131 CT and MRI have similar utility in the evalua-tion of tumor size and detection of satellite lesions.However, CT may be better for assessment of vascularencasement, identification of extrahepatic metastasis, anddetermination of resectability.17,132
Serum levels of carbohydrate antigen 19-9 (CA19-9), atumor biomarker, can aid in diagnosis, but this assaydetects iCCA with only 62% sensitivity and 63% speci-ficity.133 Moreover, increased levels of CA19-9 also havebeen observed in patients with benign diseases such asbacterial cholangitis or choledocholithiasis.5 Nonetheless,very high levels of CA19-9 (�1000 U/mL) have beenassociated with metastatic iCCA, so this assay might beused in disease staging rather than diagnosis.134 Mixedtumors are characterized by histologic and imaging fea-tures of HCC and iCCA. In these cases, immunohisto-chemical analysis for cytokeratins 7 and 19 can beuseful—tumors positive for cytokeratins can be consideredto be mixed hepatocellular CCA.17,135 A definitive diag-nosis of iCCA requires liver biopsy analysis. According tothe World Health Organization classification criteria,iCCAs can be adenocarcinomas or mucinous carcinomas.2
The treatment of choice for iCCA is surgical resection.Patients should undergo surgery only if they havepotentially resectable tumors and are appropriate surgical
candidates. After surgical resection, the median time ofdisease-free survival is 26 months; reported rates of recur-rence are 60%–65%.136,137 Approximately 60% of patientssurvive for 5 years after resection. Factors associated withrecurrence and reduced survival time after resection includevascular invasion, lymph node metastasis, multiple tumors,and cirrhosis.4,138 Nuclear expression of S100A4, a memberof the S100 family of calcium-binding proteins, inneoplastic ducts was associated with metastasis andreduced time of survival after surgical resection in a subsetof patients with CCA.139
Liver transplantation as a curative option for iCCA ishighly controversial. iCCA was reported to recur in 70% ofpatients within 5 years of liver transplantation, and themedian disease-free survival time was 8 months in a seriesof 14 patients with iCCA or mixed HCC-iCCA.135 Patientswith very small iCCAs (
-
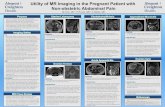
Figure 4. Diagnostic modalitiesused for cholangiocarcinoma.(A) MRI of a pCCA mass (out-lined in circle). (B) CT image of apCCA mass with right portalvein encasement (black arrow).(C) Magnetic resonance chol-angiopancreatography image ofcommon hepatic duct involve-ment by tumor (white arrow). (D)ERC image showing excludedsegmental ducts (white arrows)in a patient with a hilar biliarystricture extending into the rightmain hepatic duct.
Figure 5. Time to diagnosis of a cholangiocarcinoma based on FISHanalysis and CA19-9 levels. Reprinted with permission from WileyInterScience and Barr et al.146
1224 RIZVI AND GORES GASTROENTEROLOGY Vol. 145, No. 6
REV
IEWS
AND
PER
SPEC
TIVES
be performed on the primary tumor because it candisseminate the tumor.143 ERC serves a diagnostic andtherapeutic purpose—it is used to assess and sample thebiliary tree via brush cytology and endoscopic biopsy, aswell as dilatation and stent placement in cases of biliaryobstruction.
Fluorescence in situ hybridization (FISH) analysis in-creases the sensitivity of cytology in diagnosing pCCA.144
FISH can detect polysomy or amplification of at least 2chromosomes: tetrasomy and trisomy 7. Of these, polys-omy in the presence of a dominant stricture is consideredsufficient for the diagnosis of pCCA, especially if thepolysomy can be confirmed over time.145 Tetrasomy canbe seen during the M phase of mitosis and should beinterpreted with caution.5 Trisomy 7 often is observedwith inflammation of the biliary tree. Detection of polys-omy by FISH also has been shown to predict the devel-opment of malignancies in patients with PSC with nomass and equivocal cytology. In a recent study, patientswith PSC who had polysomy and levels of CA19-9 greaterthan 129 U/mL all went on to develop cancer, mainlywithin 2 years (Figure 5).146
The only curative options for pCCA are surgical resec-tion and neoadjuvant chemoradiation followed by livertransplantation. The Bismuth–Corlette staging classifica-tion is based on the anatomic location of the CCA withinthe biliary tree and is meant to help guide decision mak-ing. Recently, this classification was expanded to take into
account vascular encasement and parenchymal value ofthe potential remnant lobe.3 Surgical resection entailslobar hepatic and bile duct resection, regional lymphade-nectomy, and Roux-en-Y hepaticojejunostomy. Potentialcontraindications to curative surgical resection includecontralateral or bilateral vascular encasement and pCCAextension bilaterally to the level of the secondary biliarybranches. The presence of regional lymphadenopathy does
-
Table 2. Criteria for Liver Transplantation in pCCA
Diagnosis of cholangiocarcinoma
Positive transluminal biopsyPositive biliary brush cytologyMalignant-appearing stricture on ERC with a CA 19-9 level> 100 U/mL
and/or FISH polysomyMass lesion on cross-sectional imaging and malignant-appearing
stricture on ERC/MRCPTumor size
Radial tumor diameter of �3 cmTumor confined to biliary tree
Absence of intrahepatic or extrahepatic metastasisUnresectability
Unresectable hilar tumor (above the cystic duct)CCA in a PSC patient (owing to skip lesions, the field defect, and
parenchymal liver disease)
MRCP, magnetic resonance cholangiopancreatography.
December 2013 CHOLANGIOCARCINOMA 1225
REV
IEW
SAND
PER
SPEC
TIVES
not necessarily preclude surgery.147 Occasionally, a tumormay be resectable but the remnant lobe has limited vol-ume. In such cases, resectability can be achieved by pre-operative relief of biliary obstruction and portal veinembolization of the affected lobe with resultant compen-satory hyperplasia of the contralateral unaffected liverlobe.147 Rates of 5-year survival after surgical resectionwith negative margins range from 11% to 41%.147
With the advent of new liver transplantation protocols,neoadjuvant chemoradiation followed by transplantationhas become an appealing option for patients selected care-fully using stringent criteria (Table 2). Sixty-five percent ofpatientswhowere treatedwithneoadjuvant therapy followedby liver transplantation at12 large-volume transplant centerssurvived for 5 years.148 Rigorous selection is imperative forsuccessful outcomes. Eligibility criteria include radial diam-eter of tumor of less than 3 cm, absence of intrahepatic orextrahepatic metastasis, and, in the case of patients withoutPSC, unresectability.149 Because of the presence of paren-chymal liver disease, patients with PSC typically require livertransplantation rather than surgical resection.
For patients who are not candidates for surgical resectionor liver transplantation, systemic chemotherapy with gem-citabine and cisplatin is recommended. For patients withbiliary obstruction, adequate drainage is essential to relievecholestasis and increase tolerance to chemotherapy.17
dCCA
Intraductal papillary neoplasm and biliary intra-
epithelial neoplasia are the precursor lesions of dCCA.30
dCCA arises from the point of insertion of the cysticduct to the ampulla of Vater and therefore can be difficultto distinguish from early pancreatic cancer.17 Analogousto pCCA, patients typically present with painless jaundice,and laboratory analysis is consistent with biliary obstruc-tion. Although pCCA and dCCA are distinct with respectto their pathogenesis and treatment, most studies evalu-ating diagnostic modalities have grouped these as extra-hepatic CCAs. Cross-sectional imaging, endoscopicultrasound, and ERC therefore are used in the samemanner in the diagnosis of dCCA as with pCCA.
Diagnosis is made on the basis of the presence of adominant stricture and positive cytology and/or detectionof polysomy by FISH.2 Surgical treatment of dCCA typi-cally entails a Whipple procedure. Only 27% of patientssurvive for 5 years after surgical resection that attainsnegative margins.4 The role of neoadjuvant chemo-radiation is limited. For patients who are not candidatesfor surgical resection, chemotherapy may be considered.17
Future Directions
Treatment options for CCA are limited and overall
survival rates are low. Earlier detection of CCA increasesthe chance of having curative treatment options. However,despite recent advances in diagnosis, such as improvedimaging and cytology techniques, including FISH, furtherwork is necessary to overcome the challenge of diagnosingCCA at an earlier stage. CCA often still is diagnosed basedon clinical criteria, such as a malignant-appearing bileduct stricture, increased serum levels of CA19-9, appear-ance of a mass during MRI, normal serum levels of IgG4level, and so forth.
There are significant geographic and ethnic variationsin the incidence of CCA, so genetic factors are likely tocontribute to its pathogenesis. Inflammatory and onco-genic signaling pathways also are involved in chol-angiocarcinogenesis, and are potential therapeutic targets.Further studies are necessary to elucidate the role of ge-netic aberrations, particularly in regions encoding keycomponents of signaling pathways. In addition, the role ofmiRs as biomarkers remains to be fully elucidated. CCAsare heterogeneous; treatments are likely to be designedbased on features of each individual tumor.150 Potentialtherapeutic targets could include the MET tyrosine re-ceptor kinase, FGFR2, the PI3K–Akt–mTOR pathway, andIDH mutations. Molecular profiling of tumors, to identifytheir specific mutations, could make it possible to offertargeted therapies in personalized treatments (Figure 2B).
Although cancer cells containmanygenetic and functionalaberrations, the tumor stroma appears to be more uniformand has strong potential as a target for new combinationtherapies. Further work is needed to highlight the dynamicreciprocal communication between tumor and stroma.
References
1. Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification ofhilar cholangiocarcinomas (Klatskin tumors) on the incidence ofintra- and extrahepatic cholangiocarcinoma in the United States.J Natl Cancer Inst 2006;98:873–875.
2. Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis andstaging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol2011;8:512–522.
3. Deoliveira ML, Schulick RD, Nimura Y, et al. New staging systemand a registry for perihilar cholangiocarcinoma. Hepatology 2011;53:1363–1371.
4. DeOliveira ML, Cunningham SC, Cameron JL, et al. Chol-angiocarcinoma: thirty-one-year experience with 564 patients at asingle institution. Ann Surg 2007;245:755–762.
5. Blechacz BG, Gores, GJ. Tumors of the bile ducts, gallbladder, andampulla. In: Feldman, ed. Sleisenger and Fordtran’s
-
1226 RIZVI AND GORES GASTROENTEROLOGY Vol. 145, No. 6
REV
IEWS
AND
PER
SPEC
TIVES
gastrointestinal and liver disease. Volume 1. 9th ed. Philadelphia:Saunders, 2010:1171–1176.
6. Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diag-nosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657–1669.
7. Everhart JE, Ruhl CE. Burden of digestive diseases in the UnitedStates part III: liver, biliary tract, and pancreas. Gastroenterology2009;136:1134–1144.
8. Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hep-atology 2011;54:173–184.
9. Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma.Semin Liver Dis 2004;24:115–125.
10. Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand.Curr Opin Gastroenterol 2008;24:349–356.
11. Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P,Thomas HC. Changing international trends in mortality rates forliver, biliary and pancreatic tumours. J Hepatol 2002;37:806–813.
12. Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, riskfactors, and pathogenesis of cholangiocarcinoma. HPB (Oxford)2008;10:77–82.
13. McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in theincidence of hepatocellular carcinoma and intrahepatic chol-angiocarcinoma in the United States. Cancer Epidemiol BiomarkersPrev 2006;15:1198–1203.
14. Patel T. Increasing incidence and mortality of primary intrahepaticcholangiocarcinoma in the United States. Hepatology 2001;33:1353–1357.
15. Patel T. Worldwide trends in mortality from biliary tract malig-nancies. BMC Cancer 2002;2:10.
16. Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends incholangiocarcinoma: is the ICD classification system misleadingus? J Hepatol 2012;56:848–854.
17. Razumilava N, Gores GJ. Classification, diagnosis, and manage-ment of cholangiocarcinoma. Clin Gastroenterol Hepatol 2013;11:13–21 e1; quiz e3–e4.
18. Shin HR, Oh JK, Lim MK, et al. Descriptive epidemiology of chol-angiocarcinoma and clonorchiasis in Korea. J Korean Med Sci2010;25:1011–1016.
19. Huang MH, Chen CH, Yen CM, et al. Relation of hepatolithiasis tohelminthic infestation. J Gastroenterol Hepatol 2005;20:141–146.
20. Edil BH, Cameron JL, Reddy S, et al. Choledochal cyst disease inchildren and adults: a 30-year single-institution experience. J AmColl Surg 2008;206:1000–1005; discussion 1005–1008.
21. Mabrut JY, Bozio G, Hubert C, et al. Management of congenital bileduct cysts. Dig Surg 2010;27:12–18.
22. Kato I, Kido C. Increased risk of death in thorotrast-exposed patientsduring the late follow-upperiod. Jpn JCancerRes1987;78:1187–1192.
23. Lee TY, Lee SS, Jung SW, et al. Hepatitis B virus infection andintrahepatic cholangiocarcinoma in Korea: a case-control study. AmJ Gastroenterol 2008;103:1716–1720.
24. Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepaticand extrahepatic cholangiocarcinoma: a hospital-based case-controlstudy. Am J Gastroenterol 2007;102:1016–1021.
25. Sorensen HT, Friis S, Olsen JH, et al. Risk of liver and other types ofcancer in patients with cirrhosis: a nationwide cohort study inDenmark. Hepatology 1998;28:921–925.
26. Palmer WC, Patel T. Are common factors involved in the patho-genesis of primary liver cancers? A meta-analysis of risk factors forintrahepatic cholangiocarcinoma. J Hepatol 2012;57:69–76.
27. Shaib YH, El-Serag HB, Davila JA, et al. Risk factors of intrahepaticcholangiocarcinoma in the United States: a case-control study.Gastroenterology 2005;128:620–626.
28. Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intra-hepatic and extrahepatic cholangiocarcinoma in the United States:a population-based case-control study. Clin Gastroenterol Hepatol2007;5:1221–1228.
29. Welzel TM, Mellemkjaer L, Gloria G, et al. Risk factors for intra-hepatic cholangiocarcinoma in a low-risk population: a nationwidecase-control study. Int J Cancer 2007;120:638–641.
30. Nakanuma Y, Sato Y, Harada K, et al. Pathological classification ofintrahepatic cholangiocarcinoma based on a new concept. World JHepatol 2010;2:419–427.
31. Komuta M, Spee B, Vander Borght S, et al. Clinicopathologicalstudy on cholangiolocellular carcinoma suggesting hepatic pro-genitor cell origin. Hepatology 2008;47:1544–1556.
32. Tsuchiya A, Kamimura H, Tamura Y, et al. Hepatocellular carcinomawith progenitor cell features distinguishable by the hepatic stem/progenitor cell marker NCAM. Cancer Lett 2011;309:95–103.
33. Cardinale V, Carpino G, Reid L, et al. Multiple cells of origin incholangiocarcinoma underlie biological, epidemiological and clin-ical heterogeneity. World J Gastrointest Oncol 2012;4:94–102.
34. Komuta M, Govaere O, Vandecaveye V, et al. Histological diversity incholangiocellular carcinoma reflects the different cholangiocytephenotypes. Hepatology 2012;55:1876–1888.
35. Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originatefrom hepatocytes in mice. J Clin Invest 2012;122:2911–2915.
36. Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise fromNotch-mediated conversion of hepatocytes. J Clin Invest 2012;122:3914–3918.
37. Holczbauer A, Factor VM, Andersen JB, et al. Modeling pathogen-esis of primary liver cancer in lineage-specific mouse cell types.Gastroenterology 2013;145:221–231.
38. Jaiswal M, LaRusso NF, Burgart LJ, et al. Inflammatory cytokinesinduce DNA damage and inhibit DNA repair in cholangiocarcinomacells by a nitric oxide-dependent mechanism. Cancer Res 2000;60:184–190.
39. Park J, Tadlock L, Gores GJ, et al. Inhibition of interleukin6-mediated mitogen-activated protein kinase activation attenuatesgrowth of a cholangiocarcinoma cell line. Hepatology 1999;30:1128–1133.
40. Kobayashi S, Werneburg NW, Bronk SF, et al. Interleukin-6 con-tributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology2005;128:2054–2065.
41. Taniai M, Grambihler A, Higuchi H, et al. Mcl-1 mediates tumor ne-crosis factor-related apoptosis-inducing ligand resistance in humancholangiocarcinoma cells. Cancer Res 2004;64:3517–3524.
42. Isomoto H, Kobayashi S, Werneburg NW, et al. Interleukin 6 upre-gulates myeloid cell leukemia-1 expression through a STAT3pathway in cholangiocarcinoma cells. Hepatology 2005;42:1329–1338.
43. Meng F, Yamagiwa Y, Ueno Y, et al. Over-expression of interleukin-6enhances cell survival and transformed cell growth in human ma-lignant cholangiocytes. J Hepatol 2006;44:1055–1065.
44. Sia D, Tovar V, Moeini A, et al. Intrahepatic cholangiocarcinoma:pathogenesis and rationale for molecular therapies. Oncogene2013;32:4861–4870.
45. Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysisof intrahepatic cholangiocarcinoma reveals 2 classes that havedifferent outcomes. Gastroenterology 2013;144:829–840.
46. Isomoto H, Mott JL, Kobayashi S, et al. Sustained IL-6/STAT-3signaling in cholangiocarcinoma cells due to SOCS-3 epigeneticsilencing. Gastroenterology 2007;132:384–396.
47. Yoon JH, Gwak GY, Lee HS, et al. Enhanced epidermal growth factorreceptor activation in human cholangiocarcinoma cells. J Hepatol2004;41:808–814.
48. Kiguchi K, Carbajal S, Chan K, et al. Constitutive expression ofErbB-2 in gallbladder epithelium results in development ofadenocarcinoma. Cancer Res 2001;61:6971–6976.
49. Matsumoto K, Nakamura T. Hepatocyte growth factor and the Metsystem as a mediator of tumor-stromal interactions. Int J Cancer2006;119:477–483.
50. Nishimura K, Kitamura M, Miura H, et al. Prostate stromal cell-derived hepatocyte growth factor induces invasion of prostatecancer cell line DU145 through tumor-stromal interaction. Prostate1999;41:145–153.
51. Nakamura T, Matsumoto K, Kiritoshi A, et al. Induction of hepato-cyte growth factor in fibroblasts by tumor-derived factors affects
-
December 2013 CHOLANGIOCARCINOMA 1227
REV
IEW
SAND
PER
SPEC
TIVES
invasive growth of tumor cells: in vitro analysis of tumor-stromalinteractions. Cancer Res 1997;57:3305–3313.
52. Comoglio PM, Giordano S, Trusolino L. Drug development of METinhibitors: targeting oncogene addiction and expedience. Nat RevDrug Discov 2008;7:504–516.
53. Lai GH, Radaeva S, Nakamura T, et al. Unique epithelial cell pro-duction of hepatocyte growth factor/scatter factor by putativeprecancerous intestinal metaplasias and associated “intestinal-type” biliary cancer chemically induced in rat liver. Hepatology2000;31:1257–1265.
54. Miyamoto M, Ojima H, Iwasaki M, et al. Prognostic significance ofoverexpression of c-Met oncoprotein in cholangiocarcinoma. Br JCancer 2011;105:131–138.
55. Radaeva S, Ferreira-Gonzalez A, Sirica AE. Overexpression of C-NEUand C-MET during rat liver cholangiocarcinogenesis: a link betweenbiliary intestinalmetaplasiaandmucin-producing cholangiocarcinoma.Hepatology 1999;29:1453–1462.
56. Yoon JH, Higuchi H, Werneburg NW, et al. Bile acids inducecyclooxygenase-2 expression via the epidermal growth factorreceptor in a human cholangiocarcinoma cell line. Gastroenter-ology 2002;122:985–993.
57. Yoon JH, Canbay AE, Werneburg NW, et al. Oxysterols inducecyclooxygenase-2 expression in cholangiocytes: implications forbiliary tract carcinogenesis. Hepatology 2004;39:732–738.
58. Kuver R. Mechanisms of oxysterol-induced disease: insights fromthe biliary system. Clin Lipidol 2012;7:537–548.
59. Nachtergaele S, Mydock LK, Krishnan K, et al. Oxysterols areallosteric activators of the oncoprotein Smoothened. Nat Chem Biol2012;8:211–220.
60. Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derivedPDGF-BB promotes Hedgehog survival signaling in chol-angiocarcinoma cells. Hepatology 2011;54:2076–2088.
61. Andersen JB, Thorgeirsson SS. Genetic profiling of intrahepaticcholangiocarcinoma. Curr Opin Gastroenterol 2012;28:266–272.
62. McKay SC, Unger K, Pericleous S, et al. Array comparative genomichybridization identifies novel potential therapeutic targets in chol-angiocarcinoma. HPB (Oxford) 2011;13:309–319.
63. Koo SH, Ihm CH, Kwon KC, et al. Genetic alterations in hepato-cellular carcinoma and intrahepatic cholangiocarcinoma. CancerGenet Cytogenet 2001;130:22–28.
64. Uhm KO, Park YN, Lee JY, et al. Chromosomal imbalances inKorean intrahepatic cholangiocarcinoma by comparative genomichybridization. Cancer Genet Cytogenet 2005;157:37–41.
65. Lee JY, Park YN, Uhm KO, et al. Genetic alterations in intrahepaticcholangiocarcinoma as revealed by degenerate oligonucleotideprimed PCR-comparative genomic hybridization. J Korean Med Sci2004;19:682–687.
66. Wong N, Li L, Tsang K, et al. Frequent loss of chromosome 3p andhypermethylation of RASSF1A in cholangiocarcinoma. J Hepatol2002;37:633–639.
67. Homayounfar K, Gunawan B, Cameron S, et al. Pattern of chro-mosomal aberrations in primary liver cancers identified bycomparative genomic hybridization. Hum Pathol 2009;40:834–842.
68. OngCK, SubimerbC,Pairojkul C, et al. Exomesequencing of liver fluke-associated cholangiocarcinoma. Nat Genet 2012;44:690–693.
69. Xu RF, Sun JP, Zhang SR, et al. KRAS and PIK3CA but not BRAFgenes are frequently mutated in Chinese cholangiocarcinoma pa-tients. Biomed Pharmacother 2011;65:22–26.
70. Ohashi K, Nakajima Y, Kanehiro H, et al. Ki-ras mutations and p53protein expressions in intrahepatic cholangiocarcinomas: relation togross tumor morphology. Gastroenterology 1995;109:1612–1617.
71. Andersen JB, Spee B, Blechacz BR, et al. Genomic and geneticcharacterization of cholangiocarcinoma identifies therapeutic tar-gets for tyrosine kinase inhibitors. Gastroenterology 2012;142:1021–1031 e15.
72. Tada M, Omata M, Ohto M. High incidence of ras gene mutation inintrahepatic cholangiocarcinoma. Cancer 1992;69:1115–1118.
73. Khan SA, Thomas HC, Toledano MB, et al. p53 Mutations in humancholangiocarcinoma: a review. Liver Int 2005;25:704–716.
74. Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2mutations in cholangiocarcinoma. HumPathol 2012;43:1552–1558.
75. Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of iso-citrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinomaidentified through broad-based tumor genotyping. Oncologist 2012;17:72–79.
76. Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydroge-nase 1 and 2 occur frequently in intrahepatic cholangiocarcinomasand share hypermethylation targets with glioblastomas. Oncogene2013;32:3091–3100.
77. Reitman ZJ, Parsons DW, Yan H. IDH1 and IDH2: not your typicaloncogenes. Cancer Cell 2010;17:215–216.
78. Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutantIDH1 delays growth and promotes differentiation of glioma cells.Science 2013;340:626–630.
79. Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutantIDH2 in leukemia cells induces cellular differentiation. Science2013;340:622–626.
80. Oishi N, Kumar MR, Roessler S, et al. Transcriptomic profiling re-veals hepatic stem-like gene signatures and interplay of miR-200cand epithelial-mesenchymal transition in intrahepatic chol-angiocarcinoma. Hepatology 2012;56:1792–1803.
81. Chen L, Yan HX, Yang W, et al. The role of microRNA expressionpattern in human intrahepatic cholangiocarcinoma. J Hepatol2009;50:358–369.
82. Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetableFGFRgene fusions in diverse cancers. CancerDiscov2013;3:636–647.
83. Yamanaka S, Olaru AV, An F, et al. MicroRNA-21 inhibits Serpini1, agene with novel tumour suppressive effects in gastric cancer. DigLiver Dis 2012;44:589–596.
84. Meng F, Henson R, Lang M, et al. Involvement of human micro-RNAin growth and response to chemotherapy in human chol-angiocarcinoma cell lines. Gastroenterology 2006;130:2113–2129.
85. Hofmann JJ, Zovein AC, Koh H, et al. Jagged1 in the portal veinmesenchyme regulates intrahepatic bile duct development: in-sights into Alagille syndrome. Development 2010;137:4061–4072.
86. Zender S, Nickeleit I, Wuestefeld T, et al. A critical role for notchsignaling in the formation of cholangiocellular carcinomas. CancerCell 2013;23:784–795.
87. Jinawath A, Akiyama Y, Sripa B, et al. Dual blockade of theHedgehog and ERK1/2 pathways coordinately decreases prolifer-ation and survival of cholangiocarcinoma cells. J Cancer Res ClinOncol 2007;133:271–278.
88. El Khatib M, Kalnytska A, Palagani V, et al. Inhibition of hedgehogsignaling attenuates carcinogenesis in vitro and increases ne-crosis of cholangiocellular carcinoma. Hepatology 2013;57:1035–1045.
89. Sirica AE, Nathanson MH, Gores GJ, et al. Pathobiology of biliaryepithelia and cholangiocarcinoma: proceedings of the Henry M.and Lillian Stratton Basic Research Single-Topic Conference. Hep-atology 2008;48:2040–2046.
90. Tanaka S, Sugimachi K, Kameyama T, et al. Human WISP1v, amember of the CCN family, is associated with invasive chol-angiocarcinoma. Hepatology 2003;37:1122–1129.
91. Junttila MR, de Sauvage FJ. Influence of tumour micro-environmentheterogeneity on therapeutic response. Nature 2013;501:346–354.
92. Sirica AE. The role of cancer-associated myofibroblasts in intra-hepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2012;9:44–54.
93. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392–401.
94. Dranoff JA, Wells RG. Portal fibroblasts: underappreciated media-tors of biliary fibrosis. Hepatology 2010;51:1438–1444.
95. Okabe H, Beppu T, Hayashi H, et al. Hepatic stellate cells may relateto progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol2009;16:2555–2564.
-
1228 RIZVI AND GORES GASTROENTEROLOGY Vol. 145, No. 6
REV
IEWS
AND
PER
SPEC
TIVES
96. Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibro-blasts contribute to the mesenchymal stem cell niche and promotetumor growth. Cancer Cell 2011;19:257–272.
97. Li T, Li D, Cheng L, et al. Epithelial-mesenchymal transition inducedby hepatitis C virus core protein in cholangiocarcinoma. Ann SurgOncol 2010;17:1937–1944.
98. Sato Y, Harada K, Itatsu K, et al. Epithelial-mesenchymal transitioninduced by transforming growth factor-{beta}1/Snail activation ag-gravates invasive growth of cholangiocarcinoma. Am J Pathol 2010;177:141–152.
99. Korita PV, Wakai T, Ajioka Y, et al. Aberrant expression of vimentincorrelates with dedifferentiation and poor prognosis in patientswith intrahepatic cholangiocarcinoma. Anticancer Res 2010;30:2279–2285.
100. Cadamuro M, Nardo G, Indraccolo S, et al. Platelet-derived growthfactor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013;58:1042–1053.
101. Fingas CD, Mertens JC, Razumilava N, et al. Targeting PDGFR-betain cholangiocarcinoma. Liver Int 2012;32:400–409.
102. Utispan K, Thuwajit P, Abiko Y, et al. Gene expression profiling ofcholangiocarcinoma-derived fibroblast reveals alterations related totumor progression and indicates periostin as a poor prognosticmarker. Mol Cancer 2010;9:13.
103. Baril P, Gangeswaran R, Mahon PC, et al. Periostin promotesinvasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway.Oncogene 2007;26:2082–2094.
104. Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2pathways in hepatocyte growth factor-induced cholangiocarcinomacell invasion. World J Gastroenterol 2010;16:713–722.
105. Ohira S, SasakiM, HaradaK, et al. Possible regulation ofmigration ofintrahepatic cholangiocarcinoma cells by interaction of CXCR4expressed in carcinoma cells with tumor necrosis factor-alpha andstromal-derived factor-1 released in stroma. Am J Pathol 2006;168:1155–1168.
106. Leelawat K, Leelawat S, Narong S, et al. Roles of the MEK1/2 andAKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cellinvasion. World J Gastroenterol 2007;13:1561–1568.
107. Terada T, Okada Y, Nakanuma Y. Expression of immunoreactivematrix metalloproteinases and tissue inhibitors of matrix metal-loproteinases in human normal livers and primary liver tumors.Hepatology 1996;23:1341–1344.
108. Prakobwong S, Yongvanit P, Hiraku Y, et al. Involvement of MMP-9 inperibiliary fibrosis and cholangiocarcinogenesis via Rac1-dependentDNA damage in a hamstermodel. Int J Cancer 2010;127:2576–2587.
109. Cohen SJ, Alpaugh RK, Palazzo I, et al. Fibroblast activation proteinand its relationship to clinical outcome in pancreatic adenocarci-noma. Pancreas 2008;37:154–158.
110. Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic effects ofdeleting cancer-associated fibroblasts in cholangiocarcinoma.Cancer Res 2013;73:897–907.
111. Ko KS, Peng J, Yang H. Animal models of cholangiocarcinoma. CurrOpin Gastroenterol 2013;29:312–318.
112. Fava G, Marucci L, Glaser S, et al. gamma-Aminobutyric acid in-hibits cholangiocarcinoma growth by cyclic AMP-dependent regu-lation of the protein kinase A/extracellular signal-regulated kinase1/2 pathway. Cancer Res 2005;65:11437–11446.
113. Pawar P, Ma L, Byon CH, et al. Molecular mechanisms of tamoxifentherapy for cholangiocarcinoma: role of calmodulin. Clin CancerRes 2009;15:1288–1296.
114. Tang T, Zheng JW, Chen B, et al. Effects of targeting magnetic drugnanoparticles on human cholangiocarcinoma xenografts in nudemice. Hepatobiliary Pancreat Dis Int 2007;6:303–307.
115. Zhang J, Han C, Wu T. MicroRNA-26a promotes chol-angiocarcinoma growth by activating beta-catenin. Gastroenter-ology 2012;143:246–256 e8.
116. Olaru AV, Ghiaur G, Yamanaka S, et al. MicroRNA down-regulated inhuman cholangiocarcinoma control cell cycle through multiple
targets involved in the G1/S checkpoint. Hepatology 2011;54:2089–2098.
117. Zhang K, Chen D, Wang X, et al. RNA interference targeting slugincreases cholangiocarcinoma cell sensitivity to cisplatin via upre-gulating PUMA. Int J Mol Sci 2011;12:385–400.
118. Obchoei S, Weakley SM, Wongkham S, et al. Cyclophilin A en-hances cell proliferation and tumor growth of liver fluke-associatedcholangiocarcinoma. Mol Cancer 2011;10:102.
119. Hou YJ, Dong LW, Tan YX, et al. Inhibition of active autophagy in-duces apoptosis and increases chemosensitivity in chol-angiocarcinoma. Lab Invest 2011;91:1146–1157.
120. Xu X, Kobayashi S, Qiao W, et al. Induction of intrahepatic chol-angiocellular carcinoma by liver-specific disruption of Smad4 andPten in mice. J Clin Invest 2006;116:1843–1852.
121. Farazi PA, Zeisberg M, Glickman J, et al. Chronic bile duct injuryassociated with fibrotic matrix microenvironment provokes chol-angiocarcinoma in p53-deficient mice. Cancer Res 2006;66:6622–6627.
122. Song H, Mak KK, Topol L, et al. Mammalian Mst1 and Mst2 ki-nases play essential roles in organ size control and tumor sup-pression. Proc Natl Acad Sci U S A 2010;107:1431–1436.
123. Lee KP, Lee JH, Kim TS, et al. The Hippo-Salvador pathway restrainshepatic oval cell proliferation, liver size, and liver tumorigenesis.Proc Natl Acad Sci U S A 2010;107:8248–8253.
124. O’Dell MR, Huang JL, Whitney-Miller CL, et al. Kras(G12D) and p53mutation cause primary intrahepatic cholangiocarcinoma. CancerRes 2012;72:1557–1567.
125. Sirica AE, Zhang Z, Lai GH, et al. A novel “patient-like” model ofcholangiocarcinoma progression based on bile duct inoculation oftumorigenic rat cholangiocyte cell lines. Hepatology 2008;47:1178–1190.
126. Campbell DJ, Dumur CI, Lamour NF, et al. Novel organotypic culturemodel of cholangiocarcinoma progression. Hepatol Res 2012;42:1119–1130.
127. Fava G, Alpini G, Rychlicki C, et al. Leptin enhances chol-angiocarcinoma cell growth. Cancer Res 2008;68:6752–6761.
128. Yang H, Li TW, Peng J, et al. A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involvedin progression. Gastroenterology 2011;141:378–388, 388 e1–e4.
129. Plengsuriyakarn T, Eursitthichai V, Labbunruang N, et al.Ultrasonography as a tool for monitoring the development andprogression of cholangiocarcinoma in Opisthorchis viverrini/dimethylnitrosamine-induced hamsters. Asian Pac J Cancer Prev2012;13:87–90.
130. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic typeand stage classification. J Hepatobiliary Pancreat Surg 2003;10:288–291.
131. Rimola J, Forner A, Reig M, et al. Cholangiocarcinoma in cirrhosis:absence of contrast washout in delayed phases by magneticresonance imaging avoids misdiagnosis of hepatocellular carci-noma. Hepatology 2009;50:791–798.
132. Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB(Oxford) 2008;10:106–109.
133. Blechacz B, Gores GJ. Cholangiocarcinoma: advances in patho-genesis, diagnosis, and treatment. Hepatology 2008;48:308–321.
134. Patel AH, Harnois DM, Klee GG, et al. The utility of CA 19-9 in thediagnoses of cholangiocarcinoma in patients without primarysclerosing cholangitis. Am J Gastroenterol 2000;95:204–207.
135. Sapisochin G, Fidelman N, Roberts JP, et al. Mixed hepatocellularcholangiocarcinoma and intrahepatic cholangiocarcinoma in pa-tients undergoing transplantation for hepatocellular carcinoma.Liver Transpl 2011;17:934–942.
136. Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma:rising frequency, improved survival, and determinants of outcomeafter resection. Ann Surg 2008;248:84–96.
137. Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcomeof intrahepatic cholangiocarcinoma following surgical resection:
-
December 2013 CHOLANGIOCARCINOMA 1229
REV
IEW
SAND
PER
SPEC
TIVES
association of lymph node metastasis and lymph node dissectionwith survival. Ann Surg Oncol 2009;16:3048–3056.
138. Li YY, Li H, Lv P, et al. Prognostic value of cirrhosis for intrahepaticcholangiocarcinoma after surgical treatment. J Gastrointest Surg2011;15:608–613.
139. Fabris L, Cadamuro M, Moserle L, et al. Nuclear expression ofS100A4 calcium-binding protein increases cholangiocarcinomainvasiveness and metastasization. Hepatology 2011;54:890–899.
140. Kuhlmann JB, Blum HE. Locoregional therapy for chol-angiocarcinoma. Curr Opin Gastroenterol 2013;29:324–328.
141. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabineversus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–1281.
142. Yamashita Y, Takahashi M, Kanazawa S, et al. Hilar chol-angiocarcinoma. An evaluation of subtypes with CT and angiog-raphy. Acta Radiol 1992;33:351–355.
143. Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fineneedle aspiration biopsy of hilar cholangiocarcinoma is associatedwith disease dissemination. HPB (Oxford) 2011;13:356–360.
144. Moreno Luna LE, Kipp B, et al. Advanced cytologic techniques forthe detection of malignant pancreatobiliary strictures. Gastroen-terology 2006;131:1064–1072.
145. Barr Fritcher EG, Kipp BR, Voss JS, et al. Primary sclerosing chol-angitis patients with serial polysomy fluorescence in situ hybridi-zation results are at increased risk of cholangiocarcinoma. Am JGastroenterol 2011;106:2023–2028.
146. Barr Fritcher EG, Voss JS, Jenkins SM, et al. Primary sclerosingcholangitis with equivocal cytology: fluorescence in situ hybridiza-tion and serum CA 19–9 predict risk of malignancy. Cancer Cyto-pathol 2013. Epub ahead of print.
147. Nagorney DM, Kendrick ML. Hepatic resection in the treatment ofhilar cholangiocarcinoma. Adv Surg 2006;40:159–171.
148. Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neo-adjuvant chemoradiation, followed by liver transplantation, forperihilar cholangiocarcinoma at 12 US centers. Gastroenterology2012;143:88–98 e3; quiz e14.
149. Hong JC, Jones CM, Duffy JP, et al. Comparative analysis ofresection and liver transplantation for intrahepatic and hilar chol-angiocarcinoma: a 24-year experience in a single center. Arch Surg2011;146:683–689.
150. Geynisman DM, Catenacci DV. Toward personalized treatmentof advanced biliary tract cancers. Discov Med 2012;14:41–57.
Received August 8, 2013. Accepted October 10, 2013.
Reprint requestsAddress requests for reprints to: Gregory J. Gores, MD, Professor of
Medicine and Physiology, Mayo Clinic, 200 First Street SW,Rochester, Minnesota 55905. e-mail: [email protected];fax: (507) 284-0762.
AcknowledgmentsThe authors would like to thank Dr Thomas Smyrk for kindly
providing the stromal cholangiocarcinoma photomicrograph and MsCourtney Hoover for outstanding secretarial support.
Conflicts of interestThe authors disclose no conflicts.
FundingThis work was supported by National Institutes of Health grants
DK59427 (G.J.G.) and T32 DK007198 (S.R.), and the Mayo Foundation.
mailto:[email protected]
Pathogenesis, Diagnosis, and Management of CholangiocarcinomaEpidemiologyRisk FactorsCells of OriginInflammationGeneticsDevelopmental PathwaysTumor MicroenvironmentAnimal ModelsDiagnosis and ManagementiCCApCCAdCCA
Future DirectionsReferencesAcknowledgments